Deck 24: Nuclear Reactions and Their Applications
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/81
العب
ملء الشاشة (f)
Deck 24: Nuclear Reactions and Their Applications
1
Who discovered radioactivity?
A) Geiger
B) Curie
C) Roentgen
D) Becquerel
E) Rutherford
A) Geiger
B) Curie
C) Roentgen
D) Becquerel
E) Rutherford
Becquerel
2
Select the nuclide that completes the following nuclear reaction. 
A)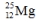
B)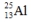
C)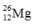
D)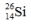
E)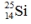

A)
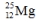
B)
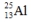
C)
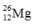
D)
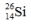
E)
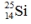
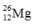
3
Which one of the following is a subatomic particle closely related to the positron?
A) proton
B) electron
C) negatron
D) neutron
E) neutrino
A) proton
B) electron
C) negatron
D) neutron
E) neutrino
electron
4
Select the nuclide that completes the following nuclear reaction. 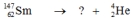
A)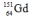
B)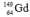
C)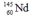
D)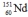
E)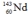
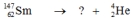
A)
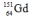
B)
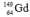
C)
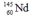
D)
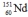
E)
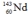

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
5
Select the nuclide that completes the following nuclear reaction. 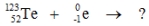
A)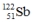
B)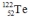
C)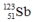
D)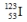
E) None of these choices are correct.
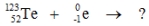
A)
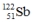
B)
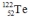
C)
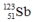
D)
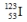
E) None of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
6
An isotope with a low value of N/Z will generally decay through
A) α decay.
B) β decay.
C)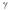 decay.
decay.
D) electron capture.
E) spontaneous fission.
A) α decay.
B) β decay.
C)
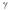 decay.
decay.D) electron capture.
E) spontaneous fission.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which one of the following equations correctly represents electron capture by the 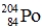 nucleus?
nucleus?
A)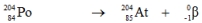
B)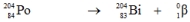
C)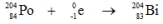
D)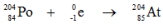
E)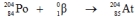
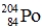 nucleus?
nucleus?A)
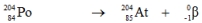
B)
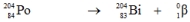
C)
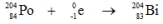
D)
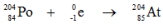
E)
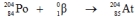

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
8
Select the nuclide that completes the following nuclear reaction. 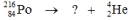
A)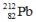
B)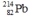
C)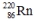
D)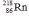
E)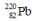
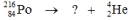
A)
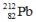
B)
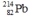
C)
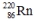
D)
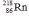
E)
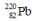

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which one of the following equations correctly represents alpha decay of 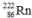 ?
?
A)
B)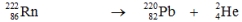
C)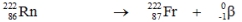
D)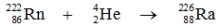
E)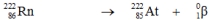
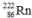 ?
?A)

B)
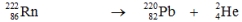
C)
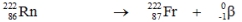
D)
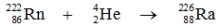
E)
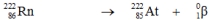

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
10
An isotope with a high value of N/Z will tend to decay through
A) α decay.
B) β decay.
C) positron decay.
D) electron capture.
E) decay.
decay.
A) α decay.
B) β decay.
C) positron decay.
D) electron capture.
E)
 decay.
decay.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
11
Select the nuclide that completes the following nuclear reaction. 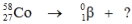
A)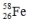
B)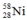
C)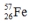
D)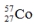
E)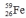
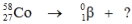
A)
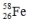
B)
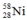
C)
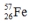
D)
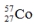
E)
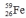

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which one of the following is an incorrect representation of the indicated particle or nucleus?
A) positron: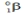
B) neutron:
C) helium-3: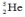
D) alpha particle: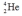
E) proton: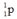
A) positron:
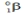
B) neutron:

C) helium-3:
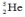
D) alpha particle:
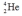
E) proton:
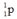

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
13
The radioisotope 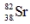 will decay through
will decay through
A) α decay.
B) β decay.
C) decay.
D) electron capture.
E) spontaneous fission.
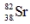 will decay through
will decay throughA) α decay.
B) β decay.
C) decay.
D) electron capture.
E) spontaneous fission.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
14
Select the nuclide that completes the following nuclear reaction. 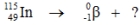
A)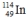
B)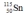
C)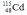
D)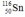
E)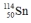
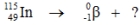
A)
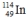
B)
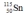
C)
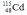
D)
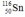
E)
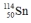

