Deck 41: Atomic Physics
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/38
العب
ملء الشاشة (f)
Deck 41: Atomic Physics
1
The normalized wave function for a hydrogen atom in the 1s state is given by ψ(r)= 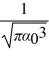 e-r/α0 where α0 is the Bohr radius,which is equal to 5.29 × 10-11 m.What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
e-r/α0 where α0 is the Bohr radius,which is equal to 5.29 × 10-11 m.What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
A)2.3 × 10-5
B)1.2 × 10-5
C)1.7 × 10-5
D)4.6 × 10-5
E)3.5 × 10-5
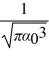 e-r/α0 where α0 is the Bohr radius,which is equal to 5.29 × 10-11 m.What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
e-r/α0 where α0 is the Bohr radius,which is equal to 5.29 × 10-11 m.What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?A)2.3 × 10-5
B)1.2 × 10-5
C)1.7 × 10-5
D)4.6 × 10-5
E)3.5 × 10-5
2.3 × 10-5
2
An electron in a hydrogen atom has orbital quantum number l = 7.How many possible values of the magnetic quantum number ml could it have?
A)6
B)7
C)15
D)33
E)98
A)6
B)7
C)15
D)33
E)98
15
3
For each value of the principal quantum number n,what are the possible values of the electron spin quantum number ms? (There may be more than one correct choice.)
A)0
B)+1/2
C)-1/2
D)+3/2
E)-3/2
A)0
B)+1/2
C)-1/2
D)+3/2
E)-3/2
+1/2
-1/2
-1/2
4
The magnitude of the orbital angular momentum L of an electron in a certain atom is equal to 3.464ħ.Which one of the following numbers could be the principal quantum number n of the electron?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
5
What is the greatest magnitude of the orbital angular momentum L for an electron in a state with principal quantum number n = 5?
A)4.47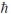
B)4.90
C)5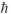
D)5.48
A)4.47
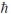
B)4.90

C)5
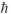
D)5.48


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
6
If two electrons in an atom have the same energy,then they must have the same four quantum numbers.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
7
The binding energy of the hydrogen atom in its ground state is -13.6 eV.What is the energy when it is in the n = 5 state?
A)2.72 eV
B)-2.72 eV
C)0.544 eV
D)-0.544 eV
E)-68 eV
A)2.72 eV
B)-2.72 eV
C)0.544 eV
D)-0.544 eV
E)-68 eV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
8
If the orbital quantum number is l = 4,which one of the following is a possible value for the principal quantum number n?
A)1
B)2
C)3
D)4
E)8
A)1
B)2
C)3
D)4
E)8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
9
Consider the four quantum numbers of an electron in an atom,n,l,ml,and ms.The energy of an electron in an isolated atom depends on
A)n only.
B)n and l only.
C)n,l,and ml only.
D)l,ml,and ms only.
E)all four quantum numbers.
A)n only.
B)n and l only.
C)n,l,and ml only.
D)l,ml,and ms only.
E)all four quantum numbers.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
10
The magnitude of the orbital angular momentum L of an electron in a certain atom is equal to 3.464ħ.What is the orbital quantum number l of the electron?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
11
An electron in a hydrogen atom is in the n = 7 shell.How many possible values of the orbital quantum number l could it have?
A)6
B)7
C)15
D)33
E)98
A)6
B)7
C)15
D)33
E)98

