Deck 4: Reactions in Aqueous Solution
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/47
العب
ملء الشاشة (f)
Deck 4: Reactions in Aqueous Solution
1
Which of the following ionic compounds is not soluble in water?
A) K2CO3
B) AgNO3
C) CaBr2
D) CsI
E) PbI2
A) K2CO3
B) AgNO3
C) CaBr2
D) CsI
E) PbI2
PbI2
2
What reaction occurs when aqueous solutions of silver nitrate and potassium iodide are mixed?
A) Ag+(aq)+ K+(aq)→ AgK(s)
B) NO3−(aq)+ I−(aq)→ NO3I(s)
C) NO3−(aq)+ K+(aq)→ KNO3(s)
D) Ag+(aq)+ NO3−(aq)+ K+(aq)+ I−(aq)→ AgI(s)+ 2KNO3(s)
E) Ag+(aq)+ I−(aq)→ AgI(s)
A) Ag+(aq)+ K+(aq)→ AgK(s)
B) NO3−(aq)+ I−(aq)→ NO3I(s)
C) NO3−(aq)+ K+(aq)→ KNO3(s)
D) Ag+(aq)+ NO3−(aq)+ K+(aq)+ I−(aq)→ AgI(s)+ 2KNO3(s)
E) Ag+(aq)+ I−(aq)→ AgI(s)
Ag+(aq)+ I−(aq)→ AgI(s)
3
Which of the following ionic compounds are likely to be soluble in water: Mg(OH)2,Pb(NO3)2,AgI,Na2CO3,and Cu3(PO4)3?
A) Na2CO3 only
B) Pb(NO3)2 and Na2CO3
C) Mg(OH)2,Na2CO3,and Cu3(PO4)3
D) Mg(OH)2,AgI,and Cu3(PO4)3
E) Pb(NO3)2,AgI,Na2CO3,and Cu3(PO4)3
A) Na2CO3 only
B) Pb(NO3)2 and Na2CO3
C) Mg(OH)2,Na2CO3,and Cu3(PO4)3
D) Mg(OH)2,AgI,and Cu3(PO4)3
E) Pb(NO3)2,AgI,Na2CO3,and Cu3(PO4)3
Pb(NO3)2 and Na2CO3
4
Precipitation reactions occur
A) when group 1 cations are mixed with group 17 anions.
B) when insoluble reactants are mixed.
C) when ionic compounds react to form non-ionic products.
D) predominantly with halide salts.
E) when soluble ionic reactants combine to form insoluble products.
A) when group 1 cations are mixed with group 17 anions.
B) when insoluble reactants are mixed.
C) when ionic compounds react to form non-ionic products.
D) predominantly with halide salts.
E) when soluble ionic reactants combine to form insoluble products.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which of the following compounds is least soluble in water?
A) Fe(OH)3.
B) Sr(OH)2.
C) Al(NO3)3.
D) KI.
E) CuCl2.
A) Fe(OH)3.
B) Sr(OH)2.
C) Al(NO3)3.
D) KI.
E) CuCl2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which of the following reagents can be added to silver nitrate to precipitate the silver ion?
A) Pb(NO3)2
B) NH4ClO4
C) KNO3
D) NaI
E) KClO4
A) Pb(NO3)2
B) NH4ClO4
C) KNO3
D) NaI
E) KClO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following reagents can be added to a solution of sodium carbonate to precipitate the carbonate ion?
A) FeCl3
B) MgBr2
C) Cu(ClO4)2
D) Pb(NO3)2
E) all of the above
A) FeCl3
B) MgBr2
C) Cu(ClO4)2
D) Pb(NO3)2
E) all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
8
What mass of precipitate is formed when 25.00 mL of 0.200 M BaCl2 and 35.00 mL of 0.125 M Na2SO4 are mixed?
A) 0.510 g
B) 1.17 g
C) 1.02 g
D) 2.04 g
E) 0.583 g
A) 0.510 g
B) 1.17 g
C) 1.02 g
D) 2.04 g
E) 0.583 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
9
What is the net ionic equation for the reaction of aqueous sodium hydroxide and aqueous iron(II)chloride?