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
15
The radioisotope 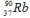 will decay through
will decay through
A) α decay.
B) β decay.
C) positron decay.
D) electron capture.
E)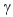 decay.
decay.
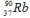 will decay through
will decay throughA) α decay.
B) β decay.
C) positron decay.
D) electron capture.
E)
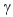 decay.
decay.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which of the following types of radioactive decay does not produce new element?
A) gamma emission
B) electron capture
C) beta emission
D) alpha emission
E) double beta emission
A) gamma emission
B) electron capture
C) beta emission
D) alpha emission
E) double beta emission

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
17
Select the nuclide that completes the following nuclear reaction. 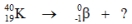
A)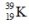
B)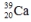
C)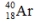
D)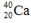
E)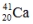
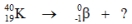
A)
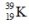
B)
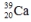
C)
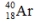
D)
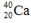
E)
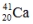

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which one of the following descriptions relating to nuclear reactions is correct?
A) The ratio of neutrons to protons remains constant.
B) The number of protons plus neutrons remains constant.
C) The number of electron remains constant.
D) The total charge changes.
E) The total number of nucleons changes.
A) The ratio of neutrons to protons remains constant.
B) The number of protons plus neutrons remains constant.
C) The number of electron remains constant.
D) The total charge changes.
E) The total number of nucleons changes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which one of the following equations correctly represents positron decay of 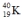 ?
?
A)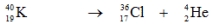
B)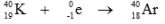
C)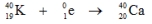
D)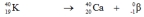
E)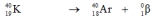
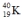 ?
?A)
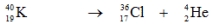
B)
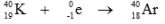
C)
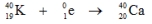
D)
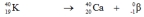
E)
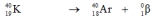

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
20
Select the nuclide that completes the following nuclear reaction. 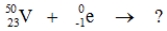
A)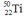
B)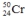
C)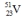
D)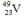
E) None of these choices are correct.
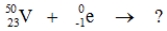
A)
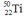
B)
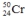
C)
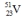
D)
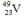
E) None of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which of the following series of radioactive decays would convert Pa-234 to Ra-226?
A) beta, alpha, beta
B) alpha, alpha
C) beta, alpha, alpha, beta
D) beta, alpha, alpha
E) alpha, beta, gamma
A) beta, alpha, beta
B) alpha, alpha
C) beta, alpha, alpha, beta
D) beta, alpha, alpha
E) alpha, beta, gamma

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
22
The radiochemist, Will I. Glow, studied thorium-232 and found that 2.82 × 10-7 moles emitted 8.42 × 106 α particles in one year. What is the decay constant for thorium-232?
A) 3.35 × 10-14 yr-1
B) 4.96 × 10-11 yr-1
C) 1.40 × 1010 yr-1
D) 2.99 × 1013 yr-1
E) None of these choices are correct.
A) 3.35 × 10-14 yr-1
B) 4.96 × 10-11 yr-1
C) 1.40 × 1010 yr-1
D) 2.99 × 1013 yr-1
E) None of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which of the following isotopes is definitely unstable?
A)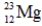
B)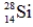
C)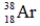
D)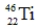
E)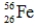
A)
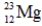
B)
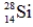
C)
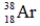
D)
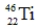
E)
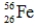

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
24
The isotopes of promethium, 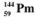 and
and 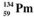 are unstable, and lie on opposite sides of the "line of stability". Which of the following combinations is most likely to represent the type of decay for these isotopes?
are unstable, and lie on opposite sides of the "line of stability". Which of the following combinations is most likely to represent the type of decay for these isotopes?
A) promethium-144, β decay; promethium-134, positron decay
B) promethium-144, positron decay; promethium-134, β decay
C) promethium-144, positron decay; promethium-134, electron capture
D) promethium-144, electron capture; promethium-134, positron decay
E) promethium-144, β decay; promethium-134,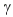 decay
decay
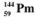 and
and 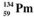 are unstable, and lie on opposite sides of the "line of stability". Which of the following combinations is most likely to represent the type of decay for these isotopes?
are unstable, and lie on opposite sides of the "line of stability". Which of the following combinations is most likely to represent the type of decay for these isotopes?A) promethium-144, β decay; promethium-134, positron decay
B) promethium-144, positron decay; promethium-134, β decay
C) promethium-144, positron decay; promethium-134, electron capture
D) promethium-144, electron capture; promethium-134, positron decay
E) promethium-144, β decay; promethium-134,
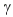 decay
decay
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which of the following isotopes is most likely to be unstable?
A)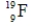
B)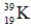
C)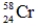
D)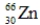
E)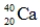
A)
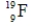
B)
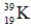
C)
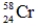
D)
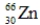
E)
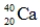

