Deck 2: The Chemical Basis of Life
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/74
العب
ملء الشاشة (f)
Deck 2: The Chemical Basis of Life
1
After a neutral atom accepts an additional electron,it becomes
A)positively charged.
B)negatively charged.
C)an ion.
D)a molecule.
E)both negatively charged and an ion at the same time.
A)positively charged.
B)negatively charged.
C)an ion.
D)a molecule.
E)both negatively charged and an ion at the same time.
E
2
The atomic number of an atom is equal to
A)the number of neutrons in the atom.
B)the number of protons in the atom.
C)the sum of the number of protons plus the number of neutrons.
D)the sum of the number of protons plus the number of electrons.
E)the sum of the number of neutrons plus the number of electrons.
A)the number of neutrons in the atom.
B)the number of protons in the atom.
C)the sum of the number of protons plus the number of neutrons.
D)the sum of the number of protons plus the number of electrons.
E)the sum of the number of neutrons plus the number of electrons.
B
3
Subatomic particles that possess a negative charge,and move around the nucleus of an atom are called
A)protons.
B)electrons.
C)neutrons.
D)photons
E)quarks
A)protons.
B)electrons.
C)neutrons.
D)photons
E)quarks
B
4
Every atom of the element carbon has the same number of
A)protons.
B)neutrons.
C)electrons.
D)photons.
E)quarks.
A)protons.
B)neutrons.
C)electrons.
D)photons.
E)quarks.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
5
______ is a form of potential energy resulting from positions and interactions among subatomic particles.
A)Chemical
B)Mechanical
C)Radiant
D)Electric
E)Heat
A)Chemical
B)Mechanical
C)Radiant
D)Electric
E)Heat

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
6
If an iron atom (Fe)lost three electrons,what would be the charge of the resulting ion?
A)Fe-3
B)Fe+6
C)Fe+1
D)Fe+2
E)Fe+3
A)Fe-3
B)Fe+6
C)Fe+1
D)Fe+2
E)Fe+3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
7
The smallest particle of an element that has the chemical characteristics of that element is a(n)
A)neutron.
B)proton.
C)electron.
D)atom.
E)electron cloud.
A)neutron.
B)proton.
C)electron.
D)atom.
E)electron cloud.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
8
Atom Y has 11 protons,11 electrons,and 12 neutrons.What is the atomic number of Atom Y?
A)11
B)12
C)22
D)23
E)24
A)11
B)12
C)22
D)23
E)24

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
9
The weight of an object is determined by:
A)the arrangement of the atoms within the object
B)the force of gravity pulling on or acting on its mass
C)its change in mass when placed in a vacuum
D)the amount of space it occupies
E)all of the above
A)the arrangement of the atoms within the object
B)the force of gravity pulling on or acting on its mass
C)its change in mass when placed in a vacuum
D)the amount of space it occupies
E)all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
10
The mass number of an element is:
A)the number of neutrons in the atom.
B)the number of protons in the atom.
C)the sum of the number of protons plus the number of neutrons.
D)the sum of the number of protons plus the number of electrons.
E)the sum of the number of neutrons plus the number of electrons
A)the number of neutrons in the atom.
B)the number of protons in the atom.
C)the sum of the number of protons plus the number of neutrons.
D)the sum of the number of protons plus the number of electrons.
E)the sum of the number of neutrons plus the number of electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
11
The amount of matter in an object is its
A)mass.
B)weight.
C)atomic number.
D)element.
E)ionic charge.
A)mass.
B)weight.
C)atomic number.
D)element.
E)ionic charge.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
12
Atoms that have gained or lost electrons are called
A)ions.
B)covalents.
C)nonpolars.
D)molecules.
E)neutrons.
A)ions.
B)covalents.
C)nonpolars.
D)molecules.
E)neutrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
13
The chemical notation for Magnesium ions is Mg+2.The designation 2+ indicates that:
A)two electrons have been lost
B)two protons have been gained
C)the ion is negatively charged
D)the atomic number is two
E)the number of electrons equals the number of protons.
A)two electrons have been lost
B)two protons have been gained
C)the ion is negatively charged
D)the atomic number is two
E)the number of electrons equals the number of protons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
14
The chemical behavior of an atom is largely determined by
A)the number of neutrons it has.
B)the size of its nucleus.
C)the electrons closest to the nucleus.
D)the size of neutrons it has.
E)its outermost electrons.
A)the number of neutrons it has.
B)the size of its nucleus.
C)the electrons closest to the nucleus.
D)the size of neutrons it has.
E)its outermost electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
15
The chemical symbol Ca2+ indicates that a calcium atom has
A)two protons in its nucleus.
B)lost two neutrons.
C)gained two protons.
D)lost two electrons.
E)an atomic number greater than 2.
A)two protons in its nucleus.
B)lost two neutrons.
C)gained two protons.
D)lost two electrons.
E)an atomic number greater than 2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
16
Subatomic particles located in the nucleus of an atom are called
A)protons.
B)neutrons.
C)electrons.
D)orbitals.
E)Both protons and neutrons are correct names.
A)protons.
B)neutrons.
C)electrons.
D)orbitals.
E)Both protons and neutrons are correct names.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
17
Two atoms with the same number of protons and electrons,but different numbers of neutrons,are called
A)isotopes.
B)ions.
C)electrolytes.
D)compounds.
E)Both ions and electrolytes are correct names.
A)isotopes.
B)ions.
C)electrolytes.
D)compounds.
E)Both ions and electrolytes are correct names.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which of the following is responsible for most of the mass of an atom?
A)neutron.
B)proton.
C)electron.
D)both neutrons and protons
E)both electrons and neutrons
A)neutron.
B)proton.
C)electron.
D)both neutrons and protons
E)both electrons and neutrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
19
Atom X has an atomic number of 20 and has a mass number of 40.The number of protons in atom X is equal to
A)10.
B)20.
C)30.
D)40.
E)60.
A)10.
B)20.
C)30.
D)40.
E)60.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
20
Atom Y has 11 protons,11 electrons,and 12 neutrons.What is the mass number of Atom Y?
A)11
B)12
C)22
D)23
E)24
A)11
B)12
C)22
D)23
E)24

