Deck 14: Carboxylic Acids and Carboxylic Acid Derivatives
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/83
العب
ملء الشاشة (f)
Deck 14: Carboxylic Acids and Carboxylic Acid Derivatives
1
The ester shown can be produced by heating together a carboxylic acid and an alcohol in the presence of a trace of acid.What carboxylic acid and alcohol are needed? 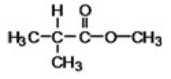
A)propanoic acid and methanol
B)methanoic acid and isopropyl alcohol
C)2-methylpropanoic acid and methanol
D)acetic acid and 2-methyl propanol
E)2-methyl butanoic acid and methanol

A)propanoic acid and methanol
B)methanoic acid and isopropyl alcohol
C)2-methylpropanoic acid and methanol
D)acetic acid and 2-methyl propanol
E)2-methyl butanoic acid and methanol
2-methylpropanoic acid and methanol
2
What is the name of the spherical particles formed in solution when specks of oil or grease are surrounded by soap molecules?
A)monomers
B)micelles
C)detergents
D)polymers
E)triglycerides
A)monomers
B)micelles
C)detergents
D)polymers
E)triglycerides
micelles
3
The general structure of a soap molecule is shown below.The carboxylate "head" of the soap molecule is best described as which of the following? 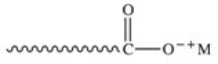
A)hydrophobic
B)lipophilic
C)hydrophilic
D)nonpolar
E)polymeric

A)hydrophobic
B)lipophilic
C)hydrophilic
D)nonpolar
E)polymeric
hydrophilic
4
Long chain carboxylic acids are known as which of the following?
A)fatty acids
B)polymers
C)polycarboxylic acids
D)esters
E)poly acids
A)fatty acids
B)polymers
C)polycarboxylic acids
D)esters
E)poly acids

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
5
What carboxylic acid is produced by the oxidation reaction shown?
A)CH3CH2CH2COOH
B)
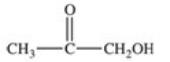
C)
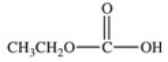
D)
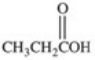
E)
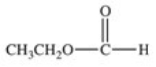
A)CH3CH2CH2COOH
B)
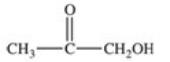
C)
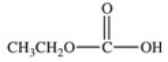
D)
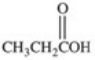
E)
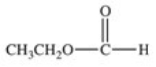

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
6
Hydrolysis of an ester produced the two organic products shown.What is the structure of the original ester? 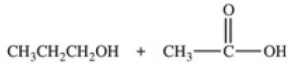
A)
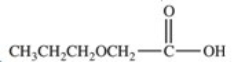
B)
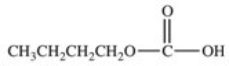
C)
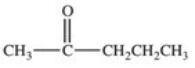
D)
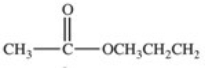
E)
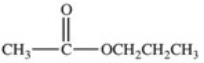
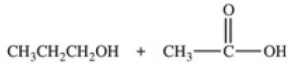
A)
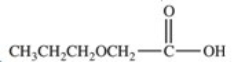
B)
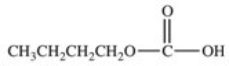
C)
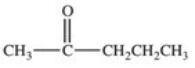
D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
7
What type of organic compound is produced in the reaction below? carboxylic acid + alcohol 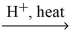 H2O + ?
H2O + ?
A)ester
B)enol
C)carboxylate salt
D)anhydride
E)acetal
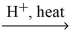 H2O + ?
H2O + ?A)ester
B)enol
C)carboxylate salt
D)anhydride
E)acetal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which statement concerning the compound ethanoic acid is FALSE?
A)It contains a carboxyl group.
B)Its condensed structure is CH3CH2COOH.
C)Its common name is acetic acid.
D)It contains two carbons.
E)It is a very polar compound.
A)It contains a carboxyl group.
B)Its condensed structure is CH3CH2COOH.
C)Its common name is acetic acid.
D)It contains two carbons.
E)It is a very polar compound.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which of the following compounds would have the greatest solubility in water?
A)
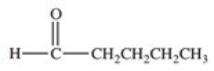
B)
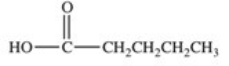
C)
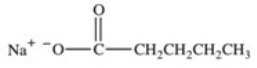
D)HOCH2CH2CH2CH2CH3
E)All would have the same solubility.
A)
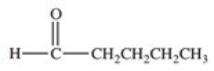
B)
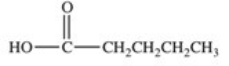
C)
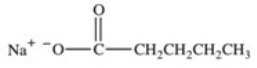
D)HOCH2CH2CH2CH2CH3
E)All would have the same solubility.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which term is used to describe the hydrolysis of an ester by an aqueous base?
A)esterification
B)isomerization
C)enolization
D)tautomerization
E)saponification
A)esterification
B)isomerization
C)enolization
D)tautomerization
E)saponification