A) Na+(aq)+ OH-(aq)→ NaOH(s)
B) Na+(aq)+ Cl-(aq)→ NaCl(s)
C) Fe2+(aq)+ 2OH-(aq)→ Fe(OH)2(s)
D) Fe2+(aq)+ OH-(aq)→ FeOH+(s)
E) Fe2+(aq)+ 2Cl-(aq)→ FeCl2(s)
A) Na+(aq)+ OH-(aq)→ NaOH(s)
B) Na+(aq)+ Cl-(aq)→ NaCl(s)
C) Fe2+(aq)+ 2OH-(aq)→ Fe(OH)2(s)
D) Fe2+(aq)+ OH-(aq)→ FeOH+(s)
E) Fe2+(aq)+ 2Cl-(aq)→ FeCl2(s)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
10
What volume of 0.250 M KCl(aq)will completely react with 50.0 mL of 0.115 M Pb(NO3)2(aq)? Pb2+(aq)+ 2Cl−(aq)→ PbCl2(s)
A) 23.0 mL
B) 46.0 mL
C) 11.5 mL
D) 109 mL
E) 218 mL
A) 23.0 mL
B) 46.0 mL
C) 11.5 mL
D) 109 mL
E) 218 mL

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
11
Write a balanced net ionic equation for the reaction of aqueous solutions of lead(II)nitrate and potassium chloride.
A) Pb(NO3)2(aq)+ 2KCl(aq)→ PbCl2(s)+ 2KNO3(aq)
B) Pb2+(aq)+ 2K+(aq)→ PbK2(s)
C) Pb2+(aq)+ 2Cl−(aq)→ PbCl2(s)
D) NO3−(aq)+ K+(aq)→ KNO3(s)
E) no precipitation occurs.
A) Pb(NO3)2(aq)+ 2KCl(aq)→ PbCl2(s)+ 2KNO3(aq)
B) Pb2+(aq)+ 2K+(aq)→ PbK2(s)
C) Pb2+(aq)+ 2Cl−(aq)→ PbCl2(s)
D) NO3−(aq)+ K+(aq)→ KNO3(s)
E) no precipitation occurs.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
12
What reaction occurs when aqueous solutions of sodium hydroxide and copper(II)bromide are mixed?
A) Na+(aq)+ OH−(aq)+ Cu2+(aq)+ 2Br−(aq)→ NaCu(s)+ Br2OH(aq)
B) Na+(aq)+ OH−(aq)+ CuBr2(s)→ NaBr2(aq)+ CuOH(s)
C) Na+(aq)+ Br−(aq)→ NaBr(s)
D) 2Na+(aq)+ Br2(aq)→ 2NaBr(s)
E) 2OH−(aq)+ Cu2+(aq)→ Cu(OH)2(s)
A) Na+(aq)+ OH−(aq)+ Cu2+(aq)+ 2Br−(aq)→ NaCu(s)+ Br2OH(aq)
B) Na+(aq)+ OH−(aq)+ CuBr2(s)→ NaBr2(aq)+ CuOH(s)
C) Na+(aq)+ Br−(aq)→ NaBr(s)
D) 2Na+(aq)+ Br2(aq)→ 2NaBr(s)
E) 2OH−(aq)+ Cu2+(aq)→ Cu(OH)2(s)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
13
Write a balanced net ionic equation for the reaction of manganese(II)hydroxide,Mn(OH)2,with hydrochloric acid.
A) Mn(OH)2(s)+ 2H+(aq)→ Mn2+(aq)+ 2H2O(l)
B) Mn(OH)2(s)+ 2H+(aq)→ MnH2(s)+ 2OH−(aq)
C) (OH)3(aq)+ 3H+(aq)→ 3H2O(l)
D) 3OH−(aq)+ 3H+(aq)→ 3H2O(l)
E) Mn2+(aq)+ 2Cl−(aq)→ MnCl2(aq)
A) Mn(OH)2(s)+ 2H+(aq)→ Mn2+(aq)+ 2H2O(l)
B) Mn(OH)2(s)+ 2H+(aq)→ MnH2(s)+ 2OH−(aq)
C) (OH)3(aq)+ 3H+(aq)→ 3H2O(l)
D) 3OH−(aq)+ 3H+(aq)→ 3H2O(l)
E) Mn2+(aq)+ 2Cl−(aq)→ MnCl2(aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
14
If aqueous solutions of sodium sulfate and barium chloride are mixed,a white precipitate forms.What is the identity of the precipitate?