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
21
When the hydrogen bonds that maintain a protein's three-dimensional shape are broken,the protein becomes nonfunctional,and is said to be
A)essential.
B)denatured.
C)structural.
D)unsaturated.
E)saturated.
A)essential.
B)denatured.
C)structural.
D)unsaturated.
E)saturated.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
22
The conversion between different states of energy (e.g.potential energy to kinetic energy):
A)is not 100% efficent
B)is 100% efficent
C)typically generates heat
D)is not possible,energy can not change its state.
E)both A and C are correct.
A)is not 100% efficent
B)is 100% efficent
C)typically generates heat
D)is not possible,energy can not change its state.
E)both A and C are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
23
According to the law of conservation of energy,the total energy of the universe is:
A)constant
B)increasing exponentially
C)decreasing exponentially
D)increasing linearly
E)decreasing linearly.
A)constant
B)increasing exponentially
C)decreasing exponentially
D)increasing linearly
E)decreasing linearly.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
24
If the products of a chemical reaction contain less potential energy than the reactants,
A)energy has been stored in the molecular bonds of the product.
B)energy has been released by the breaking of molecular bonds.
C)the reaction will be reversible without additional energy input.
D)a synthesis reaction is likely to have occurred.
E)All of these choices are correct.
A)energy has been stored in the molecular bonds of the product.
B)energy has been released by the breaking of molecular bonds.
C)the reaction will be reversible without additional energy input.
D)a synthesis reaction is likely to have occurred.
E)All of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
25
The chemical compound that is represented by the acronym DNA
A)contains the sugar deoxyribose.
B)has two chains that form a double helix.
C)is composed of nucleotides.
D)is responsible for controlling cell activities.
E)has all of the properties listed here.
A)contains the sugar deoxyribose.
B)has two chains that form a double helix.
C)is composed of nucleotides.
D)is responsible for controlling cell activities.
E)has all of the properties listed here.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which of the following is NOT considered a compound?
A)water (H20).
B)sodium chloride (NaCl).
C)hydrogen chloride (HCl)
D)a hydrogen molecule (H2)
E)all of the above are compounds.
A)water (H20).
B)sodium chloride (NaCl).
C)hydrogen chloride (HCl)
D)a hydrogen molecule (H2)
E)all of the above are compounds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
27
Ionic compounds
A)are held together by the force of attraction between oppositely charged ions.
B)are not considered to be molecules.
C)do not have distinct units.
D)All of these choices are correct.
E)None of these choices are correct.
A)are held together by the force of attraction between oppositely charged ions.
B)are not considered to be molecules.
C)do not have distinct units.
D)All of these choices are correct.
E)None of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
28
Given that sodium bicarbonate dissociates to form Na+ and HCO3- when mixed with water,which of these would be part of the explanation for taking bicarbonate (NaHCO3)for excess stomach acid?
A)NaHCO3 will not release hydrogen ions when mixed with water.
B)HCO3- will be a hydrogen ion acceptor.
C)Free hydrogen ions increase the acidity of a solution.
D)When bicarbonate ions combine with hydrogen ions,the pH increases.
E)All of these are necessary to fully explain how sodium bicarbonate works to counter excess stomach acid.
A)NaHCO3 will not release hydrogen ions when mixed with water.
B)HCO3- will be a hydrogen ion acceptor.
C)Free hydrogen ions increase the acidity of a solution.
D)When bicarbonate ions combine with hydrogen ions,the pH increases.
E)All of these are necessary to fully explain how sodium bicarbonate works to counter excess stomach acid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
29
Energy
A)is the capacity to do work.
B)can neither be created nor destroyed.
C)is constantly being converted into different forms by the body.
D)can be stored in the chemical bonds between molecules/subatomic particles.
E)All of these choices are correct.
A)is the capacity to do work.
B)can neither be created nor destroyed.
C)is constantly being converted into different forms by the body.
D)can be stored in the chemical bonds between molecules/subatomic particles.
E)All of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
30
When one atom loses an electron and another atom accepts that electron a(n)__________ bond between the two atoms results.
A)covalent
B)hydrogen
C)ionic
D)explosive
E)radioactive
A)covalent
B)hydrogen
C)ionic
D)explosive
E)radioactive