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
12
What is the energy of an incident photon that is just enough to excite a hydrogen atom from its ground state to its n = 4 state?
A)12.75 eV
B)10.20 eV
C)3.40 eV
D)0.85 eV
A)12.75 eV
B)10.20 eV
C)3.40 eV
D)0.85 eV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
13
How fast must a hydrogen atom be traveling for its kinetic energy to be just enough to excite the ground-state atom to its first excited state in a collision? (1 eV = 1.60 × 10-19 J, mh ≈ mproton = 1.67 × 10-27 kg)
A)44.2 km/s
B)21.7 km/s
C)66.5 km/s
D)113 km/s
E)51.0 km/s
A)44.2 km/s
B)21.7 km/s
C)66.5 km/s
D)113 km/s
E)51.0 km/s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
14
In the ground state,the quantum numbers (n,l,ml,ms)for hydrogen are,respectively
A)1,1,1,1.
B)1,0,0,0.
C)1,0,0,± .
.
D)1,1,1,± .
.
E)1,1,0,± .
.
A)1,1,1,1.
B)1,0,0,0.
C)1,0,0,±
 .
.D)1,1,1,±
 .
.E)1,1,0,±
 .
.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
15
An electron in a hydrogen atom has orbital quantum number l = 4.How many possible values of the magnetic quantum number ml could it have?
A)4
B)10
C)5
D)9
E)3
A)4
B)10
C)5
D)9
E)3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
16
What is the minimum speed needed by a ground-state hydrogen atom for its kinetic energy to be enough to ionize the atom in a collision? (1 eV = 1.60 × 10-19 J,mh ≈ mproton = 1.67 × 10-27 kg)
A)44.2 km/s
B)21.7 km/s
C)66.5 km/s
D)113 km/s
E)51.0 km/s
A)44.2 km/s
B)21.7 km/s
C)66.5 km/s
D)113 km/s
E)51.0 km/s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
17
If an electron has spin quantum number ms = -  ,what is the possible value for the orbital quantum number l of the electron?
,what is the possible value for the orbital quantum number l of the electron?
A)0
B)1
C)2
D)11
E)All of the above numbers are possible.
 ,what is the possible value for the orbital quantum number l of the electron?
,what is the possible value for the orbital quantum number l of the electron?A)0
B)1
C)2
D)11
E)All of the above numbers are possible.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which of the following are characteristics of laser light? (There may be more than one correct choice.)
A)It is coherent.
B)It is produced by an inverted population of atoms.
C)It contains a full spectrum of wavelengths.
A)It is coherent.
B)It is produced by an inverted population of atoms.
C)It contains a full spectrum of wavelengths.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
19
An electron in a hydrogen atom has principal quantum number n = 4.How many possible values of the orbital quantum number l could it have?
A)8
B)9
C)3
D)4
E)10
A)8
B)9
C)3
D)4
E)10

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
20
If the principal quantum number of an electron is n = 5,which one of the following is NOT an allowed magnetic quantum number ml for the electron?
A)0
B)2
C)3
D)4
E)5
A)0
B)2
C)3
D)4
E)5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
21
The only VALID electron state and shell designation among the following is
A)1p,K.
B)2s,K.
C)1s,L.
D)2p,L.
E)3f,M.
A)1p,K.
B)2s,K.
C)1s,L.
D)2p,L.
E)3f,M.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
22
How many photons per second emerge from a laser of power 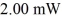 with wavelength
with wavelength 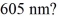 (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s)
(c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s)
A)6.09 × 1015 photons/s
B)5.03 × 1012 photons/s
C)3.07 × 1015 photons/s
D)3.07 × 1013 photons/s
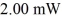 with wavelength
with wavelength 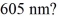 (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s)
(c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s)A)6.09 × 1015 photons/s
B)5.03 × 1012 photons/s
C)3.07 × 1015 photons/s
D)3.07 × 1013 photons/s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
23
A neutral atom has an electron configuration of 1s22s22p63s23p2.How many protons does it have in its nucleus?
A)5
B)11
C)14
D)20
E)26
A)5
B)11
C)14
D)20
E)26

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
24
The magnitude of the orbital angular momentum L of an electron in a certain atom is equal to 3.464ħ.Which of the following angles could NOT be the angle between the orbital angular momentum vector of the electron and an arbitrary z-direction?
A)107°
B)90.0°
C)73.2°
D)54.7°
E)0.00°
A)107°
B)90.0°
C)73.2°
D)54.7°
E)0.00°

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
25
An atom has completely filled inner shells and a single valence electron in an excited p state.The filled inner shells have an orbital momentum equal to zero.What is the magnitude of the orbital angular momentum L of the atom?
A)1.0ħ
B)1.2ħ
C)1.4ħ
D)1.7ħ
E)2.0ħ
A)1.0ħ
B)1.2ħ
C)1.4ħ
D)1.7ħ
E)2.0ħ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
26
How many possible sets of quantum numbers (electron states)are there in the 5f subshell?
A)2
B)6
C)8
D)10
E)14
A)2
B)6
C)8
D)10
E)14