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which one of the following nuclei has a magic number of neutrons and/or protons?
A)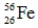
B)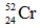
C)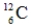
D)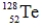
E)
A)
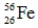
B)
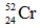
C)
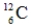
D)
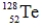
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
27
A scintillation counter
A) measures the signal coming from an ionized gas.
B) measures light emissions from excited atoms.
C) depends on an avalanche of electrons generated as a particle moves through a tube of argon gas.
D) detects high energy radiation better than low energy radiation.
E) detects an electric current in a gas.
A) measures the signal coming from an ionized gas.
B) measures light emissions from excited atoms.
C) depends on an avalanche of electrons generated as a particle moves through a tube of argon gas.
D) detects high energy radiation better than low energy radiation.
E) detects an electric current in a gas.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which of the following isotopes is most likely to be unstable?
A)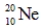
B)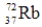
C)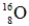
D)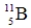
E)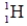
A)
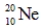
B)
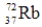
C)
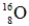
D)
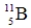
E)
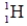

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
29
The isotopes 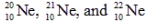 are all stable, while
are all stable, while 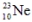 is radioactive. The mode of decay for
is radioactive. The mode of decay for 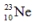 is most likely to be
is most likely to be
A) positron decay.
B) α decay.
C)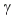 decay.
decay.
D) electron capture.
E) β decay.
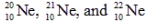 are all stable, while
are all stable, while 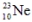 is radioactive. The mode of decay for
is radioactive. The mode of decay for 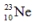 is most likely to be
is most likely to beA) positron decay.
B) α decay.
C)
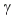 decay.
decay.D) electron capture.
E) β decay.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
30
An isotope with Z > 83, which lies close to the band of stability, will generally decay through
A) α decay.
B) β decay.
C)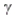 decay.
decay.
D) positron decay.
E) electron capture.
A) α decay.
B) β decay.
C)
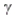 decay.
decay.D) positron decay.
E) electron capture.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
31
The nuclide Pb-210 undergoes three successive decays (beta, alpha, and beta, respectively) to form a stable nuclide. What are the three nuclides that form from Pb-210 in this decay series?
A) Tl-210, Au-206, Pt-206
B) Bi-210, Tl-206, Pb-206
C) Pb-209, Hg-205, Hg-204
D) Bi-210, Pb-206, Bi-206
E) None of these choices are correct.
A) Tl-210, Au-206, Pt-206
B) Bi-210, Tl-206, Pb-206
C) Pb-209, Hg-205, Hg-204
D) Bi-210, Pb-206, Bi-206
E) None of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
32
A certain isotope has a specific activity of 7.29 × 10-4 Ci/g. How many α particles will a 75.0 mg sample emit in one hour?
A) 9.99 × 104
B) 2.02 × 106
C) 7.28 × 109
D) 1.29 × 1012
E) None of these choices are correct.
A) 9.99 × 104
B) 2.02 × 106
C) 7.28 × 109
D) 1.29 × 1012
E) None of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
33
The isotopes 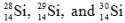 are all stable, while
are all stable, while 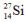 is radioactive. The mode of decay for
is radioactive. The mode of decay for 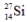 is most likely to be
is most likely to be
A) positron decay.
B) alpha decay.
C) beta decay.
D) gamma decay.
E) fission.
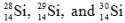 are all stable, while
are all stable, while 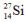 is radioactive. The mode of decay for
is radioactive. The mode of decay for 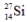 is most likely to be
is most likely to beA) positron decay.
B) alpha decay.
C) beta decay.
D) gamma decay.
E) fission.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
34
What is the specific activity (in Ci/g) of an isotope if 3.56 mg emits 4.26 × 108 β particles per second?
A) 0.003232 Ci/g
B) 0.0115 Ci/g
C) 0.309 Ci/g
D) 3.23 Ci/g
E) None of these choices are correct.
A) 0.003232 Ci/g
B) 0.0115 Ci/g
C) 0.309 Ci/g
D) 3.23 Ci/g
E) None of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
35
The isotope 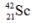 is unstable. This is predictable because
is unstable. This is predictable because
A) the number of neutrons is too large in relation to the number of protons.
B) the number of neutrons is too small in relation to the number of protons.
C) the atomic number is too large.
D) the mass number is too large.
E) Sc isotopes are all unstable.
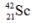 is unstable. This is predictable because
is unstable. This is predictable becauseA) the number of neutrons is too large in relation to the number of protons.
B) the number of neutrons is too small in relation to the number of protons.
C) the atomic number is too large.
D) the mass number is too large.
E) Sc isotopes are all unstable.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which of the following isotopes is most likely to be unstable?
A)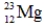
B)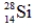
C)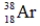
D)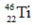
E)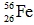
A)
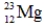
B)
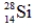
C)
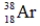
D)
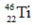
E)
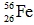