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
11
What organic product is formed in the neutralization of benzoic acid? 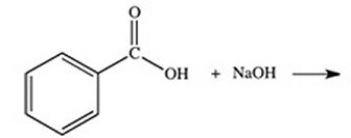
A)
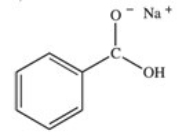
B)
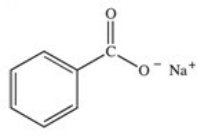
C)
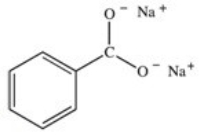
D)
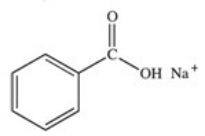
E)
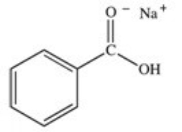

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which of the following is a generic acyl group?
A)
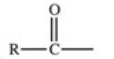
B)
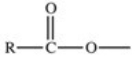
C)
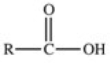
D)
E)
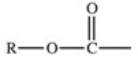
A)

B)

C)
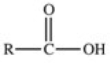
D)

E)
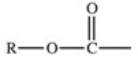

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
13
What product is formed when cinnamaldehyde is oxidized? ![<strong>What product is formed when cinnamaldehyde is oxidized? \stackrel { [ \mathrm { O } ] } { \longrightarrow } </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7201/11edd210_d8bf_03fc_b674_31935e36174a_TB7201_11.jpg)
A)
![<strong>What product is formed when cinnamaldehyde is oxidized? \stackrel { [ \mathrm { O } ] } { \longrightarrow } </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7201/11ead7a6_4506_11a2_ad99_0b1040f6acda_TB7201_00.jpg)
B)
![<strong>What product is formed when cinnamaldehyde is oxidized? \stackrel { [ \mathrm { O } ] } { \longrightarrow } </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7201/11ead7a6_4506_38b3_ad99_618be45c5c7c_TB7201_00.jpg)
C)
![<strong>What product is formed when cinnamaldehyde is oxidized? \stackrel { [ \mathrm { O } ] } { \longrightarrow } </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7201/11ead7a6_4506_38b4_ad99_cba440f90a6e_TB7201_00.jpg)
D)
![<strong>What product is formed when cinnamaldehyde is oxidized? \stackrel { [ \mathrm { O } ] } { \longrightarrow } </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7201/11ead7a6_4506_38b5_ad99_7fcffca1a413_TB7201_00.jpg)
E)
![<strong>What product is formed when cinnamaldehyde is oxidized? \stackrel { [ \mathrm { O } ] } { \longrightarrow } </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7201/11ead7a6_4506_38b6_ad99_fb7efea46d02_TB7201_00.jpg)
![<strong>What product is formed when cinnamaldehyde is oxidized? \stackrel { [ \mathrm { O } ] } { \longrightarrow } </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7201/11edd210_d8bf_03fc_b674_31935e36174a_TB7201_11.jpg)
A)
![<strong>What product is formed when cinnamaldehyde is oxidized? \stackrel { [ \mathrm { O } ] } { \longrightarrow } </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7201/11ead7a6_4506_11a2_ad99_0b1040f6acda_TB7201_00.jpg)
B)
![<strong>What product is formed when cinnamaldehyde is oxidized? \stackrel { [ \mathrm { O } ] } { \longrightarrow } </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7201/11ead7a6_4506_38b3_ad99_618be45c5c7c_TB7201_00.jpg)
C)
![<strong>What product is formed when cinnamaldehyde is oxidized? \stackrel { [ \mathrm { O } ] } { \longrightarrow } </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7201/11ead7a6_4506_38b4_ad99_cba440f90a6e_TB7201_00.jpg)
D)
![<strong>What product is formed when cinnamaldehyde is oxidized? \stackrel { [ \mathrm { O } ] } { \longrightarrow } </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7201/11ead7a6_4506_38b5_ad99_7fcffca1a413_TB7201_00.jpg)
E)
![<strong>What product is formed when cinnamaldehyde is oxidized? \stackrel { [ \mathrm { O } ] } { \longrightarrow } </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB7201/11ead7a6_4506_38b6_ad99_fb7efea46d02_TB7201_00.jpg)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of the following is the condensed formula for a generic carboxylic acid?
A)RCOH
B)ROOC
C)RCOOR
D)RCOOH
E)RCOHO
A)RCOH
B)ROOC
C)RCOOR
D)RCOOH
E)RCOHO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
15
What is the name of the functional group found in all carboxylic acids?
A)acetal group
B)enol group
C)carboxyl group
D)carbinol group
E)hemiacetal group
A)acetal group
B)enol group
C)carboxyl group
D)carbinol group
E)hemiacetal group