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
27
What is the correct electronic configuration for the ground state sodium atom,which has 11 electrons?
A)1s12s23p62s2
B)1s22s13p62s2
C)1s12s22p6
D)1s22s22p63s2
E)1s22s22p63s1
A)1s12s23p62s2
B)1s22s13p62s2
C)1s12s22p6
D)1s22s22p63s2
E)1s22s22p63s1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
28
A collection of atoms has 20% of the sample in a state 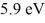 above the ground state.If these emit coherent radiation,what is the wavelength of the laser light produced? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
above the ground state.If these emit coherent radiation,what is the wavelength of the laser light produced? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
A)210 nm
B)91 nm
C)340 nm
D)34 nm
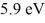 above the ground state.If these emit coherent radiation,what is the wavelength of the laser light produced? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
above the ground state.If these emit coherent radiation,what is the wavelength of the laser light produced? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)A)210 nm
B)91 nm
C)340 nm
D)34 nm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
29
The wavelength of a ruby laser is 694.3 nm.What is the energy difference between the two energy states involved in laser action? (c = 2.9979 × 108 m/s,h = 6.626 × 10-34 J ∙ s, 1 eV = 1.6022 × 10-19 J)
A)1.537 eV
B)1.646 eV
C)1.786 eV
D)1.812 eV
E)3.572 eV
A)1.537 eV
B)1.646 eV
C)1.786 eV
D)1.812 eV
E)3.572 eV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
30
How many electrons does it take to fill the d subshell?
A)10
B)6
C)14
D)4
E)8
A)10
B)6
C)14
D)4
E)8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
31
What is the correct electronic configuration for ground state carbon,which has 6 electrons?
A)1s22s22p2
B)1s12p1
C)1s12s22p1
D)1s12s12p1
E)1s22s22p4
A)1s22s22p2
B)1s12p1
C)1s12s22p1
D)1s12s12p1
E)1s22s22p4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
32
The only INVALID electron state and shell designation among the following is
A)1s,K.
B)2s,L.
C)2d,L.
D)3s,M.
E)3d,M.
A)1s,K.
B)2s,L.
C)2d,L.
D)3s,M.
E)3d,M.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
33
How many electrons can be found with principal quantum number n = 3 in a suitably heavy atom?
A)18
B)6
C)20
D)9
A)18
B)6
C)20
D)9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
34
In a ruby laser,an electron jumps from a higher energy level to a lower one.If the energy difference between the two levels is 1.8 eV,what is the wavelength of the emitted photon? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
A)350 nm
B)470 nm
C)650 nm
D)690 nm
E)960 nm
A)350 nm
B)470 nm
C)650 nm
D)690 nm
E)960 nm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
35
An atom has completely filled inner shells and a single valence electron in an excited p state.The filled inner shells have an orbital momentum equal to zero.A magnetic field is applied,defining the z-axis along the field.Which of the following sets of angles are possible angles between the magnetic field and the orbital angular momentum?
A)45°
B)90°
C)45°,90°
D)45°,135°
E)45°,90°,135°
A)45°
B)90°
C)45°,90°
D)45°,135°
E)45°,90°,135°

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
36
What is the electron configuration for ground state Li,which has 3 electrons?
A)1s3
B)1s12s2
C)1s22s1
D)1s21p1
E)1s11p2
A)1s3
B)1s12s2
C)1s22s1
D)1s21p1
E)1s11p2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
37
The correct ground state electron configuration of boron,which has 5 electrons,is
A)1s22s22p.
B)1s22s22p3.
C)1s21p22s.
D)1s22p23s.
E)1s22p3.
A)1s22s22p.
B)1s22s22p3.
C)1s21p22s.
D)1s22p23s.
E)1s22p3.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
38
You need 14 W of infrared laser light power with wavelength 1270 nm to bore a hole in a diamond.How many downward atomic transitions per second must occur in the laser if all of them result in light directed onto the diamond? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J ∙ s)
A)8.9 × 1019
B)8.9 × 1018
C)5.9 × 1019
D)2.7 × 1018
A)8.9 × 1019
B)8.9 × 1018
C)5.9 × 1019
D)2.7 × 1018

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck