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
31
The unequal,asymmetric sharing of electrons which results in one end (pole)of the molecule having a small electrical charge opposite the other end is called
A)hydrogen bonding.
B)polar covalent bonding.
C)double covalent bonding.
D)ionic bonding.
E)non-polar covalent bonding.
A)hydrogen bonding.
B)polar covalent bonding.
C)double covalent bonding.
D)ionic bonding.
E)non-polar covalent bonding.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
32
Which of the following analogies does not illustrate the energy type it is paired with?
A)the cocking back of the trigger on a starters pistol before a race - potential energy
B)picking up speed as you roll down a snow covered hill in winter - kinetic energy
C)the stretching of a bungee chord without releasing it - mechanical energy
D)the spring up you get when you jump on a pogo stick - kinetic energy
E)basketball players bending their knees before they do a lay-up - mechanical energy
A)the cocking back of the trigger on a starters pistol before a race - potential energy
B)picking up speed as you roll down a snow covered hill in winter - kinetic energy
C)the stretching of a bungee chord without releasing it - mechanical energy
D)the spring up you get when you jump on a pogo stick - kinetic energy
E)basketball players bending their knees before they do a lay-up - mechanical energy

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following is considered a compound but not a molecule?
A)water (H20).
B)sodium chloride (NaCl).
C)calcium (Ca2+)
D)glucose (C6H12O6)
E)all of the above are compounds and molecules.
A)water (H20).
B)sodium chloride (NaCl).
C)calcium (Ca2+)
D)glucose (C6H12O6)
E)all of the above are compounds and molecules.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
34
When there is an equal sharing of electrons between atoms,the bond that is formed is called:
A)an ionic bond.
B)a polar covalent bond.
C)a non-polar covalent bond.
D)a hydrogen bond.
E)none of the above.
A)an ionic bond.
B)a polar covalent bond.
C)a non-polar covalent bond.
D)a hydrogen bond.
E)none of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
35
Covalent bonds occur when
A)one atom loses an electron.
B)two substances dissociate in water.
C)two atoms share electrons.
D)ions are formed.
E)one atom gains an electron.
A)one atom loses an electron.
B)two substances dissociate in water.
C)two atoms share electrons.
D)ions are formed.
E)one atom gains an electron.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which of the following statements is FALSE about molecules?
A)In order to be considered a molecule,a structure must be an independent unit.
B)All compounds are automatically considered molecules.
C)Molecules are formed when two or more atoms chemically combine to form a structure that behaves as an independent unit.
D)The atoms that make up a molecule can either be the same or different.
E)The atoms that make up a molecule must be chemically bound to one another.
A)In order to be considered a molecule,a structure must be an independent unit.
B)All compounds are automatically considered molecules.
C)Molecules are formed when two or more atoms chemically combine to form a structure that behaves as an independent unit.
D)The atoms that make up a molecule can either be the same or different.
E)The atoms that make up a molecule must be chemically bound to one another.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
37
If a molecule consists of two or more different kinds of atoms,it is a(n)
A)atom.
B)ion.
C)isotope.
D)compound.
E)Both atom and ion are correct names.
A)atom.
B)ion.
C)isotope.
D)compound.
E)Both atom and ion are correct names.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
38
The conversion of ATP into ADP
A)adds a phosphate group.
B)stores energy in the release of an inorganic phosphate group.
C)is an example of a exchange reaction.
D)is highly reversible.
E)requires the input of energy.
A)adds a phosphate group.
B)stores energy in the release of an inorganic phosphate group.
C)is an example of a exchange reaction.
D)is highly reversible.
E)requires the input of energy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
39
A(n)__________ is formed when one atom loses an electron and another atom accepts that electron.
A)ion
B)ionic bond
C)hydrogen bond
D)covalent bond
E)atom
A)ion
B)ionic bond
C)hydrogen bond
D)covalent bond
E)atom

