Deck 19: Complex Ions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/51
العب
ملء الشاشة (f)
Deck 19: Complex Ions
1
What is the coordination number of the central metal ion in [Ni(NH3)4(H2O)2]Cl2?
A) 0
B) 2
C) 4
D) 6
E) 8
A) 0
B) 2
C) 4
D) 6
E) 8
6
2
Identify the ligands and their charges in [Os(NH3)4Cl2]+.
A) ammonia (no charge),chloride ion (charge = −1)
B) chloride ion (charge = −1)
C) ammonia (no charge)
D) osmium (charge = +3)
E) osmium (charge = +3),chloride ion (charge = −1)
A) ammonia (no charge),chloride ion (charge = −1)
B) chloride ion (charge = −1)
C) ammonia (no charge)
D) osmium (charge = +3)
E) osmium (charge = +3),chloride ion (charge = −1)
ammonia (no charge),chloride ion (charge = −1)
3
What is the formula for potassium diamminetetrachlorovanadate(III)?
A) [KV(NH3)2Cl4]
B) K2[V(NH3)2Cl4]
C) K[V(NH3)2Cl4]
D) [KV(NH3)2]Cl4
E) K[V(NH3)2]Cl4
A) [KV(NH3)2Cl4]
B) K2[V(NH3)2Cl4]
C) K[V(NH3)2Cl4]
D) [KV(NH3)2]Cl4
E) K[V(NH3)2]Cl4
K[V(NH3)2Cl4]
4
What is the coordination number of the copper in [Cu(NH3)2]Cl?
A) 1
B) 2
C) 3
D) 4
E) 8
A) 1
B) 2
C) 3
D) 4
E) 8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
5
What is the name of the compound having the formula K2[PtCl4]?
A) potassium chloroplatinate(II)
B) potassium tetrachloroplatinate(II)
C) potassium chloroplatinate(IV)
D) potassium platanotetrachlorate(II)
E) dipotassium tetrachloroplatnum(II)
A) potassium chloroplatinate(II)
B) potassium tetrachloroplatinate(II)
C) potassium chloroplatinate(IV)
D) potassium platanotetrachlorate(II)
E) dipotassium tetrachloroplatnum(II)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
6
What is the oxidation number of molybdenum in [Mo(H2O)5OH]Cl2?
A) +1
B) +2
C) +3
D) +4
E) +6
A) +1
B) +2
C) +3
D) +4
E) +6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
7
A Lewis base is defined as a species that
A) increases the OH− concentration in water.
B) accepts a proton.
C) donates a pair of electrons to a bond.
D) has a negative charge.
E) is two electrons short of an octet in the valence shell.
A) increases the OH− concentration in water.
B) accepts a proton.
C) donates a pair of electrons to a bond.
D) has a negative charge.
E) is two electrons short of an octet in the valence shell.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
8
What is the name of the compound having the formula (NH4)2[Cu(CN)4]?
A) ammonium tetracyanocuprate(II)
B) diammonium tetracyanocuprate(II)
C) bisamminetetracyanocuprate(II)
D) tetracyanobisamminecuprate(II)
E) tetracyanocuprate(II)ammonia
A) ammonium tetracyanocuprate(II)
B) diammonium tetracyanocuprate(II)
C) bisamminetetracyanocuprate(II)
D) tetracyanobisamminecuprate(II)
E) tetracyanocuprate(II)ammonia

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
9
What is the coordination number of the central metal ion in [Cr(en)2(OH)2]NO3? (en = H2NCH2CH2NH2)
A) 0
B) 2
C) 4
D) 6
E) 7
A) 0
B) 2
C) 4
D) 6
E) 7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
10
What is the correct formula for a nickel(II)complex which contains 4 ammonia molecules and 2 water molecules?