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
16
What product,in addition to methanol,is produced in the hydrolysis of methyl ethanoate?
A)ethanol
B)ethanal
C)ethanone
D)ethanoic acid
E)formic acid
A)ethanol
B)ethanal
C)ethanone
D)ethanoic acid
E)formic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
17
When a carboxylic acid dissolves in water,the ion shown below is formed.What is the name of this type of ion? 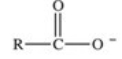
A)enolate ion
B)acyl anion
C)carboxylate anion
D)oxalate ion
E)acetate ion

A)enolate ion
B)acyl anion
C)carboxylate anion
D)oxalate ion
E)acetate ion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which equation best represents the dissociation of propanoic acid in water?
A)
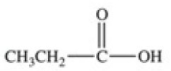
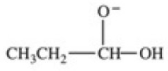
B)
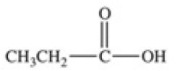
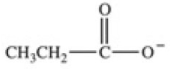
C)
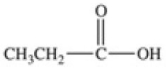
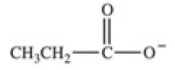
D)
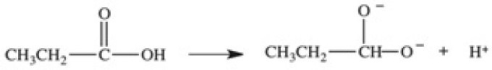
E)

A)
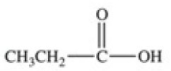
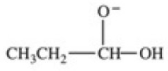
B)
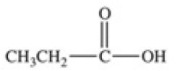
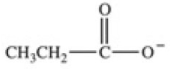
C)
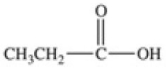
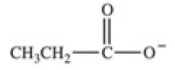
D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
19
What two compounds will react to produce hexyl acetate when heated together in the presence of a trace of acid?
A)hexane and ethanol
B)hexene and acetic acid
C)ethanol and hexanoic acid
D)hexanol and acetic acid
E)hexane and acetic acid
A)hexane and ethanol
B)hexene and acetic acid
C)ethanol and hexanoic acid
D)hexanol and acetic acid
E)hexane and acetic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
20
What is the IUPAC name for the missing product in the following equation? propanoic acid + ethanol 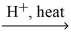 H2O + ?
H2O + ?
A)propyl ethanoate
B)propyl acetate
C)ethyl propate
D)ethyl propanoate
E)ethyl propanoic acid anhydride
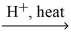 H2O + ?
H2O + ?A)propyl ethanoate
B)propyl acetate
C)ethyl propate
D)ethyl propanoate
E)ethyl propanoic acid anhydride

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
21
What carboxylic acid is produced in muscle cells during strenuous exercise?
A)adipic acid
B)citric acid
C)oxalic acid
D)lactic acid
E)None of the choices are correct.
A)adipic acid
B)citric acid
C)oxalic acid
D)lactic acid
E)None of the choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
22
What is the common name of the following compound? 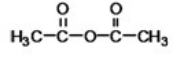
A)acyl anhydride
B)methyl anhydride
C)acetic anhydride
D)diethyl anhydride
E)formic anhydride
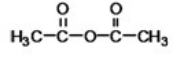
A)acyl anhydride
B)methyl anhydride
C)acetic anhydride
D)diethyl anhydride
E)formic anhydride