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
40
Non polar molecules:
A)are created when the bonding atoms share electrons equally between themselves.
B)have an asymmetrical electrical charge.
C)are also considered ions.
D)result from polar covalent bonds.
E)all of the above.
A)are created when the bonding atoms share electrons equally between themselves.
B)have an asymmetrical electrical charge.
C)are also considered ions.
D)result from polar covalent bonds.
E)all of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
41
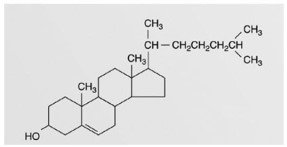 This figure represents an example of a(n)
This figure represents an example of a(n)A)steroid.
B)triglyceride.
C)phospholipids.
D)wax.
E)fatty acid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
42
The building blocks for proteins are
A)monosaccharides.
B)disaccharides.
C)glycerol + fatty acids.
D)nucleotides.
E)amino acids.
A)monosaccharides.
B)disaccharides.
C)glycerol + fatty acids.
D)nucleotides.
E)amino acids.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
43
A solution with a pH of 4 would have _____ hydrogen ions than a solution with a pH of 6.
A)2 times more
B)2 times fewer
C)20 times more
D)20 times fewer
E)100 times more
A)2 times more
B)2 times fewer
C)20 times more
D)20 times fewer
E)100 times more

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
44
__________ is formed by the reaction of an acid and a base.
A)An acid
B)A base
C)A salt
D)A buffer
A)An acid
B)A base
C)A salt
D)A buffer

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
45
Nucleotides are the building blocks for
A)carbohydrates.
B)fats (triglycerides).
C)nucleic acids.
D)proteins.
A)carbohydrates.
B)fats (triglycerides).
C)nucleic acids.
D)proteins.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
46
Given that MgCl2 is composed of Mg+2 ions and Cl- ions,MgCl2 would be considered to be
A)an acid.
B)a base.
C)a salt.
D)a buffer.
A)an acid.
B)a base.
C)a salt.
D)a buffer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
47
A solution with a greater concentration of hydroxide ions than hydrogen ions is
A)a buffer.
B)a salt.
C)basic.
D)acidic.
E)hydrophobic.
A)a buffer.
B)a salt.
C)basic.
D)acidic.
E)hydrophobic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
48
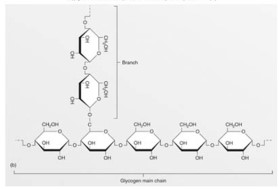 This figure represents an example of a(n)
This figure represents an example of a(n)A)protein.
B)nucleic acid.
C)lipid.
D)carbohydrate.
E)ATP molecule.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
49
The chemical compound that is represented by the acronym ATP
A)is synthesized using energy released during the breakdown of food molecules.
B)can be broken down to ADP and a fatty acid.
C)has nothing to do with stored energy.
D)is a common temporary storage form of immediately usable energy within cells.
E)is synthesized using energy released during the breakdown of food molecules and is a common temporary storage form of immediately usable energy within cells.
A)is synthesized using energy released during the breakdown of food molecules.
B)can be broken down to ADP and a fatty acid.
C)has nothing to do with stored energy.
D)is a common temporary storage form of immediately usable energy within cells.
E)is synthesized using energy released during the breakdown of food molecules and is a common temporary storage form of immediately usable energy within cells.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
50
Substances that donate hydrogen ions (protons)to a solution are called
A)acids.
B)bases.
C)alkaline.
D)salts.
A)acids.
B)bases.
C)alkaline.
D)salts.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
51
The macromolecules that function as the genetic material and are involved in protein synthesis are
A)carbohydrates
B)lipids
C)proteins
D)nucleic acids
A)carbohydrates
B)lipids
C)proteins
D)nucleic acids