A) Ni(NH3)4(H2O)2
B) Ni(NH3)4(H2O)22+
C) [Ni(NH3)4](H2O)2
D) Ni(NH3)4(H2O)22−
E) Ni(NH3)4(H2O)24−
A) Ni(NH3)4(H2O)2
B) Ni(NH3)4(H2O)22+
C) [Ni(NH3)4](H2O)2
D) Ni(NH3)4(H2O)22−
E) Ni(NH3)4(H2O)24−

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
11
What is the correct formula for a square planar copper(II)complex that is bonded to ethylenediamine ligands?
A) Cu(en)2+
B) Cu(en)22+
C) Cu(en)32+
D) Cu(en)42+
E) Cu(en)62+
A) Cu(en)2+
B) Cu(en)22+
C) Cu(en)32+
D) Cu(en)42+
E) Cu(en)62+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
12
What is the oxidation number of the iron in [Fe(CN)6]3−?
A) −3
B) −2
C) +2
D) +3
E) +4
A) −3
B) −2
C) +2
D) +3
E) +4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
13
What is the formula for dicyanobis(ethylenediamine)zirconium(IV)nitrate?
A) Zr[(CN)2(NO3)2(en)2]
B) [Zr(CN)2(NO3)2(en)2]
C) (NO3)2[Zr(CN)2(en)2]
D) [Zr(CN)2(en)2](NO3)2
E) [Zr(NO3)2(en)2](CN)2
A) Zr[(CN)2(NO3)2(en)2]
B) [Zr(CN)2(NO3)2(en)2]
C) (NO3)2[Zr(CN)2(en)2]
D) [Zr(CN)2(en)2](NO3)2
E) [Zr(NO3)2(en)2](CN)2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
14
What is the oxidation number of the chromium in [Cr(H2O)2Cl4]−?
A) 0
B) +1
C) +2
D) +3
E) +5
A) 0
B) +1
C) +2
D) +3
E) +5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which molecule or ion does NOT act as a ligand?
A) H2O
B) NH4+
C) Cl-
D) C2O42-
E) CN-
A) H2O
B) NH4+
C) Cl-
D) C2O42-
E) CN-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
16
Identify the ligands and their charges in [Co(en)2Br2]+.
A) ethylene (charge = −1),bromide (charge = 0)
B) ethylene (charge = −1),bromide ion (charge = −1)
C) ethylenediamine (charge = 0),bromide ion (charge = −1),cobalt (charge = +3)
D) ethylenediamine (charge = 0),bromide (charge = 0)
E) ethylenediamine (charge = 0),bromide ion (charge = −1)
A) ethylene (charge = −1),bromide (charge = 0)
B) ethylene (charge = −1),bromide ion (charge = −1)
C) ethylenediamine (charge = 0),bromide ion (charge = −1),cobalt (charge = +3)
D) ethylenediamine (charge = 0),bromide (charge = 0)
E) ethylenediamine (charge = 0),bromide ion (charge = −1)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
17
Identify the ligands and their charges in [Cr(en)4(CN)2]NO3.
A) ethylenediamine (no charge)
B) cyanide (charge = no charge)
C) nitrate ion (charge = −1)
D) ethylenediamine (no charge),cyanide ion (charge = −1)
E) ethylenediamine (no charge),cyanide ion (charge = −1),nitrate ion (charge = −1)
A) ethylenediamine (no charge)
B) cyanide (charge = no charge)
C) nitrate ion (charge = −1)
D) ethylenediamine (no charge),cyanide ion (charge = −1)
E) ethylenediamine (no charge),cyanide ion (charge = −1),nitrate ion (charge = −1)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
18
What is the oxidation number of the copper in [Cu(NH3)3Cl]NO3?
A) 0
B) +1
C) +2
D) +4
E) +5
A) 0
B) +1
C) +2
D) +4
E) +5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
19
Ions such as [Co(H2O)6]3+ and [Ag(CN)2]- are called ____.
A) ligands
B) Lewis bases
C) chelates
D) alloys
E) coordination complexes
A) ligands
B) Lewis bases
C) chelates
D) alloys
E) coordination complexes

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
20
What is the name of the compound having the formula [Cr(en)2(H2O)2]SO4?
A) dihydroxydiethlyenediamminechromate(II)sulfate
B) diaquabis(ethylenediamine)sulfatochromate(IV)
C) bis(ethylenediamine)diaquachromium(II)sulfato
D) diaquabis(ethylenediamine)sulfatochromium(II)
E) diaquabis(ethylenediamine)chromium(II)sulfate
A) dihydroxydiethlyenediamminechromate(II)sulfate
B) diaquabis(ethylenediamine)sulfatochromate(IV)
C) bis(ethylenediamine)diaquachromium(II)sulfato
D) diaquabis(ethylenediamine)sulfatochromium(II)
E) diaquabis(ethylenediamine)chromium(II)sulfate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
21
The complex FeF64− is paramagnetic.Which of the following set of terms best describes the complex?
A) high-spin,Δo large
B) high-spin,Δo small
C) low-spin,Δo large
D) low-spin,Δo small
E) none of the above
A) high-spin,Δo large
B) high-spin,Δo small
C) low-spin,Δo large
D) low-spin,Δo small
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
22
If an octahedral iron(II)complex is diamagnetic,which of the following sets of conditions best describes the complex?
A) low-spin,Δo small
B) low-spin,Δo large
C) high-spin,Δo small
D) high-spin,Δo large
E) none of these
A) low-spin,Δo small
B) low-spin,Δo large
C) high-spin,Δo small
D) high-spin,Δo large
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which of the following species have geometric isomers: [Fe(en)Cl4]-,[Fe(en)2Cl2]+,and [Fe(en)2BrCl]+?
A) [Fe(en)Cl4]- only
B) [Fe(en)2Cl2]+ only
C) [Fe(en)2BrCl]+ only
D) [Fe(en)2Cl2]+ and [Fe(en)2BrCl]+
E) [Fe(en)Cl4]-,[Fe(en)2Cl2]+,and [Fe(en)2BrCl]+
A) [Fe(en)Cl4]- only
B) [Fe(en)2Cl2]+ only
C) [Fe(en)2BrCl]+ only
D) [Fe(en)2Cl2]+ and [Fe(en)2BrCl]+
E) [Fe(en)Cl4]-,[Fe(en)2Cl2]+,and [Fe(en)2BrCl]+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
24
Potassium hexacyanoiron(II),K4[Fe(CN)6],is water soluble.In an aqueous solution,it will decompose into
A) 2 ions.
B) 4 ions.
C) 5 ions.
D) 6 ions.
E) 10 ions.
A) 2 ions.
B) 4 ions.
C) 5 ions.
D) 6 ions.
E) 10 ions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
25
How many geometric isomers may exist for the square-planar complex ion [Pt(OH)2(NH3)2]2-?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
26
What are the possible formulas of all the tetracoordinate complex ions formed by copper(II)with NH3 and/or ethylenediamine (en)molecules as ligands?
A) Cu(NH3)42+,Cu(en)(NH3)32+,Cu(en)2(NH3)22+,Cu(en)3(NH3)2+,Cu(en)42+
B) Cu(NH3)42+,Cu(en)2(NH3)42+,Cu(en)22+
C) Cu(NH3)42+,Cu(en)(NH3)22+,Cu(en)22+
D) Cu(NH3)62+,Cu(en)2(NH3)22+,Cu(en)32+
E) Cu(NH3)42+,Cu(en)22+
A) Cu(NH3)42+,Cu(en)(NH3)32+,Cu(en)2(NH3)22+,Cu(en)3(NH3)2+,Cu(en)42+
B) Cu(NH3)42+,Cu(en)2(NH3)42+,Cu(en)22+
C) Cu(NH3)42+,Cu(en)(NH3)22+,Cu(en)22+
D) Cu(NH3)62+,Cu(en)2(NH3)22+,Cu(en)32+
E) Cu(NH3)42+,Cu(en)22+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
27
Determine the number of unpaired electrons in an octahedral,strong-field Mn2+ complex ion.