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
37
The isotope 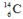 is unstable. This is predictable because
is unstable. This is predictable because
A) N/Z ≠ 1.
B) N/Z is relatively low and Z < 20.
C) N/Z is relatively large and Z < 20.
D) Z is small.
E) N is large.
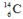 is unstable. This is predictable because
is unstable. This is predictable becauseA) N/Z ≠ 1.
B) N/Z is relatively low and Z < 20.
C) N/Z is relatively large and Z < 20.
D) Z is small.
E) N is large.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
38
So-called "magic numbers" of particles are thought to convey extra stability to certain nuclei. These magic numbers refer to which of the following particles?
A) protons only
B) electrons only
C) positrons only
D) neutrons only
E) protons and neutrons
A) protons only
B) electrons only
C) positrons only
D) neutrons only
E) protons and neutrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
39
Detection of radiation by a Geiger-Müller counter depends on
A) the emission of a photon from an excited atom.
B) the ability of an ionized gas to carry an electrical current.
C) the emission of a photon of light by the radioactive particle.
D) the ability of a photomultiplier tube to amplify the electrical signal from a phosphor.
E) the detection of the sound made by decay particles.
A) the emission of a photon from an excited atom.
B) the ability of an ionized gas to carry an electrical current.
C) the emission of a photon of light by the radioactive particle.
D) the ability of a photomultiplier tube to amplify the electrical signal from a phosphor.
E) the detection of the sound made by decay particles.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
40
The radioisotope 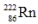 will decay through
will decay through
A) α decay.
B) β decay.
C)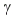 decay.
decay.
D) positron decay.
E) electron capture.
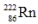 will decay through
will decay throughA) α decay.
B) β decay.
C)
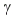 decay.
decay.D) positron decay.
E) electron capture.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
41
Assuming that no other particles are produced, which of the following particles could be used to bombard nitrogen-14 in order to make fluorine-18?
A) alpha particle
B) beta particle
C) neutron
D) proton
E) positron
A) alpha particle
B) beta particle
C) neutron
D) proton
E) positron

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
42
A N-14 nucleus is hit by a particle, forming a C-14 nucleus and a proton as the only products. Identify the type of particle which struck the N-14 nucleus.
A) alpha
B) proton
C) electron
D) neutron
E) deuterium
A) alpha
B) proton
C) electron
D) neutron
E) deuterium