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
23
The contents on the bottle of a hand lotion lists octyl palmitate as an ingredient.Based on its name,what type of compound is octyl palmitate?
A)carboxylic acid
B)ester
C)carboxylate salt
D)fatty acid
E)It is impossible to tell from the name alone.
A)carboxylic acid
B)ester
C)carboxylate salt
D)fatty acid
E)It is impossible to tell from the name alone.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
24
What is the IUPAC name of the simplest dicarboxylic acid?
A)ethanoic acid
B)diethanoic acid
C)methanoic acid
D)methanedioic acid
E)ethanedioic acid
A)ethanoic acid
B)diethanoic acid
C)methanoic acid
D)methanedioic acid
E)ethanedioic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
25
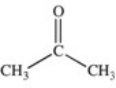
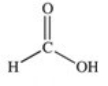
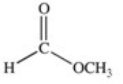
Which of the compounds below has the strongest intermolecular forces of attraction?
A)CH3OH
B)
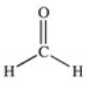
C)
D)
E)
A)CH3OH
B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
26
What is the name of the missing product in the reaction shown below? acetic acid + sodium hydroxide → ? + water
A)sodium acetate
B)sodium chloride
C)acetic hydroxide
D)acetal
E)sodium acetic acid
A)sodium acetate
B)sodium chloride
C)acetic hydroxide
D)acetal
E)sodium acetic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
27
What is the common name of the acid formed in the following reaction?
A)formic acid
B)acetic acid
C)oxalic acid
D)methanoic acid
E)propanoic acid
A)formic acid
B)acetic acid
C)oxalic acid
D)methanoic acid
E)propanoic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
28
Soap molecules form micelles when dissolved in water.What part of the soap molecule is oriented on the inside of the micelle?
A)the negatively charged carboxylate "head"
B)the positively charged carboxylate "head"
C)the hydrophilic carboxylate group
D)the hydrophobic hydrocarbon "tail"
E)the polar hydrocarbon chain
A)the negatively charged carboxylate "head"
B)the positively charged carboxylate "head"
C)the hydrophilic carboxylate group
D)the hydrophobic hydrocarbon "tail"
E)the polar hydrocarbon chain

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
29
Lactic acid,also known as α-hydroxy propionic acid,is an α-hydroxy acid (AHA)found in some skin care products.What is its structure?
A)
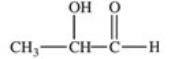
B)
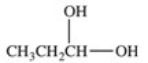
C)
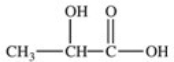
D)
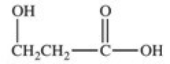
E)
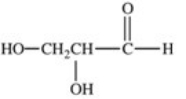
A)
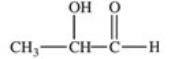
B)

C)
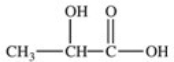
D)
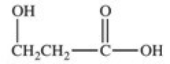
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which of the following is the general structure for a thioester?
A)R−S−R
B)R−S−S−R
C)
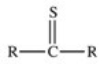
D)
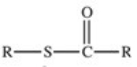
E)
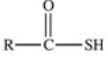
A)R−S−R
B)R−S−S−R
C)
D)
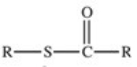
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
31
What suffix is used in the names of organic esters?
A)-ol
B)-al
C)-ate
D)-one
E)-ide
A)-ol
B)-al
C)-ate
D)-one
E)-ide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
32
What is the IUPAC name of the following compound? 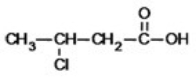
A)3-chlorobutanoic acid
B)2-chlorobutanoic acid
C)2-chloropropanoic acid
D)3-chloropropanoic acid
E)3-chloro-1-propanoic acid
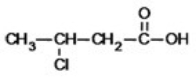
A)3-chlorobutanoic acid
B)2-chlorobutanoic acid
C)2-chloropropanoic acid
D)3-chloropropanoic acid
E)3-chloro-1-propanoic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which statement best explains why propanoic acid boils at a much higher temperature than hexane?
A)Lower molecular weights tend to boil at higher temperatures.
B)Propanoic acid has weaker intermolecular forces than hexane.
C)Propanoic acid molecules can hydrogen bond with other propanoic molecules but hexane is nonpolar and cannot hydrogen bond.
D)Chains with fewer carbon atoms tend to boil at higher temperatures than ones with more carbon atoms.
E)None of the choices are correct.
A)Lower molecular weights tend to boil at higher temperatures.
B)Propanoic acid has weaker intermolecular forces than hexane.
C)Propanoic acid molecules can hydrogen bond with other propanoic molecules but hexane is nonpolar and cannot hydrogen bond.
D)Chains with fewer carbon atoms tend to boil at higher temperatures than ones with more carbon atoms.
E)None of the choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
34
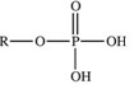
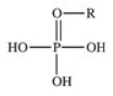
The reaction shown below between an alcohol and phosphoric acid produces a phosphoester.What is the general structure of a phosphoester? 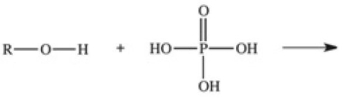
A)
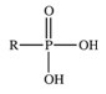
B)
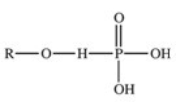
C)
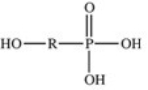
D)
E)

