Deck 16: Spontaneity of Reaction
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/49
العب
ملء الشاشة (f)
Deck 16: Spontaneity of Reaction
1
The dissolution of ammonium nitrate occurs spontaneously in water at 25°C.As NH4NO3 dissolves,the temperature of the water decreases.What are the signs of ΔH,ΔS,and ΔG for this process?
A) ΔH > 0,ΔS < 0,ΔG > 0
B) ΔH > 0,ΔS > 0,ΔG > 0
C) ΔH > 0,ΔS > 0,ΔG < 0
D) ΔH < 0,ΔS < 0,ΔG < 0
E) ΔH < 0,ΔS > 0,ΔG > 0
A) ΔH > 0,ΔS < 0,ΔG > 0
B) ΔH > 0,ΔS > 0,ΔG > 0
C) ΔH > 0,ΔS > 0,ΔG < 0
D) ΔH < 0,ΔS < 0,ΔG < 0
E) ΔH < 0,ΔS > 0,ΔG > 0
ΔH > 0,ΔS > 0,ΔG < 0
2
Calculate ΔS° for the following reaction, H2(g)+ Br2(l)→ 2HBr(g)
Given S°[H2(g)] = +131 J/mol⋅K,S°[Br2(l)] = +152 J/mol⋅K,and S°[HBr(g)] = +199 J/mol⋅K.
A) −84 J/K
B) +84 J/K
C) +115 J/K
D) +482 J/K
E) +681 J/K
Given S°[H2(g)] = +131 J/mol⋅K,S°[Br2(l)] = +152 J/mol⋅K,and S°[HBr(g)] = +199 J/mol⋅K.
A) −84 J/K
B) +84 J/K
C) +115 J/K
D) +482 J/K
E) +681 J/K
−84 J/K
3
If a chemical reaction has a negative change in entropy,ΔS,then
A) the reaction is endothermic.
B) the reaction is spontaneous.
C) the equilibrium constant is greater than 1.
D) there is an increase in the order of the system.
E) the change in Gibbs free energy,ΔG,is negative.
A) the reaction is endothermic.
B) the reaction is spontaneous.
C) the equilibrium constant is greater than 1.
D) there is an increase in the order of the system.
E) the change in Gibbs free energy,ΔG,is negative.
there is an increase in the order of the system.
4
All of the following statements are true EXCEPT
A) a reaction is spontaneous if ΔG < 0.
B) if ΔG = 0,the system is at equilibrium.
C) if ΔG = 0,then ΔS = ΔH.
D) if ΔG > 0,then a reaction is not spontaneous.
E) ΔG is referred to as Gibbs free energy.
A) a reaction is spontaneous if ΔG < 0.
B) if ΔG = 0,the system is at equilibrium.
C) if ΔG = 0,then ΔS = ΔH.
D) if ΔG > 0,then a reaction is not spontaneous.
E) ΔG is referred to as Gibbs free energy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
5
All of the following events result in an increase in entropy EXCEPT
A) the melting of candle wax.
B) the combustion of carbon.
C) the dissolution of sodium chloride in water.
D) the evaporation of ethanol.
E) the formation of N2O4(g)from NO2(g).
A) the melting of candle wax.
B) the combustion of carbon.
C) the dissolution of sodium chloride in water.
D) the evaporation of ethanol.
E) the formation of N2O4(g)from NO2(g).

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
6
For which of the following reactions will the entropy of the system decrease?
A) 2NH3(g)→ N2(g)+ 3H2(g)
B) 2C(s)+ O2(g)→ 2CO(g)
C) CaCO3(s)→ CaO(s)+ CO2(g)
D) 2NO2(g)→ N2O4(g)
E) NaOH(s)→ Na+(aq)+ OH-(aq)
A) 2NH3(g)→ N2(g)+ 3H2(g)
B) 2C(s)+ O2(g)→ 2CO(g)
C) CaCO3(s)→ CaO(s)+ CO2(g)
D) 2NO2(g)→ N2O4(g)
E) NaOH(s)→ Na+(aq)+ OH-(aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
7
If ΔG is positive at all temperatures,then which of the following statements must be true?
A) ΔH < 0 and ΔS < 0
B) ΔH < 0 and ΔS = 0
C) ΔH < 0 and ΔS > 0
D) ΔH > 0 and ΔS > 0
E) ΔH > 0 and ΔS < 0
A) ΔH < 0 and ΔS < 0
B) ΔH < 0 and ΔS = 0
C) ΔH < 0 and ΔS > 0
D) ΔH > 0 and ΔS > 0
E) ΔH > 0 and ΔS < 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
8
When a real gas is compressed from low pressure to a higher pressure,its temperature increases.Predict the signs of ΔH and ΔS.
A) ΔH < 0 and ΔS < 0
B) ΔH < 0 and ΔS > 0
C) ΔH > 0 and ΔS < 0
D) ΔH > 0 and ΔS > 0
E) ΔH < 0 and ΔS = 0
A) ΔH < 0 and ΔS < 0
B) ΔH < 0 and ΔS > 0
C) ΔH > 0 and ΔS < 0
D) ΔH > 0 and ΔS > 0
E) ΔH < 0 and ΔS = 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
9
All of the following statements are true EXCEPT
A) ΔS > 0 for systems that become more disorderly.
B) reactions are spontaneous when ΔG < 0.
C) reactions are spontaneous when ΔH < 0.
D) ΔH < 0 for exothermic reactions.
E) generally,nature tends to move from more ordered to more random states.
A) ΔS > 0 for systems that become more disorderly.
B) reactions are spontaneous when ΔG < 0.
C) reactions are spontaneous when ΔH < 0.
D) ΔH < 0 for exothermic reactions.
E) generally,nature tends to move from more ordered to more random states.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
10
All of the following statements are true EXCEPT
A) for a given material,the gas has greater entropy than the solid.
B) the entropy for gaseous elements is zero at 298 K.
C) at 0 K,an ordered pure crystalline solid has an entropy of zero.
D) for a given material,the liquid has greater entropy than the solid.
E) increasing the temperature of a substance increases its entropy.
A) for a given material,the gas has greater entropy than the solid.
B) the entropy for gaseous elements is zero at 298 K.
C) at 0 K,an ordered pure crystalline solid has an entropy of zero.
D) for a given material,the liquid has greater entropy than the solid.
E) increasing the temperature of a substance increases its entropy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which of the following linear chain alcohols is likely to have the largest standard molar entropy in the liquid state?
A) CH3OH
B) CH3CH2OH
C) CH3CH2CH2OH
D) CH3CH2CH2CH2OH
E) CH3CH2CH2CH2CH2OH
A) CH3OH
B) CH3CH2OH
C) CH3CH2CH2OH
D) CH3CH2CH2CH2OH
E) CH3CH2CH2CH2CH2OH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
12
If a chemical reaction is exothermic,but not spontaneous,which of the following must be true?
A) ΔG > 0,ΔS > 0 and ΔH > 0
B) ΔG < 0,ΔS > 0 and ΔH > 0
C) ΔG > 0,ΔS < 0 and ΔH > 0
D) ΔG < 0,ΔS < 0 and ΔH < 0
E) ΔG > 0,ΔS < 0 and ΔH < 0
A) ΔG > 0,ΔS > 0 and ΔH > 0
B) ΔG < 0,ΔS > 0 and ΔH > 0
C) ΔG > 0,ΔS < 0 and ΔH > 0
D) ΔG < 0,ΔS < 0 and ΔH < 0
E) ΔG > 0,ΔS < 0 and ΔH < 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
13
Calculate ΔS° for the following reaction, 2SO2(g)+ O2(g)→ 2SO3(g)
Given S°[SO2(g)] = 248.2 J/mol⋅K,S°[O2(g)] = 205.1 J/mol⋅K,and S°[SO3(g)] = 256.8 J/mol⋅K.
A) -196.5 J/K
B) -94.0 J/K
C) -187.9 J/K
D) +187.9 J/K
E) +196.5 J/K
Given S°[SO2(g)] = 248.2 J/mol⋅K,S°[O2(g)] = 205.1 J/mol⋅K,and S°[SO3(g)] = 256.8 J/mol⋅K.
A) -196.5 J/K
B) -94.0 J/K
C) -187.9 J/K
D) +187.9 J/K
E) +196.5 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
14
Calculate ΔS° for the evaporation of water,given S°[H2O(l)] = +69.9 J/mol⋅K and S°[H2O(g)] = +188.7 J/mol⋅K.
A) −258.6 J/K
B) −118.8 J/K
C) 0.0000 J/K
D) +118.8 J/K
E) +258.6 J/K
A) −258.6 J/K
B) −118.8 J/K
C) 0.0000 J/K
D) +118.8 J/K
E) +258.6 J/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which of the following reactions is likely to have the most positive change in entropy?
A) N2(g)+ 3H2(g)→ 2NH3(g)
B) N2(g)+ 2O2(g)→ 2NO2(g)
C) 2C(s)+ O2(g)→ 2CO(g)
D) C(s)+ O2(g)→ CO2(g)
E) CaO(s)+ CO2(g)→ CaCO3(s)
A) N2(g)+ 3H2(g)→ 2NH3(g)
B) N2(g)+ 2O2(g)→ 2NO2(g)
C) 2C(s)+ O2(g)→ 2CO(g)
D) C(s)+ O2(g)→ CO2(g)
E) CaO(s)+ CO2(g)→ CaCO3(s)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
16
Predict the signs of ΔS and ΔH for the evaporation of water at 295 K.
A) ΔH = 0 and ΔS > 0
B) ΔH > 0 and ΔS < 0
C) ΔH > 0 and ΔS > 0
D) ΔH < 0 and ΔS > 0
E) ΔH < 0 and ΔS < 0
A) ΔH = 0 and ΔS > 0
B) ΔH > 0 and ΔS < 0
C) ΔH > 0 and ΔS > 0
D) ΔH < 0 and ΔS > 0
E) ΔH < 0 and ΔS < 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
17
All of the following statements are false for the freezing of water at 273 K EXCEPT
A) ΔH < 0.
B) ΔH > 0.
C) ΔH = 0.
D) ΔS = 0.
E) ΔS > 0.
A) ΔH < 0.
B) ΔH > 0.
C) ΔH = 0.
D) ΔS = 0.
E) ΔS > 0.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
18
Predict the sign of ΔS and ΔH for the combustion of gasoline at 500 K.
A) ΔH > 0 and ΔS > 0
B) ΔH > 0 and ΔS = 0
C) ΔH < 0 and ΔS > 0
D) ΔH < 0 and ΔS < 0
E) ΔH < 0 and ΔS = 0
A) ΔH > 0 and ΔS > 0
B) ΔH > 0 and ΔS = 0
C) ΔH < 0 and ΔS > 0
D) ΔH < 0 and ΔS < 0
E) ΔH < 0 and ΔS = 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
19
The second law of thermodynamics states that
A) in a spontaneous process,there is a net increase in entropy,taking into account both the system and the surroundings.
B) there is no disorder in a perfect crystal at 0 K.
C) the total energy of the universe is always increasing.
D) the total energy of the universe is constant.
E) mass and energy are conserved in all chemical reactions.
A) in a spontaneous process,there is a net increase in entropy,taking into account both the system and the surroundings.
B) there is no disorder in a perfect crystal at 0 K.
C) the total energy of the universe is always increasing.
D) the total energy of the universe is constant.
E) mass and energy are conserved in all chemical reactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
20
Diluting concentrated sulfuric acid with water can be dangerous.The temperature of the solution can increase rapidly.What are the signs of ΔH,ΔS,and ΔG for this process?