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
43
A 7.85 × 10-5 mol sample of copper-61 emits 1.47 × 1019 positrons in 90.0 minutes. What is the decay constant for copper-61?
A) 0.00230 h-1
B) 0.00346 h-1
C) 0.207 h-1
D) 0.311 h-1
E) None of these choices are correct.
A) 0.00230 h-1
B) 0.00346 h-1
C) 0.207 h-1
D) 0.311 h-1
E) None of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
44
A 9.52 × 10-5 mol sample of rubidium-86 emits 8.87 × 1016 β particles in one hour. What is the half-life of rubidium-86?
A) 2.23 × 10-3 h
B) 1.55 × 10-3 h
C) 448 h
D) 645 h
E) None of these choices are correct.
A) 2.23 × 10-3 h
B) 1.55 × 10-3 h
C) 448 h
D) 645 h
E) None of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
45
The isotope 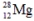 has a half-life of 21 hours. If a sample initially contains exactly 10000 atoms of
has a half-life of 21 hours. If a sample initially contains exactly 10000 atoms of 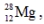 approximately how many of these atoms will remain after one week?
approximately how many of these atoms will remain after one week?
A) 1250
B) 78
C) 39
D) 0
E) None of these choices are correct.
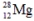 has a half-life of 21 hours. If a sample initially contains exactly 10000 atoms of
has a half-life of 21 hours. If a sample initially contains exactly 10000 atoms of 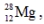 approximately how many of these atoms will remain after one week?
approximately how many of these atoms will remain after one week?A) 1250
B) 78
C) 39
D) 0
E) None of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
46
A 55-kg person exposed to thorium-234 receives 7.5 × 104 β particles, each with an energy of 1.6 × 10-14 J. How many rads does the person receive?
A) 2.1 × 10-19
B) 1.2 × 10-17
C) 2.2 × 10-9
D) 1.2 × 10-9
E) None of these choices are correct.
A) 2.1 × 10-19
B) 1.2 × 10-17
C) 2.2 × 10-9
D) 1.2 × 10-9
E) None of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
47
A 30.0-kg child receives 2.65 × 107 β particles, each with an energy of 4.60 × 10-13 J. If the RBE = 0.78, how many millirem did the child receive?
A) 3.2 × 10-7
B) 5.2 × 10-7
C) 5.2 × 10-4
D) 3.2 × 10-2
E) None of these choices are correct.
A) 3.2 × 10-7
B) 5.2 × 10-7
C) 5.2 × 10-4
D) 3.2 × 10-2
E) None of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
48
In living organisms, C-14 atoms disintegrate at a rate of 15.3 atoms per minute per gram of carbon. A charcoal sample from an archaeological site has a C-14 disintegration rate of 9.16 atoms per minute per gram of carbon. Estimate the age of this sample. The half-life of C-14 is 5730 years.
A) 3170 years
B) 3430 years
C) 4020 years
D) 4790 years
E) 6750 years
A) 3170 years
B) 3430 years
C) 4020 years
D) 4790 years
E) 6750 years

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
49
An 85-kg person exposed to barium-141 receives 2.5 × 105 β particles, each with an energy of 5.2 × 10-13 J. How many rads does the person receive?
A) 2.4 × 10-20
B) 1.5 × 10-7
C) 1.8 × 10-16
D) 6.1 × 10-15
E) None of these choices are correct.
A) 2.4 × 10-20
B) 1.5 × 10-7
C) 1.8 × 10-16
D) 6.1 × 10-15
E) None of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
50
Identify the missing species in the following nuclear transmutation. 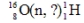
A)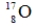
B)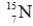
C)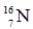
D)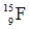
E)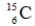
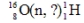
A)
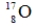
B)
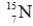
C)
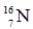
D)
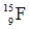
E)
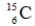

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
51
Identify the missing species in the following nuclear transmutation. 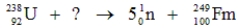
A)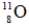
B)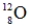
C)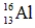
D)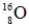
E)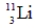
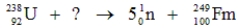
A)
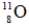
B)
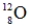
C)
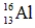
D)
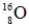
E)
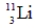

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
52
Cesium-134 is a β emitter with a half-life of 2.0 years. How much of a 2.50-g sample of cesium-134 will remain after 10 years?
A) 0.0024 g
B) 0.078 g
C) 0.25 g
D) 0.50 g
E) None of these choices are correct.
A) 0.0024 g
B) 0.078 g
C) 0.25 g
D) 0.50 g
E) None of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
53
Identify the missing species in the following nuclear transmutation. 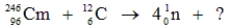
A)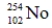
B)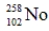
C)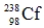
D)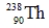
E) None of these choices are correct.
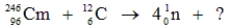
A)
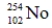
B)
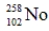
C)
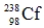
D)
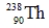
E) None of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
54
Palladium-107 undergoes β decay (t1/2 = 6.5 × 105 yr) to form silver-107. How long will it take for 0.150 mol of silver-107 to form from 1.25 mol of palladium-107?
A) 2.0 × 107 y
B) 1.4 × 107 y
C) 1.2 × 106 y
D) 8.3 × 105 y
E) 1.2 × 105 y
A) 2.0 × 107 y
B) 1.4 × 107 y
C) 1.2 × 106 y
D) 8.3 × 105 y
E) 1.2 × 105 y