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
35
What is the condensed formula for the product of the following reaction? 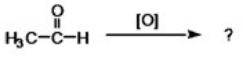
A)CH3COOH
B)CH3(OH)2
C)CH3CH2OH
D)CH3CH3
E)None of the choices are correct.
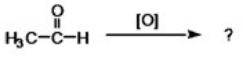
A)CH3COOH
B)CH3(OH)2
C)CH3CH2OH
D)CH3CH3
E)None of the choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
36
What type of product is formed in the reaction between an acid chloride and a carboxylate anion?
A)ester
B)acid anhydride
C)carboxylic acid
D)acetal
E)ether
A)ester
B)acid anhydride
C)carboxylic acid
D)acetal
E)ether

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
37
What role does acetyl coenzyme A (acetyl CoA)play in the body?
A)It serves as the energy source for energy-requiring reactions.
B)It serves as a carrier of two-carbon acetyl groups.
C)It serves as an identification marker on the surface of cells.
D)It functions as a catalyst for reduction reactions.
E)It is a structural component of muscle and bone.
A)It serves as the energy source for energy-requiring reactions.
B)It serves as a carrier of two-carbon acetyl groups.
C)It serves as an identification marker on the surface of cells.
D)It functions as a catalyst for reduction reactions.
E)It is a structural component of muscle and bone.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
38
What is the name of the compound shown below? 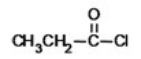
A)chloro propanoate
B)propanoyl chloride
C)ethyl chloroformate
D)ethyl formyl chloride
E)propyl chloride
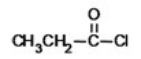
A)chloro propanoate
B)propanoyl chloride
C)ethyl chloroformate
D)ethyl formyl chloride
E)propyl chloride

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
39
What is the IUPAC name for the missing product in the following equation? ethanoic acid + methanol 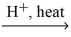 H2O + ?
H2O + ?
A)methyl ethyl ketone
B)ethyl methanoate
C)2-propanone
D)3-propanone
E)methyl ethanoate
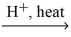 H2O + ?
H2O + ?A)methyl ethyl ketone
B)ethyl methanoate
C)2-propanone
D)3-propanone
E)methyl ethanoate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which of these is butyric acid?
A)CH3CH2CH2COOH
B)CH3CH2CH2CH2COOH
C)CH3CH2CH2CH2OH
D)CH3CH2COCH2CH3
E)None of the choices are correct.
A)CH3CH2CH2COOH
B)CH3CH2CH2CH2COOH
C)CH3CH2CH2CH2OH
D)CH3CH2COCH2CH3
E)None of the choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
41
Which species or compound is often reacted with an acid chloride to produce an acid anhydride?
A)ROH
B)R1COOR2
C)HCl
D)RCOO-
E)NaOH
A)ROH
B)R1COOR2
C)HCl
D)RCOO-
E)NaOH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
42
A phosphate ester is formed when phosphoric acid reacts with which of the following types of compounds?
A)ester
B)base
C)alcohol
D)carboxylic acid
E)thio compound
A)ester
B)base
C)alcohol
D)carboxylic acid
E)thio compound