A) ΔH < 0,ΔS > 0,ΔG < 0
B) ΔH < 0,ΔS < 0,ΔG < 0
C) ΔH < 0,ΔS > 0,ΔG > 0
D) ΔH > 0,ΔS > 0,ΔG < 0
E) ΔH > 0,ΔS < 0,ΔG > 0
A) ΔH < 0,ΔS > 0,ΔG < 0
B) ΔH < 0,ΔS < 0,ΔG < 0
C) ΔH < 0,ΔS > 0,ΔG > 0
D) ΔH > 0,ΔS > 0,ΔG < 0
E) ΔH > 0,ΔS < 0,ΔG > 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
21
Calculate ?G° for the following reaction at 298 K. S(s)+ O2(g)? SO2(g)
A) ?300.2 kJ
B) ?296.8 kJ
C) ?85.1 kJ
D) +29.3 kJ
E) +145.7 kJ
A) ?300.2 kJ
B) ?296.8 kJ
C) ?85.1 kJ
D) +29.3 kJ
E) +145.7 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
22
For the dimerization of nitrogen dioxide,ΔG° = −4.7 kJ at 25°C. 2NO2(g)→ N2O4(g)
Calculate ΔG for this reaction if the partial pressures of NO2 and N2O4 are both 0.50 atm.(R = 8.31 × 10−3 kJ/K)
A) −6.4 kJ
B) −4.8 kJ
C) −4.7 kJ
D) −4.6 kJ
E) −3.0 kJ
Calculate ΔG for this reaction if the partial pressures of NO2 and N2O4 are both 0.50 atm.(R = 8.31 × 10−3 kJ/K)
A) −6.4 kJ
B) −4.8 kJ
C) −4.7 kJ
D) −4.6 kJ
E) −3.0 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
23
Calculate ?S° for the dissociation of dinitrogen tetraoxide at 25°C. N2O4(g)? 2NO2(g)
A) ?2.10 kJ/K
B) ?0.550 kJ/K
C) ?0.208 kJ/K
D) +0.076 kJ/K
E) +2.10 kJ/K
A) ?2.10 kJ/K
B) ?0.550 kJ/K
C) ?0.208 kJ/K
D) +0.076 kJ/K
E) +2.10 kJ/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
24
The standard free energy change associated with the dissolution of ammonium nitrate in water is -6.73 kJ at 298 K. NH4NO3(s) ? NH4NO3(aq)
What is the equilibrium constant for the reaction? (R = 8.31 × 10?3 kJ/K)
A) 1.9 × 10-3
B) 6.6 × 10-2
C) 1.0
D) 15
E) 5.2 × 102
What is the equilibrium constant for the reaction? (R = 8.31 × 10?3 kJ/K)
A) 1.9 × 10-3
B) 6.6 × 10-2
C) 1.0
D) 15
E) 5.2 × 102

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
25
If ΔG is negative at all temperatures,then which of the following statements must be true?
A) ΔH < 0 and ΔS < 0
B) ΔH < 0 and ΔS = 0
C) ΔH < 0 and ΔS > 0
D) ΔH > 0 and ΔS > 0
E) ΔH > 0 and ΔS < 0
A) ΔH < 0 and ΔS < 0
B) ΔH < 0 and ΔS = 0
C) ΔH < 0 and ΔS > 0
D) ΔH > 0 and ΔS > 0
E) ΔH > 0 and ΔS < 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
26
Ammonia is synthesized from nitrogen and hydrogen gases. N2(g)+ 3H2(g)→ 2NH3(g)
If ΔH° = −92.2 kJ and ΔS° = −0.1987 kJ/K,at what temperature will ΔG° = 0?
A) 0.00216 K
B) 18.3 K
C) 92.0 K
D) 464 K
E) 672 K
If ΔH° = −92.2 kJ and ΔS° = −0.1987 kJ/K,at what temperature will ΔG° = 0?
A) 0.00216 K
B) 18.3 K
C) 92.0 K
D) 464 K
E) 672 K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
27
Calculate ΔG° for the reaction below at 25.0°C CS2(g)+ 3Cl2(g)→ S2Cl2(g)+ CCl4(g)
Given ΔH° = -231.1 kJ and ΔS° = -287.6 J/K.
A) -518.7 kJ
B) -316.9 kJ
C) -145.4 kJ
D) -56.5 kJ
E) +56.5 kJ
Given ΔH° = -231.1 kJ and ΔS° = -287.6 J/K.
A) -518.7 kJ
B) -316.9 kJ
C) -145.4 kJ
D) -56.5 kJ
E) +56.5 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
28
At 298 K,all of the following substances have a standard free energy of formation of zero EXCEPT ____.