A) Na2Ba
B) NaCl2
C) NaCl
D) BaSO4
E) Ba2SO4
A) Na2Ba
B) NaCl2
C) NaCl
D) BaSO4
E) Ba2SO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
15
Identify all of the spectator ions in the precipitation reaction below. Ca2+(aq)+ 2Br−(aq)+ 2Li+(aq)+ CO32−(aq)→ CaCO3(s)+ 2Li+(aq)+ 2Br−(aq)
A) Ca2+ and Li+
B) Br− and CO32−
C) Br− and Li+
D) CaCO3
E) Ca2+,Br−,Li+,and CO32−
A) Ca2+ and Li+
B) Br− and CO32−
C) Br− and Li+
D) CaCO3
E) Ca2+,Br−,Li+,and CO32−

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which of the following pairs of aqueous solutions will produce a precipitate when mixed?
A) NaCl(aq)and Ca(NO3)2(aq)
B) KCl(aq)and NaOH(aq)
C) Cu(NO3)2(aq)and AgNO3(aq)
D) ZnSO4(aq)and LiCl(aq)
E) K2SO4(aq)and Pb(ClO4)2(aq)
A) NaCl(aq)and Ca(NO3)2(aq)
B) KCl(aq)and NaOH(aq)
C) Cu(NO3)2(aq)and AgNO3(aq)
D) ZnSO4(aq)and LiCl(aq)
E) K2SO4(aq)and Pb(ClO4)2(aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
17
If aqueous solutions of nickel(II)chloride and potassium phosphate are mixed,which insoluble compound will form?
A) Ni3(PO4)2
B) NiCl2
C) KCl
D) K2Ni
E) K3PO4
A) Ni3(PO4)2
B) NiCl2
C) KCl
D) K2Ni
E) K3PO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
18
What net ionic reaction occurs when aqueous solutions of potassium carbonate and iron(III)bromide are mixed?
A) CO32−(aq)+ 2Fe+(aq)→ Fe2CO3(s)
B) 3CO32−(aq)+ 2Fe3+(aq)→ Fe2(CO3)3(s)
C) 3CO32−(aq)+ 6Fe+(aq)→ 3Fe2CO3(s)
D) 3K2CO3(aq)+ 2Fe3+(aq)+ 6Br−(aq)→ Fe2(CO3)3(s)+ 6KBr(s)
E) no reaction occurs
A) CO32−(aq)+ 2Fe+(aq)→ Fe2CO3(s)
B) 3CO32−(aq)+ 2Fe3+(aq)→ Fe2(CO3)3(s)
C) 3CO32−(aq)+ 6Fe+(aq)→ 3Fe2CO3(s)
D) 3K2CO3(aq)+ 2Fe3+(aq)+ 6Br−(aq)→ Fe2(CO3)3(s)+ 6KBr(s)
E) no reaction occurs

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
19
If an aqueous solution of ____ is added to a mixture of Pb2+ and Ba2+,the lead ion will precipitate,but the barium ion will remain in solution.
A) NaOH
B) Na2SO4
C) K3PO4
D) KCO3
E) Ca(CH3CO2)2
A) NaOH
B) Na2SO4
C) K3PO4
D) KCO3
E) Ca(CH3CO2)2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
20
Identify all of the spectator ions in the reaction below. Zn(OH)2(s)+ 2H+(aq)+ 2NO3−(aq)→ Zn2+(aq)+ 2NO3−(aq)+ 2H2O(l)
A) Zn(OH)2
B) NO3−
C) Zn2+
D) H+
E) H+ and NO3−
A) Zn(OH)2
B) NO3−
C) Zn2+
D) H+
E) H+ and NO3−

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
21
The principal ingredient in Tums antacid tablets is calcium carbonate,CaCO3.A single tablet contains 0.500 g CaCO3.What volume of 0.2500 M HCl is required to titrate a tablet of Tums? 2H+(aq)+ CO32−(aq)→ H2O(l)+ CO2(g)
A) 0.0100 mL
B) 0.0200 mL
C) 10.0 mL
D) 20.0 mL
E) 40.0 mL
A) 0.0100 mL
B) 0.0200 mL
C) 10.0 mL
D) 20.0 mL
E) 40.0 mL

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
22
A oxidizing agent is a species that
A) takes a proton from an Arrhenius acid.
B) is oxidized in a chemical reaction.
C) gains electrons in a chemical reaction.
D) loses electrons in a chemical reaction.
E) gives a proton to an Arrhenius base.
A) takes a proton from an Arrhenius acid.
B) is oxidized in a chemical reaction.
C) gains electrons in a chemical reaction.
D) loses electrons in a chemical reaction.
E) gives a proton to an Arrhenius base.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
23
According to the Arrhenius acid-base definition
A) acids produce H+ in aqueous solutions and bases produce OH− in aqueous solutions.
B) acids produce OH− in aqueous solutions and bases produce H+ in aqueous solutions.
C) acids only react with bases.
D) all hydrogen halides are strong acids.
E) acids and bases are strong electrolytes.
A) acids produce H+ in aqueous solutions and bases produce OH− in aqueous solutions.
B) acids produce OH− in aqueous solutions and bases produce H+ in aqueous solutions.
C) acids only react with bases.
D) all hydrogen halides are strong acids.
E) acids and bases are strong electrolytes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
24
Exactly 19.36 mL of 0.1481 M NaOH is used to titrate a 25.00 mL sample of HF.What is the concentration of the hydrofluoric acid?
A) 6.464 × 10−2 M
B) 1.147 × 10−1 M
C) 1.916 × 10−1 M
D) 2.867 × 10−3 M
E) 6.570 × 10−3 M
A) 6.464 × 10−2 M
B) 1.147 × 10−1 M
C) 1.916 × 10−1 M
D) 2.867 × 10−3 M
E) 6.570 × 10−3 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which of the following compounds is a weak base?
A) NaOH
B) H2CO3
C) LiCl
D) NH3
E) CH3CO2H
A) NaOH
B) H2CO3
C) LiCl
D) NH3
E) CH3CO2H

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
26
Identify the oxidizing and reducing agents in the redox reaction below. 3Cu(s)+ 8H+(aq)+ 2NO3−(aq)→ 3Cu2+(aq)+ 2NO(g)+ 4H2O(l)
A) reducing agent: Cu;oxidizing agent: Cu2+
B) reducing agent: NO3−;oxidizing agent: NO
C) reducing agent: Cu;oxidizing agent: NO3−
D) reducing agent: NO3−;oxidizing agent: Cu
E) reducing agent: H+;oxidizing agent: NO
A) reducing agent: Cu;oxidizing agent: Cu2+
B) reducing agent: NO3−;oxidizing agent: NO
C) reducing agent: Cu;oxidizing agent: NO3−
D) reducing agent: NO3−;oxidizing agent: Cu
E) reducing agent: H+;oxidizing agent: NO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
27
What is the balanced net ionic equation for the reaction between aqueous solutions of acetic acid and sodium hydroxide?
A) CH3CO2H(aq)+ OH−(aq)→ CH3(aq)+ CO32−(aq)+ H2(g)
B) CH3CO2H(aq)+ 3NaOH(aq)→ CCO2H3−(aq)+ 3Na+(aq)+ H2O(l)
C) CH3CO2H(aq)+ OH−(aq)→ CH3−(aq)+ CO2(g)+ H2O(l)
D) CH3CO2H(aq)+ OH−(aq)→ CH3CO2−(aq)+ H2O(l)
E) CH3CO2H(aq)+ 2Na+(aq)+ 2OH−(aq)→ CH3CO22−(aq)+ 2Na+(aq)+ H2O(l)
A) CH3CO2H(aq)+ OH−(aq)→ CH3(aq)+ CO32−(aq)+ H2(g)
B) CH3CO2H(aq)+ 3NaOH(aq)→ CCO2H3−(aq)+ 3Na+(aq)+ H2O(l)
C) CH3CO2H(aq)+ OH−(aq)→ CH3−(aq)+ CO2(g)+ H2O(l)
D) CH3CO2H(aq)+ OH−(aq)→ CH3CO2−(aq)+ H2O(l)
E) CH3CO2H(aq)+ 2Na+(aq)+ 2OH−(aq)→ CH3CO22−(aq)+ 2Na+(aq)+ H2O(l)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
28
Assign oxidation numbers to each atom in manganese(IV)oxide.
A) Mn = +4;O = 0
B) Mn = +4;O = −2
C) Mn = +2;O = 0
D) Mn = +2;O = −2
E) Mn = 0;O = 0
A) Mn = +4;O = 0
B) Mn = +4;O = −2
C) Mn = +2;O = 0
D) Mn = +2;O = −2
E) Mn = 0;O = 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
29
In the laboratory,acid spills are often neutralized by adding sodium bicarbonate.What mass of sodium bicarbonate reacts with 225 mL of 6.00 M HCl? H+(aq)+ NaHCO3(s)→ H2O(l)+ CO2(g)+ Na+(aq)
A) 1.35 g
B) 71.5 g
C) 113 g
D) 143 g
E) 2240 g
A) 1.35 g
B) 71.5 g
C) 113 g
D) 143 g
E) 2240 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
30
What is the oxidation number of iodine in potassium iodate,KIO3?
A) -1
B) 0
C) +3
D) +5
E) +7
A) -1
B) 0
C) +3
D) +5
E) +7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
31
Assign oxidation numbers to each atom in sodium hydrogen carbonate,NaHCO3?
A) Na = +1,H = -1,C = +6,O = -2
B) Na = +1,H = +1,C = +4,O = -2
C) Na = +1,H = -1,C = +2,O = -2
D) Na = -1,H = +1,C = 0,O = -2
E) Na = 0,H = 0,C = 0,O = 0
A) Na = +1,H = -1,C = +6,O = -2
B) Na = +1,H = +1,C = +4,O = -2
C) Na = +1,H = -1,C = +2,O = -2
D) Na = -1,H = +1,C = 0,O = -2
E) Na = 0,H = 0,C = 0,O = 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
32
When HCl(g)and NH3(g)are mixed,a white solid forms.What is the balanced equation for this reaction?
A) HCl(g)+ NH3(g)→ NH4Cl(s)
B) HCl(g)+ NH3(g)→ NH2Cl(g)+ H2(s)
C) HCl(g)+ NH3(g)→ NH4(s)+ Cl(g)
D) HCl(g)+ NH3(g)→ NH2Cl(s)+ H2(g)
E) 3HCl(g)+ NH3(g)→ NCl3(s)+ 3H2(g)
A) HCl(g)+ NH3(g)→ NH4Cl(s)
B) HCl(g)+ NH3(g)→ NH2Cl(g)+ H2(s)
C) HCl(g)+ NH3(g)→ NH4(s)+ Cl(g)
D) HCl(g)+ NH3(g)→ NH2Cl(s)+ H2(g)
E) 3HCl(g)+ NH3(g)→ NCl3(s)+ 3H2(g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
33
Balance the reaction and identify which species is reduced and which is oxidized. Na(s)+ H2O(l)→ Na+(aq)+ OH−(aq)+ H2(g)
A) Na(s)+ H2O(l)→ Na+(aq)+ OH−(aq)+ H2(g);Na reduced,H2O oxidized
B) 2Na(s)+ 2H2O(l)→ 2Na+(aq)+ 2OH−(aq)+ H2(g);Na reduced,H2O oxidized
C) Na(s)+ H2O(l)→ Na+(aq)+ OH−(aq)+ H2(g);H2O reduced,Na oxidized
D) 2Na(s)+ 2H2O(l)→ 2Na+(aq)+ 2OH−(aq)+ H2(g);H2O reduced,Na oxidized
E) 2Na(s)+ 2H2O(l)→ 2Na+(aq)+ 2OH−(aq)+ H2(g);not a redox reaction
A) Na(s)+ H2O(l)→ Na+(aq)+ OH−(aq)+ H2(g);Na reduced,H2O oxidized
B) 2Na(s)+ 2H2O(l)→ 2Na+(aq)+ 2OH−(aq)+ H2(g);Na reduced,H2O oxidized
C) Na(s)+ H2O(l)→ Na+(aq)+ OH−(aq)+ H2(g);H2O reduced,Na oxidized
D) 2Na(s)+ 2H2O(l)→ 2Na+(aq)+ 2OH−(aq)+ H2(g);H2O reduced,Na oxidized
E) 2Na(s)+ 2H2O(l)→ 2Na+(aq)+ 2OH−(aq)+ H2(g);not a redox reaction

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which list contains only strong acids?