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
52
Chemicals that resist changes in pH when acids or bases are added to a solution are
A)acids.
B)bases.
C)salts.
D)buffers.
A)acids.
B)bases.
C)salts.
D)buffers.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
53
A(n)__________ is formed when two atoms share electrons.
A)ion
B)ionic bond
C)hydrogen bond
D)covalent bond
E)atom
A)ion
B)ionic bond
C)hydrogen bond
D)covalent bond
E)atom

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
54
Glycerol and fatty acids are the building blocks for
A)carbohydrates.
B)fats (triglycerides).
C)nucleic acids.
D)proteins.
A)carbohydrates.
B)fats (triglycerides).
C)nucleic acids.
D)proteins.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
55
Which of these statements is true?
A)Carbohydrates are organic molecules formed from amino acid building blocks.
B)Monosaccharides become bound together by hydrolysis reactions to form polysaccharides.
C)Monosaccharides,disaccharides,and polysaccharides are large inorganic molecules.
D)The building blocks for lipids are nucleotides.
E)Essential amino acids are those that cannot be synthesized by the body.
A)Carbohydrates are organic molecules formed from amino acid building blocks.
B)Monosaccharides become bound together by hydrolysis reactions to form polysaccharides.
C)Monosaccharides,disaccharides,and polysaccharides are large inorganic molecules.
D)The building blocks for lipids are nucleotides.
E)Essential amino acids are those that cannot be synthesized by the body.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
56
___________ are a common fuel nutrient that has glycogen as a storage form.
A)Carbohydrates
B)Lipids
C)Proteins
D)Nucleic acids
A)Carbohydrates
B)Lipids
C)Proteins
D)Nucleic acids

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
57
Sucrose is an example of
A)a monosaccharide.
B)a lipid.
C)a disaccharide.
D)an inorganic molecule.
E)a polysaccharide.
A)a monosaccharide.
B)a lipid.
C)a disaccharide.
D)an inorganic molecule.
E)a polysaccharide.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
58
A solution with a pH of 7 is considered to be
A)acidic.
B)basic or alkaline.
C)neutral.
D)in equilibrium.
A)acidic.
B)basic or alkaline.
C)neutral.
D)in equilibrium.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
59
Monosaccharides are the building blocks for
A)carbohydrates.
B)fats (triglycerides).
C)nucleic acids.
D)proteins.
A)carbohydrates.
B)fats (triglycerides).
C)nucleic acids.
D)proteins.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
60
A large organic molecule was analyzed and found to contain carbon,hydrogen,oxygen,nitrogen,and sulfur.Of these choices,which would most likely have been the type of molecule analyzed?
A)carbohydrate
B)lipid
C)protein
D)nucleic acid
E)steroid
A)carbohydrate
B)lipid
C)protein
D)nucleic acid
E)steroid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
61
Which of these statements concerning lipids is NOT true?
A)The building blocks of fats (triglycerides)are fatty acids and glycerol.
B)A fatty acid that contains only single covalent bonds between the carbon atoms is called unsaturated.
C)Fats,phospholipids,and steroids are lipids.
D)Lipids are substances that dissolve in nonpolar solvents.
A)The building blocks of fats (triglycerides)are fatty acids and glycerol.
B)A fatty acid that contains only single covalent bonds between the carbon atoms is called unsaturated.
C)Fats,phospholipids,and steroids are lipids.
D)Lipids are substances that dissolve in nonpolar solvents.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
62
Which of the following processes represents a synthesis reaction?
A)glycolysis
B)the creation of a protein from amino acids
C)glycogenolysis
D)All of these are synthesis reactions.
A)glycolysis
B)the creation of a protein from amino acids
C)glycogenolysis
D)All of these are synthesis reactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
63
Enzymes
A)are globular proteins.
B)function as biological catalysts.
C)lower the activation energy of a reaction.
D)can be used to regulate chemical reactions.
E)All of these choices are correct.
A)are globular proteins.
B)function as biological catalysts.
C)lower the activation energy of a reaction.
D)can be used to regulate chemical reactions.
E)All of these choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
64
A substance that increases the rate at which a reaction proceeds,without itself being changed or depleted is a
A)catalyst.
B)reactant.
C)buffer.
D)base.
E)product.
A)catalyst.
B)reactant.
C)buffer.
D)base.
E)product.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
65
In a reversible reaction,when the rate of product formation is equal to the rate of reactant formation,the reaction is
A)stopped.
B)at equilibrium.
C)in danger of exploding.
D)a net decomposition reaction.
E)a net synthesis reaction.
A)stopped.
B)at equilibrium.
C)in danger of exploding.
D)a net decomposition reaction.
E)a net synthesis reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
66
When two or more atoms,ions,or molecules combine to form a new and larger molecule,the process is called a
A)decomposition reaction.
B)synthesis reaction.
C)reversible reaction.
D)buffer reaction.
E)condensation reaction.
A)decomposition reaction.
B)synthesis reaction.
C)reversible reaction.
D)buffer reaction.
E)condensation reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
67
In living things,which of these is most important for regulating the rate of chemical reactions?
A)changing concentration of reactants
B)changing temperature
C)changing concentration and activity of enzymes catalyzing the reactions
D)nature of reacting substances - carbohydrates react faster than lipids,for example
A)changing concentration of reactants
B)changing temperature
C)changing concentration and activity of enzymes catalyzing the reactions
D)nature of reacting substances - carbohydrates react faster than lipids,for example