A) Br2(g)
B) I2(s)
C) S8(s)
D) Cl2(g)
E) Hg(l)
A) Br2(g)
B) I2(s)
C) S8(s)
D) Cl2(g)
E) Hg(l)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
29
What is the correct form for the Gibbs-Helmholtz equation?
A) ΔG = ΔH − TΔS
B) ΔG = ΔH + TΔS
C) ΔH = ΔG − TΔS
D) ΔS = ΔH − TΔG
E) ΔG = ΔS − TΔH
A) ΔG = ΔH − TΔS
B) ΔG = ΔH + TΔS
C) ΔH = ΔG − TΔS
D) ΔS = ΔH − TΔG
E) ΔG = ΔS − TΔH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
30
In what temperature range is ΔG° greater than zero for the formation of NH4Cl(s)from NH3(g)and HCl(g)? NH3(g)+ HCl(g)→ NH4Cl(s)
For the reaction,ΔH° = −176.0 kJ and ΔS° = −0.2845 kJ/K.
A) The temperature must be greater than 619 K.
B) The temperature must be less than 619 K.
C) The temperature must be exactly 619 K.
D) ΔG° is always greater than zero.
E) ΔG° is never greater than zero.
For the reaction,ΔH° = −176.0 kJ and ΔS° = −0.2845 kJ/K.
A) The temperature must be greater than 619 K.
B) The temperature must be less than 619 K.
C) The temperature must be exactly 619 K.
D) ΔG° is always greater than zero.
E) ΔG° is never greater than zero.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
31
Ammonia is synthesized from nitrogen and hydrogen gases at a temperature of 475°C. N2(g)+ 3H2(g)→ 2NH3(g)
If ΔH° = −92.2 kJ and ΔS° = −0.1987 kJ/K,what is ΔG° for the reaction at 575°C?
A) −260.7 kJ
B) −186.6 kJ
C) −92.0 kJ
D) +2.2 kJ
E) +76.3 kJ
If ΔH° = −92.2 kJ and ΔS° = −0.1987 kJ/K,what is ΔG° for the reaction at 575°C?
A) −260.7 kJ
B) −186.6 kJ
C) −92.0 kJ
D) +2.2 kJ
E) +76.3 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
32
At what temperatures will a reaction be spontaneous if ΔH° = +62.4 kJ and ΔS° = +301 J/K?
A) All temperatures below 207 K.
B) All temperatures above 207 K.
C) Temperatures between 179 K and 235 K.
D) The reaction will be spontaneous at any temperature.
E) The reaction will never be spontaneous.
A) All temperatures below 207 K.
B) All temperatures above 207 K.
C) Temperatures between 179 K and 235 K.
D) The reaction will be spontaneous at any temperature.
E) The reaction will never be spontaneous.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
33
What is ΔG° at 555°C for a reaction with ΔH° = −152 kJ and ΔS° = 79.1 J/K?
A) −4370 kJ
B) −217 kJ
C) −196 kJ
D) +196 kJ
E) + 643 kJ
A) −4370 kJ
B) −217 kJ
C) −196 kJ
D) +196 kJ
E) + 643 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
34
For the decomposition of hydrogen peroxide to water and oxygen, H2O2(g)→ H2O(g)+ 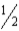 O2(g)
O2(g)
ΔH° = −106 kJ,and ΔS° = +0.0580 kJ/K.Under which of the following conditions is the reaction spontaneous (ΔG° < 0)?
A) The temperature must be greater than 1.83 × 103 K.
B) The temperature must be less than 1.83 × 103 K.
C) The temperature must be between 225 K and 1.83 × 103 K.
D) ΔG° is always less than zero.
E) ΔG° is never less than zero.
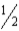 O2(g)
O2(g)ΔH° = −106 kJ,and ΔS° = +0.0580 kJ/K.Under which of the following conditions is the reaction spontaneous (ΔG° < 0)?
A) The temperature must be greater than 1.83 × 103 K.
B) The temperature must be less than 1.83 × 103 K.
C) The temperature must be between 225 K and 1.83 × 103 K.
D) ΔG° is always less than zero.
E) ΔG° is never less than zero.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
35
Calculate at 325 K for ethylene,C2H4(g),given the thermodynamic data below.