A) HCl,HNO3,HF,HClO4
B) H2SO4,H3PO4,HClO4,NH3
C) HCl,HNO3,H3PO4,HClO4
D) HCl,H2SO4,HClO4,HI
E) HNO3,H2SO4,NaOH,H3PO4
A) HCl,HNO3,HF,HClO4
B) H2SO4,H3PO4,HClO4,NH3
C) HCl,HNO3,H3PO4,HClO4
D) HCl,H2SO4,HClO4,HI
E) HNO3,H2SO4,NaOH,H3PO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
35
Which of the following are strong bases: NH3,NaOH,Ba(OH)2,and HF?
A) NH3 and HF
B) NaOH and Ba(OH)2
C) NH3 and NaOH
D) NaOH,Ba(OH)2,and HF
E) NH3,NaOH,Ba(OH)2,and HF
A) NH3 and HF
B) NaOH and Ba(OH)2
C) NH3 and NaOH
D) NaOH,Ba(OH)2,and HF
E) NH3,NaOH,Ba(OH)2,and HF

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
36
What is the net ionic equation for the reaction of aqueous hydrochloric acid and aqueous potassium hydroxide?
A) HCl(aq)+ OH-(aq)→ H2O(l)+ Cl-(aq)
B) Cl-(aq)+ K+(aq)→ KCl(s)
C) HCl(aq)+ KOH(aq)→ KCl(aq)+ H2O(l)
D) Cl-(aq)+ K+(aq)→ KCl(aq)
E) H+(aq)+ OH-(aq)→ H2O(l)
A) HCl(aq)+ OH-(aq)→ H2O(l)+ Cl-(aq)
B) Cl-(aq)+ K+(aq)→ KCl(s)
C) HCl(aq)+ KOH(aq)→ KCl(aq)+ H2O(l)
D) Cl-(aq)+ K+(aq)→ KCl(aq)
E) H+(aq)+ OH-(aq)→ H2O(l)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which of the following compounds is a weak acid?
A) HCl
B) H3PO4
C) HNO3
D) HClO4
E) H2SO4
A) HCl
B) H3PO4
C) HNO3
D) HClO4
E) H2SO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
38
What is the oxidation half-reaction for the reaction of zinc with hydrochloric acid? Zn(s)+ 2H+(aq)→ Zn2+(aq)+ H2(g)
A) Zn(s)→ Zn2+(aq)+ 2e−
B) Zn(s)+ 2e− → Zn2+(aq)
C) 2H+(aq)→ H2(g)+ 2e−
D) 2H+(aq)+ 2e− → H2(g)
E) none of the above
A) Zn(s)→ Zn2+(aq)+ 2e−
B) Zn(s)+ 2e− → Zn2+(aq)
C) 2H+(aq)→ H2(g)+ 2e−
D) 2H+(aq)+ 2e− → H2(g)
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
39
What are the spectator ions in the reaction between aqueous hydrobromic acid and aqueous sodium hydroxide?
A) Na+ only
B) H+ and OH-
C) Na+ and Br-
D) Br- only
E) H+,Br-,Na+,and OH-
A) Na+ only
B) H+ and OH-
C) Na+ and Br-
D) Br- only
E) H+,Br-,Na+,and OH-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
40
A mass of 0.4113 g of an unknown acid,HA,is titrated with NaOH(aq).If the acid reacts with 28.10 mL of 0.1055 M NaOH(aq),what is the molar mass of the acid?
A) 2.965 × 10-3 g/mol
B) 9.128 g/mol
C) 138.7 g/mol
D) 337.3 g/mol
E) 820.7 g/mol
A) 2.965 × 10-3 g/mol
B) 9.128 g/mol
C) 138.7 g/mol
D) 337.3 g/mol
E) 820.7 g/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
41
What volume of 0.2500 M cobalt(III)sulfate is required to react completely with 5.00 g of sodium carbonate?