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
55
Iodine-131, t1/2 = 8.0 days, is used in diagnosis and treatment of thyroid gland diseases. If a laboratory sample of iodine-131 initially emits 9.95 × 1018 β particles per day, how long will it take for the activity to drop to 6.22 × 1017 β particles per day?
A) 2.0 days
B) 16 days
C) 32 days
D) 128 days
E) None of these choices are correct.
A) 2.0 days
B) 16 days
C) 32 days
D) 128 days
E) None of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
56
Identify the missing species in the following nuclear transmutation. 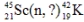
A)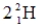
B)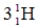
C)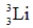
D)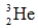
E)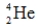
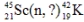
A)
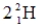
B)
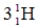
C)
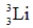
D)
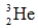
E)
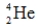

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
57
All the disintegrations of a sample of an unknown nuclide weighing 4.6 × 10-2 g were counted. In the first half-life of the sample, the total number of disintegrations counted was 4.3 × 1020. What is the atomic weight of the unknown element?
A) 32 amu
B) 16 amu
C) 8 amu
D) 4 amu
E) None of these choices are correct.
A) 32 amu
B) 16 amu
C) 8 amu
D) 4 amu
E) None of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
58
A pure sample of tritium, 3H, was prepared and sealed in a container for a number of years. Tritium undergoes β decay with a half-life of 12.32 years. How long has the container been sealed if analysis of the contents shows there are 5.25 mol of 3H and 6.35 mol of 3He present?
A) 2.34 y
B) 3.38 y
C) 9.77 y
D) 14.1 y
E) 25.6 y
A) 2.34 y
B) 3.38 y
C) 9.77 y
D) 14.1 y
E) 25.6 y

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
59
The isotope 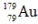 has a half-life of 7.5 seconds. If a sample contains 144 atoms of
has a half-life of 7.5 seconds. If a sample contains 144 atoms of 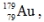 approximately how many such atoms were there present 30 seconds earlier?
approximately how many such atoms were there present 30 seconds earlier?
A) 576
B) 1152
C) 2304
D) 4320
E) 4.30 × 108
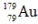 has a half-life of 7.5 seconds. If a sample contains 144 atoms of
has a half-life of 7.5 seconds. If a sample contains 144 atoms of 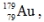 approximately how many such atoms were there present 30 seconds earlier?
approximately how many such atoms were there present 30 seconds earlier?A) 576
B) 1152
C) 2304
D) 4320
E) 4.30 × 108

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
60
A patient's thyroid gland is to be exposed to an average of 5.5 μCi for 16 days as an ingested sample of iodine-131 decays. If the energy of the β radiation is 9.7 × 10-14 J and the mass of the thyroid is 32.0 g, what is the dose received by the patient?
A) 0.027 rads
B) 1.2 rads
C) 37 rads
D) 85 rads
E) None of these choices are correct.
A) 0.027 rads
B) 1.2 rads
C) 37 rads
D) 85 rads
E) None of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
61
Which one of the following elements is formed largely in supernova explosions?
A) H
B) He
C) Mg
D) Fe
E) U
A) H
B) He
C) Mg
D) Fe
E) U