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
43
What acid chloride and carboxylate anion are necessary to produce the acid anhydride shown below? 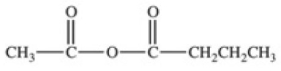
A)methanoate ion and propanoyl chloride
B)ethanoyl chloride and butanoate ion
C)ethanoate ion and propanoyl chloride
D)ethanoyl chloride and propanoate ion
E)butanoyl chloride and methanoate ion

A)methanoate ion and propanoyl chloride
B)ethanoyl chloride and butanoate ion
C)ethanoate ion and propanoyl chloride
D)ethanoyl chloride and propanoate ion
E)butanoyl chloride and methanoate ion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
44
Methylparaben is an ester used as a preservative in foods,beverages,and cosmetics.It may be prepared by the reaction shown below.What is the structure of methylparaben? 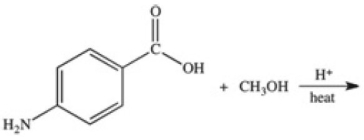
A)
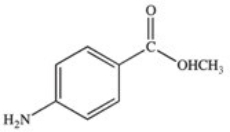
B)
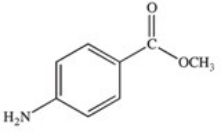
C)
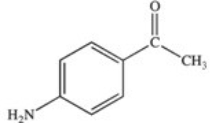
D)
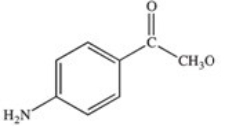
E)
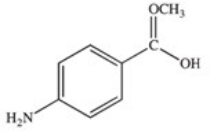

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
45
Which formula best represents the structure of acetic acid when it is dissolved in a basic solution?
A)
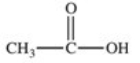
B)
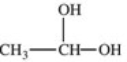
C)
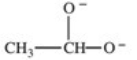
D)
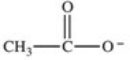
E)
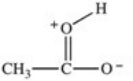
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
46
What is the common name of the carboxylic acid found in rancid butter?
A)acetic acid
B)palmitic acid
C)lactic acid
D)butyric acid
E)oleic acid
A)acetic acid
B)palmitic acid
C)lactic acid
D)butyric acid
E)oleic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
47
What is the IUPAC name of the following compound? 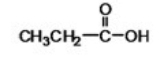
A)ethanoic acid
B)lactic acid
C)pyruvic acid
D)butyric acid
E)propanoic acid

A)ethanoic acid
B)lactic acid
C)pyruvic acid
D)butyric acid
E)propanoic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
48
What class of compounds is primarily responsible for the pleasant smell of many fruits?
A)carboxylic acids
B)esters
C)carboxylate salts
D)acid chlorides
E)alcohols
A)carboxylic acids
B)esters
C)carboxylate salts
D)acid chlorides
E)alcohols

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
49
The first step in the biochemical breakdown of sugars involves the formation of which of the following?
A)a phosphate ester
B)a thioester
C)a conventional ester
D)glycogen
E)DNA
A)a phosphate ester
B)a thioester
C)a conventional ester
D)glycogen
E)DNA

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
50
What is the name of the ester shown below? 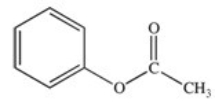
A)phenyl acetate
B)ethyl benzoate
C)benzyl acetate
D)ethyl phenoate
E)methyl benzoate

A)phenyl acetate
B)ethyl benzoate
C)benzyl acetate
D)ethyl phenoate
E)methyl benzoate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
51
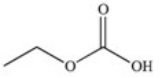
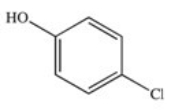
The drug Clofibrate is used to lower blood triglycerides and cholesterol.It can be made in an esterification reaction between a carboxylic acid and an alcohol.Which of the structures below represents the alcohol required for its synthesis? 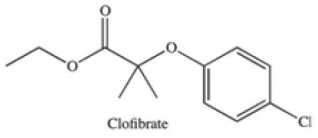
A)
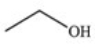
B)
C)
D)
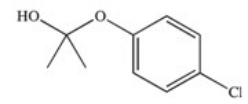
E)
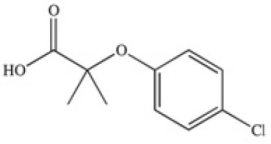

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which of the following is the term that describes a compound that acts as a pain killer?
A)analpheric
B)analgesic
C)prostaglandin
D)hydrophilic
E)pheromone
A)analpheric
B)analgesic
C)prostaglandin
D)hydrophilic
E)pheromone