A) ?47.1 kJ
B) ?19.0 kJ
C) +39.1 kJ
D) +52.1 kJ
E) +69.6 kJ
A) ?47.1 kJ
B) ?19.0 kJ
C) +39.1 kJ
D) +52.1 kJ
E) +69.6 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
36
Nitric oxide can be made from the reaction of oxygen and nitrogen gases. O2(g)+ N2(g)→ 2NO(g)
If ΔG° = 165.5 kJ,and ΔH° = 180.4 kJ,what is ΔS° at 325°C?
A) 0.0125 kJ/K
B) 0.0249 kJ/K
C) 0.0458 kJ/K
D) 0.142 kJ/K
E) 1.02 kJ/K
If ΔG° = 165.5 kJ,and ΔH° = 180.4 kJ,what is ΔS° at 325°C?
A) 0.0125 kJ/K
B) 0.0249 kJ/K
C) 0.0458 kJ/K
D) 0.142 kJ/K
E) 1.02 kJ/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
37
Calculate ?S° for the dissolution of BaCl2 in water at 25°C. BaCl2(s)? Ba2+(aq)+ 2Cl?(aq)
A) ?0.20 kJ/K
B) ?0.14 kJ/K
C) ?0.0020 kJ/K
D) +0.12 kJ/K
E) +0.29 kJ/K
A) ?0.20 kJ/K
B) ?0.14 kJ/K
C) ?0.0020 kJ/K
D) +0.12 kJ/K
E) +0.29 kJ/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
38
In a gas phase reaction,what is the effect of increasing reactant or product pressure on the standard Gibbs free energy,ΔG°?
A) ΔG° increases due to decreased entropy.
B) ΔG° decreases due to decreased entropy.
C) ΔG° increases due to increased enthalpy.
D) ΔG° may either increase or decrease.
E) ΔG° is unchanged.
A) ΔG° increases due to decreased entropy.
B) ΔG° decreases due to decreased entropy.
C) ΔG° increases due to increased enthalpy.
D) ΔG° may either increase or decrease.
E) ΔG° is unchanged.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
39
When ammonium nitrate dissolves spontaneously in water the temperature of the solution decreases.Which statement is true for this system?
A) ΔH < 0 and ΔS < 0
B) ΔH < 0 and ΔS = 0
C) ΔH = 0 and ΔS > 0
D) ΔH > 0 and ΔS > 0
E) ΔH > 0 and ΔS < 0
A) ΔH < 0 and ΔS < 0
B) ΔH < 0 and ΔS = 0
C) ΔH = 0 and ΔS > 0
D) ΔH > 0 and ΔS > 0
E) ΔH > 0 and ΔS < 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
40
Calculate ?G° for the reaction below at 25.0°C.
P4O10(s)+ 6H2O(l)? 4H3PO4(l)
A) -6119.1 kJ
B) -632.8 kJ
C) -355.6 kJ
D) +210.6 kJ
E) +6119.1 kJ
P4O10(s)+ 6H2O(l)? 4H3PO4(l)
A) -6119.1 kJ
B) -632.8 kJ
C) -355.6 kJ
D) +210.6 kJ
E) +6119.1 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
41
For the decomposition of calcium carbonate,ΔG° = +130.4 kJ at 25°C. CaCO3(s)→ CaO(s)+ CO2(g)
Calculate the partial pressure of CO2 if ΔG = 0.0 kJ.(R = 8.31 × 10−3 kJ/K)
A) 1.3 × 10−23 atm
B) 2.7 × 102 atm
C) 8.2 × 10−5 atm
D) 5.3 × 101 atm
E) 7.4 × 1022 atm
Calculate the partial pressure of CO2 if ΔG = 0.0 kJ.(R = 8.31 × 10−3 kJ/K)
A) 1.3 × 10−23 atm
B) 2.7 × 102 atm
C) 8.2 × 10−5 atm
D) 5.3 × 101 atm
E) 7.4 × 1022 atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
42
Given that S(g)+ O2(g)? SO2(g)
?G° = -300.1 kJ
2S(g)+ 3O2(g)? 2SO3(g)
?G° = -742.1 kJ
Calculate of the following reaction:
SO2(g)+ 1/2O2(g)? SO3(g)
A) -1042.2 kJ
B) -71.0 kJ
C) +2.47 kJ
D) +71.0 kJ
E) +1042.2 kJ
?G° = -300.1 kJ
2S(g)+ 3O2(g)? 2SO3(g)
?G° = -742.1 kJ
Calculate of the following reaction:
SO2(g)+ 1/2O2(g)? SO3(g)
A) -1042.2 kJ
B) -71.0 kJ
C) +2.47 kJ
D) +71.0 kJ
E) +1042.2 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
43
For a chemical reaction,ΔG and ΔG° are equal
A) when products and reactants are in standard state concentrations.
B) when K = 0.
C) when K > 1.
D) for a system at equilibrium.
E) when the entropy change is zero.
A) when products and reactants are in standard state concentrations.
B) when K = 0.
C) when K > 1.
D) for a system at equilibrium.
E) when the entropy change is zero.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
44
At 25°C,the equilibrium constant for the following reaction, Cu2+(aq)+ Zn(s)→ Cu(s)+ Zn2+(aq)
Is 1.9 × 1037.Calculate ΔG° for this reaction.(R = 8.31 × 10−3 kJ/K)
A) −213 kJ
B) −145 kJ
C) −57.3 kJ
D) +57.3 kJ
E) +213 kJ
Is 1.9 × 1037.Calculate ΔG° for this reaction.(R = 8.31 × 10−3 kJ/K)
A) −213 kJ
B) −145 kJ
C) −57.3 kJ
D) +57.3 kJ
E) +213 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
45
Rubber elasticity is an entropic phenomenon.As a rubber band is stretched the entropy of the system
A) is reduced.
B) is increased.
C) remains unchanged.
D) breaks the polymer chain.
E) causes chemical bonds to form.
A) is reduced.
B) is increased.
C) remains unchanged.
D) breaks the polymer chain.
E) causes chemical bonds to form.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
46
If ΔG° = 0,then
A) K = 0.
B) K < 0.
C) K < 1.
D) K = 1.
E) K < −1.
A) K = 0.
B) K < 0.
C) K < 1.
D) K = 1.
E) K < −1.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
47
At 25°C,the acid dissociation constant for formic acid is 6.9 × 10−4.Calculate ΔG° for this reaction.(R = 8.31 × 10−3 kJ/K)
A) −18.0 kJ
B) −1.51 kJ
C) +1.51 kJ
D) +18.0 kJ
E) +41.4 kJ
A) −18.0 kJ
B) −1.51 kJ
C) +1.51 kJ
D) +18.0 kJ
E) +41.4 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
48
The standard free energy change for a chemical reaction is +13.3 kJ.What is the equilibrium constant for the reaction at 125°C? (R = 8.31 × 10−3 kJ/K)
A) 2.8 × 10-6
B) 2.0 × 10-5
C) 4.7 × 10-3
D) 1.8 × 10-2
E) 2.1 × 102
A) 2.8 × 10-6
B) 2.0 × 10-5
C) 4.7 × 10-3
D) 1.8 × 10-2
E) 2.1 × 102

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck
49
Given that,at 25°C, C(s)+ O2(g)→ CO2(g)
ΔG° = −394.4 kJ
C(s)+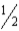 O2(g)→ CO(g)
O2(g)→ CO(g)
ΔG° = −137.2 kJ
Calculate ΔG° for the reaction below.
CO(g)+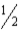 O2(g)→ CO2(g)
O2(g)→ CO2(g)
A) −531.6 kJ
B) −257.2 kJ
C) −171.5 kJ
D) +141.2 kJ
E) +531.6 kJ
ΔG° = −394.4 kJ
C(s)+
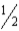 O2(g)→ CO(g)
O2(g)→ CO(g)ΔG° = −137.2 kJ
Calculate ΔG° for the reaction below.
CO(g)+
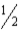 O2(g)→ CO2(g)
O2(g)→ CO2(g)A) −531.6 kJ
B) −257.2 kJ
C) −171.5 kJ
D) +141.2 kJ
E) +531.6 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 49 في هذه المجموعة.
فتح الحزمة
k this deck