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
62
After 4 half-lives, the fraction of a radioactive isotope which still remains is approximately one-eighth.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
63
Exposure to 10 nCi for 10 minutes is more hazardous for a child than for an adult because
A) the child's cells are dividing more rapidly than the adult's and are, therefore, more susceptible to the radiation.
B) the child's smaller body size makes the effective dose larger for the child than for the adult.
C) the child's immune system is not developed well enough to resist damage.
D) the child's skin is not as thick as an adult's and cannot block as much radiation.
E) None of these choices are correct.
A) the child's cells are dividing more rapidly than the adult's and are, therefore, more susceptible to the radiation.
B) the child's smaller body size makes the effective dose larger for the child than for the adult.
C) the child's immune system is not developed well enough to resist damage.
D) the child's skin is not as thick as an adult's and cannot block as much radiation.
E) None of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
64
Which of the following materials is put into a nuclear reactor to slow the chain reaction?
A) heavy water
B) moderators
C) control rods
D) reflectors
E) chlorine
A) heavy water
B) moderators
C) control rods
D) reflectors
E) chlorine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
65
Sodium-21 will emit positrons each having an energy of 4.0 × 10-13 J. What is this energy in MeV?
A) 4.0 × 10-7 MeV
B) 2.5 MeV
C) 40 MeV
D) 2.5 × 106 MeV
E) None of these choices are correct.
A) 4.0 × 10-7 MeV
B) 2.5 MeV
C) 40 MeV
D) 2.5 × 106 MeV
E) None of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
66
Gamma-rays and X-rays interact with matter, causing
A) formation of ions, but no free radicals.
B) formation of free radicals, but no ions.
C) nuclear transmutation reactions.
D) formation of ions and free radicals.
E) formation of ions and nuclear transmutation reactions.
A) formation of ions, but no free radicals.
B) formation of free radicals, but no ions.
C) nuclear transmutation reactions.
D) formation of ions and free radicals.
E) formation of ions and nuclear transmutation reactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
67
Carbon-14 will emit a β particle with an energy of 0.1565 MeV. What is this energy in joules?
A) 1.0 × 10-24 J
B) 2.5 × 10-20 J
C) 1.0 × 10-18 J
D) 2.5 × 10-14 J
E) None of these choices are correct.
A) 1.0 × 10-24 J
B) 2.5 × 10-20 J
C) 1.0 × 10-18 J
D) 2.5 × 10-14 J
E) None of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
68
Gamma rays are not deflected by an electric field.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
69
Radioactive decay follows zero-order kinetics.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
70
The difference between the rad and the rem is
A) the rem is a rad per year.
B) the rad takes into account the type of radiation.
C) the rem takes into account the effect on the particular biological tissue.
D) the rem is a rad per kilogram.
E) None of these choices are correct.
A) the rem is a rad per year.
B) the rad takes into account the type of radiation.
C) the rem takes into account the effect on the particular biological tissue.
D) the rem is a rad per kilogram.
E) None of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
71
Calcium-39 undergoes positron decay. Each positron carries 5.49 MeV of energy. How much energy will be emitted when 0.0025 mol of calcium-39 decays?
A) 13.2 kJ
B) 1.32 × 104 kJ
C) 1.32 × 106 kJ
D) 1.32 × 109 kJ
E) None of these choices are correct.
A) 13.2 kJ
B) 1.32 × 104 kJ
C) 1.32 × 106 kJ
D) 1.32 × 109 kJ
E) None of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
72
It is believed that two carbon-12 nuclei can react in the core of a supergiant star to form sodium-23 and hydrogen-1. Calculate the energy released from this reaction for each mole of hydrogen formed. The masses of carbon-12, sodium-23, and hydrogen-1 are 12.0000 amu, 22.989767 amu, and 1.007825, respectively. 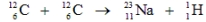
A) 2.16 × 1014 kJ
B) 2.16 × 1011 kJ
C) 2.16 × 108 kJ
D) 2.16 × 105 kJ
E) None of these choices are correct.
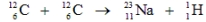
A) 2.16 × 1014 kJ
B) 2.16 × 1011 kJ
C) 2.16 × 108 kJ
D) 2.16 × 105 kJ
E) None of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
73
An alkaline earth element is radioactive. It and its daughter elements decay by emitting a total of three alpha particles in succession. In what group of the periodic table is the element resulting from the emission of the third alpha particle?
A) 4A (14)
B) 5A (15)
C) 6A (16)
D) 7A (17)
E) 8A (18)
A) 4A (14)
B) 5A (15)
C) 6A (16)
D) 7A (17)
E) 8A (18)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
74
Positron decay and electron capture have the same net effect on the Z and N values of a nucleus.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
75
No alpha decay is observed for isotopes of elements with Z < 83.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
76
The (negative) binding energy per nucleon reaches a maximum for the isotope 126C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
77
Most foodstuffs contain natural, radioactive isotopes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
78
The s-process involves a slow succession of neutron absorption and beta decay processes during the normal life of a star.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
79
Gamma rays are high energy electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck
80
The r-process occurs during supernova explosions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 81 في هذه المجموعة.
فتح الحزمة
k this deck