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
53
What product(s)are formed when an ester reacts with water upon heating in the presence of NaOH?
A)two carboxylic acids
B)a carboxylic acid and an alcohol
C)two alcohols
D)an alcohol and an ether
E)a carboxylate salt and an alcohol
A)two carboxylic acids
B)a carboxylic acid and an alcohol
C)two alcohols
D)an alcohol and an ether
E)a carboxylate salt and an alcohol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
54
Which one of the following types of compounds can react with a carboxylic acid to form an ester?
A)acyl chloride
B)acetate
C)alcohol
D)ether
E)aldehyde
A)acyl chloride
B)acetate
C)alcohol
D)ether
E)aldehyde

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
55
Which of the following statements concerning carboxylic acids is FALSE?
A)Carboxylic acids are hydrogen ion acceptors.
B)The functional group of a carboxylic acid is condensed as COOH or CO2H.
C)Carboxylic acids form strong intermolecular hydrogen bonds.
D)Carboxylic acids are weak acids.
E)The water solubility of a carboxylic acid decreases with an increase in the number of carbons in the carbon chain.
A)Carboxylic acids are hydrogen ion acceptors.
B)The functional group of a carboxylic acid is condensed as COOH or CO2H.
C)Carboxylic acids form strong intermolecular hydrogen bonds.
D)Carboxylic acids are weak acids.
E)The water solubility of a carboxylic acid decreases with an increase in the number of carbons in the carbon chain.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
56
What term describes the sodium salt of a long-chain fatty acid?
A)esterate
B)ester
C)soap
D)triglyceride
E)bile salt
A)esterate
B)ester
C)soap
D)triglyceride
E)bile salt

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
57
What is the IUPAC name of the missing product in the following reaction? 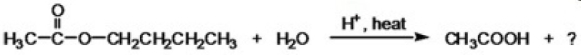
A)butane
B)butanoic acid
C)butanal
D)1-butanol
E)None of the choices are correct.
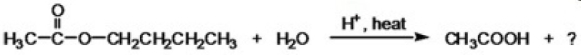
A)butane
B)butanoic acid
C)butanal
D)1-butanol
E)None of the choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
58
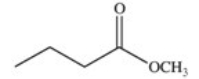
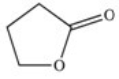
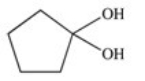
A cyclic ester (lactone)is formed when a carboxylic acid reacts with an alcohol functional group that is present in the same molecule.Which structure represents the lactone formed in the reaction below? 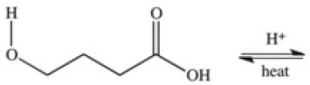
A)
B)
C)
D)
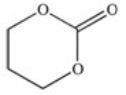
E)
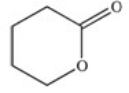

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
59
What other product is released when an anhydride is formed by the combination of two carboxylic acids?
A)alcohol
B)water
C)ester
D)HCl
E)hydrogen
A)alcohol
B)water
C)ester
D)HCl
E)hydrogen

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
60
Which of the following compounds would react similarly because they contain the same functional group? 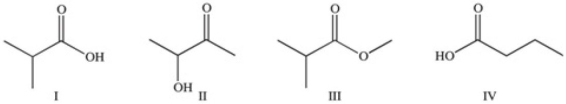
A)I and II
B)I and IV
C)II and IV
D)I,II,and III
E)I,II,and IV