A) 62.9 mL
B) 78.6 mL
C) 10.2 mL
D) 11.7 mL
E) 31.4 mL
A) 62.9 mL
B) 78.6 mL
C) 10.2 mL
D) 11.7 mL
E) 31.4 mL

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
42
Identify the reaction type for the reaction between potassium metal and hydrochloric acid. 2K(s)+ 2H+(aq)→ 2K+(aq)+ H2(g)
A) precipitation
B) acid-base
C) oxidation-reduction
D) both answers a and c
E) none of the above
A) precipitation
B) acid-base
C) oxidation-reduction
D) both answers a and c
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
43
What is the reduction half-reaction for the equation below? 5Fe2+(aq)+ MnO4−(aq)+ 8H+(aq)→ 5Fe3+(aq)+ Mn2+(aq)+ 4H2O(l)
A) MnO4−(aq)+ 5e− → Mn2+(aq)+ 2O2(g)
B) MnO4−(aq)+ 8H+(aq)+ 5e− → Mn2+(aq)+ 4H2O(l)
C) Fe2+(aq)+ e− → Fe3+(aq)
D) 8H+(aq)+ 8e− → 8H2O(l)
E) none of the above
A) MnO4−(aq)+ 5e− → Mn2+(aq)+ 2O2(g)
B) MnO4−(aq)+ 8H+(aq)+ 5e− → Mn2+(aq)+ 4H2O(l)
C) Fe2+(aq)+ e− → Fe3+(aq)
D) 8H+(aq)+ 8e− → 8H2O(l)
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
44
In the reaction given below,how many grams of sodium metal are consumed if 2.02 g of hydrogen gas are produced? 2Na(s)+ 2H2O(l)→ 2NaOH(aq)+ H2(g)
A) 92.0 g
B) 5.75 g
C) 11.5 g
D) 23.0 g
E) 46.0 g
A) 92.0 g
B) 5.75 g
C) 11.5 g
D) 23.0 g
E) 46.0 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
45
How many grams of KClO3 are needed to produce of 2.56 grams of O2? 2KClO3(s)→ 2KCl(s)+ 3O2(g)
A) 1.00 g
B) 1.71 g
C) 6.53 g
D) 9.80 g
E) 14.7 g
A) 1.00 g
B) 1.71 g
C) 6.53 g
D) 9.80 g
E) 14.7 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
46
Which of the pictorial representations best represents the precipitation reaction that occurs between aqueous solutions of Fe3+ and OH−? Assume that the circles represent cations and the squares represent anions.
A)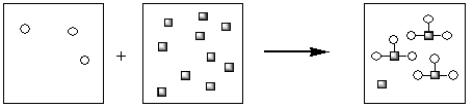
B)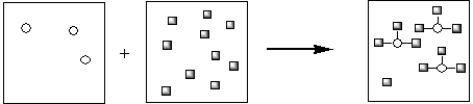
C)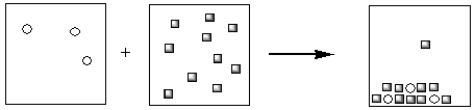
D)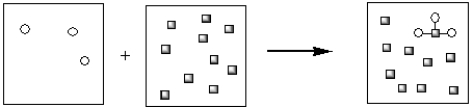
E)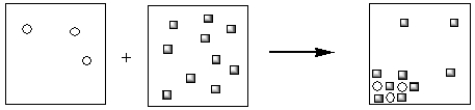
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
47
Write a balanced half-reaction for the reduction of permanganate ion,MnO7−,to Mn2+ in an acidic solution.
A) MnO4−(aq)+ 4H2O(l)→ Mn2+(aq)+ 8OH−(aq)
B) MnO4−(aq)+ 8H+(aq)+ 5e− → Mn2+(aq)+ 4H2O(l)
C) MnO4−(aq)+ 5e− → Mn2+(aq)+ 4O2-(aq)
D) MnO4−(aq)→ Mn2+(aq)+ 4O2−(aq)
E) MnO4−(aq)+ 4H+(aq)+ 5e− → Mn2+(aq)+ 4OH−(aq)
A) MnO4−(aq)+ 4H2O(l)→ Mn2+(aq)+ 8OH−(aq)
B) MnO4−(aq)+ 8H+(aq)+ 5e− → Mn2+(aq)+ 4H2O(l)
C) MnO4−(aq)+ 5e− → Mn2+(aq)+ 4O2-(aq)
D) MnO4−(aq)→ Mn2+(aq)+ 4O2−(aq)
E) MnO4−(aq)+ 4H+(aq)+ 5e− → Mn2+(aq)+ 4OH−(aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck