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
68
Which of the following pairs correctly matches the example with its classification?
A)compound - two atoms of hydrogen combined
B)molecule - sodium chloride
C)molecule - two hydrogen atoms and one oxygen atom combined
D)compound - two hydrogen atoms and one oxygen atom combined
E)Both molecule - two hydrogen atoms and one oxygen atom combined and compound - two hydrogen atoms and one oxygen atom combined are correct.
A)compound - two atoms of hydrogen combined
B)molecule - sodium chloride
C)molecule - two hydrogen atoms and one oxygen atom combined
D)compound - two hydrogen atoms and one oxygen atom combined
E)Both molecule - two hydrogen atoms and one oxygen atom combined and compound - two hydrogen atoms and one oxygen atom combined are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
69
Which of these is NOT a property of water that makes it useful for living organisms?
A)Water allows body temperature to increase or decrease rapidly.
B)Water causes ionic substances to dissociate.
C)Water acts as a lubricant.
D)Water is necessary for the transport of nutrients,gases,and waste products.
E)Water is necessary for many chemical reactions.
A)Water allows body temperature to increase or decrease rapidly.
B)Water causes ionic substances to dissociate.
C)Water acts as a lubricant.
D)Water is necessary for the transport of nutrients,gases,and waste products.
E)Water is necessary for many chemical reactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
70
Enzymes function by
A)increasing the activation energy needed to start a chemical reaction.
B)having a specific shape that allows them to bind to particular reactants.
C)each enzyme acting as a catalyst for many different reaction types.
D)greatly decreasing reaction rates.
E)doing all of these.
A)increasing the activation energy needed to start a chemical reaction.
B)having a specific shape that allows them to bind to particular reactants.
C)each enzyme acting as a catalyst for many different reaction types.
D)greatly decreasing reaction rates.
E)doing all of these.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
71
Which of the following will decrease the rate at which a reaction occurs?
A)decreasing the concentration of reactants
B)increasing the concentration of reactants
C)increasing the temperature
D)increasing the amount of the required catalyst
E)All of these will decrease the rate at which the reaction occurs.
A)decreasing the concentration of reactants
B)increasing the concentration of reactants
C)increasing the temperature
D)increasing the amount of the required catalyst
E)All of these will decrease the rate at which the reaction occurs.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
72
Which of these is an organic molecule?
A)H2O
B)H2CO3
C)CO2
D)NaCl
E)CaCl2
A)H2O
B)H2CO3
C)CO2
D)NaCl
E)CaCl2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
73
Glycogen and starch are examples of
A)monosaccharides.
B)nucleic acids.
C)proteins.
D)polysaccharides.
E)lipids.
A)monosaccharides.
B)nucleic acids.
C)proteins.
D)polysaccharides.
E)lipids.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck
74
Chemical reactions with the property of being able to proceed from reactants to products and from products to reactants are called
A)exchange reactions.
B)synthesis reactions.
C)decomposition reactions.
D)reversible reactions.
E)net reaction rates.
A)exchange reactions.
B)synthesis reactions.
C)decomposition reactions.
D)reversible reactions.
E)net reaction rates.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 74 في هذه المجموعة.
فتح الحزمة
k this deck