A)I and II
B)I and IV
C)II and IV
D)I,II,and III
E)I,II,and IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
61
Acetic acid reacts with sodium hydroxide to produce
A)sodium acetate and water,the products of a neutralization reaction.
B)sodium acetate and water,the products of an oxidation.
C)sodium acetate and water,the products of a reduction.
D)acetaldehyde and sodium metal,the products of a precipitation reaction.
E)acetaldehyde and sodium metal,the products of an oxidation-reduction reaction.
A)sodium acetate and water,the products of a neutralization reaction.
B)sodium acetate and water,the products of an oxidation.
C)sodium acetate and water,the products of a reduction.
D)acetaldehyde and sodium metal,the products of a precipitation reaction.
E)acetaldehyde and sodium metal,the products of an oxidation-reduction reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
62
The oxidation of formaldehyde produces acetic acid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
63
Carboxylic acids are generally weak acids.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
64
Carboxylic acids are too weak to neutralize strong bases such as NaOH.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
65
The carboxyl group is found in all fatty acids.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
66
A single carboxylic acid molecule may contain more than one carboxyl functional group.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
67
What is the IUPAC name of the compound formed by the oxidation of 2-bromopentanal?
A)2-bromo-1-pentanol
B)2-bromopentanoate
C)2-bromopentanoic acid
D)2-bromo-2-pentanol
E)2-bromo-2-pentene
A)2-bromo-1-pentanol
B)2-bromopentanoate
C)2-bromopentanoic acid
D)2-bromo-2-pentanol
E)2-bromo-2-pentene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
68
Name the compound formed by the reaction between butanoic acid and potassium hydroxide.
A)potassium butanal
B)potassium butanoate
C)potassium butanol
D)potassium butanone
E)potassium buterate
A)potassium butanal
B)potassium butanoate
C)potassium butanol
D)potassium butanone
E)potassium buterate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
69
When comparing the boiling point of each of the following pairs,which of the following lists the compound with the highest boiling point first,assuming the same carbon chain length?
A)aldehyde,alcohol
B)carboxylic acid,alkane
C)ether,carboxylic acid
D)ketone,carboxylic acid
E)alkane,aldehyde
A)aldehyde,alcohol
B)carboxylic acid,alkane
C)ether,carboxylic acid
D)ketone,carboxylic acid
E)alkane,aldehyde

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
70
What carboxylic acid,first synthesized by Bayer and Company,and a derivative of an extract of willow bark,launched the pharmaceutical industry?
A)tartaric acid
B)oxalic acid
C)acetylsalicylic acid
D)benzoic acid
E)salicylic acid
A)tartaric acid
B)oxalic acid
C)acetylsalicylic acid
D)benzoic acid
E)salicylic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
71
Which of the following is the structure of o-chlorobenzoic acid?
A)
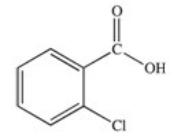
B)
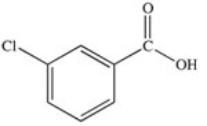
C)
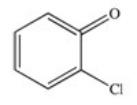
D)
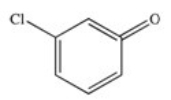
E)
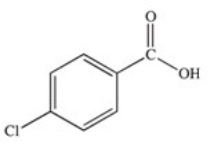
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
72
Soap is a triglyceride.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
73
Formic acid is the simplest carboxylic acid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
74
Saponification is hydrogenation of an ester under basic conditions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
75
What two compounds will react to produce methyl hexanoate in an acid catalyzed esterification reaction?
A)methane and hexane
B)methanoic acid and hexanoic acid
C)hexanol and methanol
D)hexanoic acid and methanol
E)hexanol and methanoic acid
A)methane and hexane
B)methanoic acid and hexanoic acid
C)hexanol and methanol
D)hexanoic acid and methanol
E)hexanol and methanoic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
76
Which word best describes the odors of large carboxylic acids?
A)fruity
B)floral
C)minty
D)foul
E)Both fruity and floral are correct.
A)fruity
B)floral
C)minty
D)foul
E)Both fruity and floral are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
77
The base-catalyzed hydrolysis of a triglyceride produces which of the following? 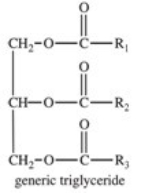
A)soap
B)polymers such as polyesters
C)acid anhydrides
D)glycerol and carboxylate salts
E)soap,glycerol,and carboxylate salts

A)soap
B)polymers such as polyesters
C)acid anhydrides
D)glycerol and carboxylate salts
E)soap,glycerol,and carboxylate salts

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
78
Traces of H+ can catalyze both the formation of an ester and its hydrolysis.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
79
Fats and oils are triesters of glycerol.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
80
What is the common name of the following compound? 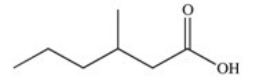
A)β-methylcaproic acid
B)2-methylvaleric acid
C)3-methylpentanoic acid
D)γ-methylcaproic acid
E)β-methylvaleric acid

A)β-methylcaproic acid
B)2-methylvaleric acid
C)3-methylpentanoic acid
D)γ-methylcaproic acid
E)β-methylvaleric acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck