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
28
How many geometric isomers exist for [Fe(H2O)4(NH3)2]3+?
A) 1
B) 2
C) 3
D) 4
E) 6
A) 1
B) 2
C) 3
D) 4
E) 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
29
All of the followings statements are true EXCEPT
A) transition metal complexes are often colored,due to 3d to 1s electronic transitions.
B) in low-spin complexes,electrons are concentrated in the lower-energy orbitals.
C) low-spin complexes contain the minimum number of unpaired electrons.
D) the crystal field splitting energy is small in high-spin complexes.
E) the crystal field splitting energy of a metal complex can sometimes be determined from the absorption spectrum.
A) transition metal complexes are often colored,due to 3d to 1s electronic transitions.
B) in low-spin complexes,electrons are concentrated in the lower-energy orbitals.
C) low-spin complexes contain the minimum number of unpaired electrons.
D) the crystal field splitting energy is small in high-spin complexes.
E) the crystal field splitting energy of a metal complex can sometimes be determined from the absorption spectrum.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
30
Chlorophylls are coordination compounds that contain a magnesium ion as the central metal.The most abundant chlorophyll,called chlorophyll a,has a mass percent of magnesium of 2.72%.What is the molar mass of chlorophyll a?
A) 2.72 g/mol
B) 57.2 g/mol
C) 112 g/mol
D) 583 g/mol
E) 893 g/mol
A) 2.72 g/mol
B) 57.2 g/mol
C) 112 g/mol
D) 583 g/mol
E) 893 g/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which of the following molecules or ions are chelating agents: NH3,H2NCH2CH2NH2,CO2,and C2O42−?
A) NH3
B) NH3 and CO2
C) CO2 and H2NCH2CH2NH2
D) H2NCH2CH2NH2 and C2O42−
E) CO2,H2NCH2CH2NH2 and C2O42−
A) NH3
B) NH3 and CO2
C) CO2 and H2NCH2CH2NH2
D) H2NCH2CH2NH2 and C2O42−
E) CO2,H2NCH2CH2NH2 and C2O42−

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
32
What is the electron configuration of Cr2+?
A) 1s22s22p63s23p6
B) 1s22s22p63s23p63d64s2
C) 1s22s22p63s23p63d34s2
D) 1s22s22p63s23p63d54s1
E) 1s22s22p63s23p63d4
A) 1s22s22p63s23p6
B) 1s22s22p63s23p63d64s2
C) 1s22s22p63s23p63d34s2
D) 1s22s22p63s23p63d54s1
E) 1s22s22p63s23p63d4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
33
What is the electron configuration for Fe3+?
A) 1s22s22p63s23p63d54s1
B) 1s22s22p63s23p63d44s2
C) 1s22s22p63s23p63d6
D) 1s22s22p63s23p63d5
E) 1s22s22p63s23p63d7
A) 1s22s22p63s23p63d54s1
B) 1s22s22p63s23p63d44s2
C) 1s22s22p63s23p63d6
D) 1s22s22p63s23p63d5
E) 1s22s22p63s23p63d7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
34
How many possible structures exist for the tetrahedral complex [Zn(en)3]2+?
A) 1
B) 2
C) 3
D) 4
E) 6
A) 1
B) 2
C) 3
D) 4
E) 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
35
How many geometric isomers exist for [Fe(en)2(CN)2]+?
A) 1
B) 2
C) 3
D) 4
E) 6
A) 1
B) 2
C) 3
D) 4
E) 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
36
What is the electron configuration for Ti4+?
A) 1s22s22p63s23p64s2
B) 1s22s22p63s23p63d2
C) 1s22s22p63s23p63d24s2
D) 1s22s22p63s23p63d10
E) 1s22s22p63s23p6
A) 1s22s22p63s23p64s2
B) 1s22s22p63s23p63d2
C) 1s22s22p63s23p63d24s2
D) 1s22s22p63s23p63d10
E) 1s22s22p63s23p6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
37
Determine the number of unpaired electrons in the low-spin complex ion Cr(CN)64−.
A) 0
B) 1
C) 2
D) 4
E) 5
A) 0
B) 1
C) 2
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
38
Determine the number of unpaired electrons in an octahedral,high-spin Cr(II)complex.
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
39
When 6 ligands approach a central metal atom along the x-,y- and z-axes,they will cause the d orbitals to split in energy.The 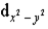 and the
and the 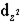 orbitals are most affected because
orbitals are most affected because
A) the electron density in these orbitals is squared.
B) unlike the other d orbitals,these orbitals contain unpaired electrons and absorb the energy of light more readily,compared to the other three d orbitals.
C) unlike the other d orbitals,these orbitals each contain a pair of electrons.
D) they have the highest electron density along the x-,y-,and z-axes,and thus feel the greatest repulsion from the ligands' unpaired electrons.
E) the shape of these orbitals is similar to that of the ligands' orbitals.
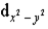 and the
and the 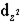 orbitals are most affected because
orbitals are most affected becauseA) the electron density in these orbitals is squared.
B) unlike the other d orbitals,these orbitals contain unpaired electrons and absorb the energy of light more readily,compared to the other three d orbitals.
C) unlike the other d orbitals,these orbitals each contain a pair of electrons.
D) they have the highest electron density along the x-,y-,and z-axes,and thus feel the greatest repulsion from the ligands' unpaired electrons.
E) the shape of these orbitals is similar to that of the ligands' orbitals.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
40
All of the following statements concerning crystal field theory are true EXCEPT
A) in low-spin complexes,electrons are concentrated in the dxy,dyz,and dxz orbitals.
B) in an isolated atom or ion,the five d orbitals have identical energy.
C) low-spin complexes contain the maximum number of unpaired electrons.
D) the crystal field splitting is larger in low-spin complexes than high-spin complexes.
E) the energy difference between d orbitals often corresponds to an energy of visible light.
A) in low-spin complexes,electrons are concentrated in the dxy,dyz,and dxz orbitals.
B) in an isolated atom or ion,the five d orbitals have identical energy.
C) low-spin complexes contain the maximum number of unpaired electrons.
D) the crystal field splitting is larger in low-spin complexes than high-spin complexes.
E) the energy difference between d orbitals often corresponds to an energy of visible light.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
41
Which of the following ligands causes the smallest splitting of d orbitals?
A) Cl−
B) en
C) CN−
D) NH3
E) NO3−
A) Cl−
B) en
C) CN−
D) NH3
E) NO3−

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
42
Heme is a coordination compound essential to your life.What is the metal ion coordinated in the heme of hemoglobin?
A) Cu2+
B) Ca2+
C) Co2+
D) Fe2+
E) Mg2+
A) Cu2+
B) Ca2+
C) Co2+
D) Fe2+
E) Mg2+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
43
Ti(NH3)63+ has a d-orbital electron transition at 399 nm.Find Δo at this wavelength.
A) 300 kJ/mol
B) 235 kJ/mol
C) 499 kJ/mol
D) 399 kJ/mol
E) 150 kJ/mol
A) 300 kJ/mol
B) 235 kJ/mol
C) 499 kJ/mol
D) 399 kJ/mol
E) 150 kJ/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
44
The spectrochemical series
A) lists metals ions in order of their oxidation state.
B) lists metals ions in order by their color.
C) lists ligands in order of their tendency to split d orbitals.
D) is used to determine the oxidation state of a central metal atom.
E) is used to calculate the crystal field splitting energy of complex ions.
A) lists metals ions in order of their oxidation state.
B) lists metals ions in order by their color.
C) lists ligands in order of their tendency to split d orbitals.
D) is used to determine the oxidation state of a central metal atom.
E) is used to calculate the crystal field splitting energy of complex ions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
45
The crystal field splitting energy for TiF63− is 203 kJ/mol.At what wavelength does the maximum absorbance of light occur for this complex?
A) 9.80 × 10−31 nm
B) 9.80 × 10−28 nm
C) 4.32 × 102 nm
D) 5.90 × 102 nm
E) 1.02 × 103 nm
A) 9.80 × 10−31 nm
B) 9.80 × 10−28 nm
C) 4.32 × 102 nm
D) 5.90 × 102 nm
E) 1.02 × 103 nm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
46
The wavelength of maximum absorption of Cu(NH3)42+ is 5.80 × 102 nm.What is the energy of the electronic transition?
A) 3.80 × 10−36 J
B) 1.16 × 10−31 J
C) 1.16 × 10−22 J
D) 3.42 × 10−19 J
E) 2.63 × 10+35 J
A) 3.80 × 10−36 J
B) 1.16 × 10−31 J
C) 1.16 × 10−22 J
D) 3.42 × 10−19 J
E) 2.63 × 10+35 J

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
47
Which of the following ligands causes the greatest splitting of d orbitals?
A) H2O
B) NO2−
C) Cl−
D) F−
E) NH3
A) H2O
B) NO2−
C) Cl−
D) F−
E) NH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
48
Alfred Werner published his theory of coordination chemistry in 1893.One of the postulates of his theory was that coordination complexes have "primary valences" and "secondary valences." He showed this by testing the ____ of solutions containing [Co(NH3)6]Cl3 vs.[Co(NH3)5 Cl]Cl2 vs.[Co(NH3)4 Cl2]Cl.
A) color
B) conductivity
C) density
D) melting point
E) taste
A) color
B) conductivity
C) density
D) melting point
E) taste

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
49
All of the following ions are able to form both low-spin and high-spin octahedral complex ions EXCEPT
A) Cr2+.
B) Cr3+.
C) Mn2+.
D) Fe3+.
E) Co3+.
A) Cr2+.
B) Cr3+.
C) Mn2+.
D) Fe3+.
E) Co3+.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
50
Why might a cyano complex of a metal appear yellow and the chloride complex of the same metal appear green?
A) The cyanide ions transmits yellow light more efficiently than the chloride ions.
B) The cyanide ions splits the d orbital energies more than the chloride ions,resulting in the cyanide complex absorbing yellow light.
C) The cyanide ions splits the d orbital energies more than chloride ions,resulting in the absorption band for the cyanide complex being shifted to shorter wavelengths.
D) The chloride ions splits the d orbital energies more than cyanide ions,resulting in the absorption band for the chloride complex being shifted to shorter wavelengths.
E) The chloride ions splits the d orbital energies more than the cyanide ions,resulting in the chloride complex absorbing yellow light.
A) The cyanide ions transmits yellow light more efficiently than the chloride ions.
B) The cyanide ions splits the d orbital energies more than the chloride ions,resulting in the cyanide complex absorbing yellow light.
C) The cyanide ions splits the d orbital energies more than chloride ions,resulting in the absorption band for the cyanide complex being shifted to shorter wavelengths.
D) The chloride ions splits the d orbital energies more than cyanide ions,resulting in the absorption band for the chloride complex being shifted to shorter wavelengths.
E) The chloride ions splits the d orbital energies more than the cyanide ions,resulting in the chloride complex absorbing yellow light.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck
51
All of the following complexes will be colored (in the visible)EXCEPT ____.
A) [Co(en)3]2+
B) [Fe(CN)6]4-
C) [Ni(H2O)6]2+
D) [Sc(NH3)6]3+
E) [Cu(NH3)4]2+
A) [Co(en)3]2+
B) [Fe(CN)6]4-
C) [Ni(H2O)6]2+
D) [Sc(NH3)6]3+
E) [Cu(NH3)4]2+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 51 في هذه المجموعة.
فتح الحزمة
k this deck








