Deck 3: Structure and Properties of Ionic and Covalent Compounds
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/93
العب
ملء الشاشة (f)
Deck 3: Structure and Properties of Ionic and Covalent Compounds
1
What is the name of the ionic compound with the formula Na3PO4?
A)silver phosphite
B)sodium phosphorus oxide
C)trisilver tetraphosphate
D)trisodium tetraphosphate
E)sodium phosphate
A)silver phosphite
B)sodium phosphorus oxide
C)trisilver tetraphosphate
D)trisodium tetraphosphate
E)sodium phosphate
sodium phosphate
2
Which statement about compounds is FALSE?
A)Compounds consist of atoms of two or more different elements that are chemically bonded together.
B)The elements in a compound cannot be separated or recovered by a physical process.
C)Ionic compounds are composed of cations and anions.
D)Covalent compounds are composed of metals and nonmetals.
E)Intermolecular forces are attractive forces between molecules of a compound,and are responsible for the compound?s physical properties.
A)Compounds consist of atoms of two or more different elements that are chemically bonded together.
B)The elements in a compound cannot be separated or recovered by a physical process.
C)Ionic compounds are composed of cations and anions.
D)Covalent compounds are composed of metals and nonmetals.
E)Intermolecular forces are attractive forces between molecules of a compound,and are responsible for the compound?s physical properties.
Covalent compounds are composed of metals and nonmetals.
3
What are the two principal classes of bonding called?
A)ionic bonding and nuclear bonding
B)covalent bonding and hydrogen bonding
C)hydrogen bonding and ionic bonding
D)polar bonding and ionic bonding
E)ionic bonding and covalent bonding
A)ionic bonding and nuclear bonding
B)covalent bonding and hydrogen bonding
C)hydrogen bonding and ionic bonding
D)polar bonding and ionic bonding
E)ionic bonding and covalent bonding
ionic bonding and covalent bonding
4
What does it mean if an atom is said to have a high electronegativity?
A)The atom requires a large amount of energy to remove an electron from its structure.
B)The atom is highly susceptible to losing a valence electron.
C)The atom has a strong attraction for electrons in a chemical bond.
D)The atom releases a large amount of energy when it gains an electron.
E)The atom has more electrons around it than necessary; the atom has an expanded octet.
A)The atom requires a large amount of energy to remove an electron from its structure.
B)The atom is highly susceptible to losing a valence electron.
C)The atom has a strong attraction for electrons in a chemical bond.
D)The atom releases a large amount of energy when it gains an electron.
E)The atom has more electrons around it than necessary; the atom has an expanded octet.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
5
Considering the electronegativity values indicated in the table,which covalent bond is the most polar?
A)C?H
B)N?O
C)Cl?F
D)H?F
E)F?F
A)C?H
B)N?O
C)Cl?F
D)H?F
E)F?F

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
6
In what region on the periodic table are elements with the lowest electronegativity values found?
A)upper right corner
B)bottom right corner
C)upper left corner
D)bottom left corner
E)middle
A)upper right corner
B)bottom right corner
C)upper left corner
D)bottom left corner
E)middle

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following compounds contains a polyatomic ion?
A)NaOH
B)HCl
C)Ca3N2
D)MgBr2
E)NH3
A)NaOH
B)HCl
C)Ca3N2
D)MgBr2
E)NH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
8
A covalent bond involves which of the following?
A)sharing of electrons between two atoms
B)transfer of electrons between two atoms
C)donation of protons from one atom to another
D)electrostatic attraction between opposite charges
E)sharing of protons between two atoms
A)sharing of electrons between two atoms
B)transfer of electrons between two atoms
C)donation of protons from one atom to another
D)electrostatic attraction between opposite charges
E)sharing of protons between two atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
9
Emergency treatment of cardiac arrest victims sometimes involves injection of a calcium chloride (CaCl2)solution directly into the heart muscle.Which statement about the compound CaCl2 is FALSE?
A)This compound is an ionic compound.
B)The cation in this compound is Ca2+.
C)The anion in this compound is Cl2−.
D)There are two chloride ions in this compound.
E)The net charge on this compound is zero.
A)This compound is an ionic compound.
B)The cation in this compound is Ca2+.
C)The anion in this compound is Cl2−.
D)There are two chloride ions in this compound.
E)The net charge on this compound is zero.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
10
A molecule of deoxyribose,an essential part of DNA,contains five carbon atoms,ten hydrogen atoms,and four oxygen atoms.How would the formula of deoxyribose be represented?
A)5C10H4O
B)C5H10O4
C)C5H10O4
D)C5H10O4
E)All formulas are acceptable.
A)5C10H4O
B)C5H10O4
C)C5H10O4
D)C5H10O4
E)All formulas are acceptable.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which statement about chemical bonds is FALSE?
A)The sharing of electrons between two nonmetals results in a covalent bond.
B)The attraction between oppositely charged ions results in an ionic bond.
C)The electrons in covalent bonds may be shared equally or unequally between the atoms.
D)A bond dipole is the separation of charge that results when atoms sharing electrons have different electronegativity values.
E)A nonpolar covalent bond is one in which electrons are not shared equally between the atoms.
A)The sharing of electrons between two nonmetals results in a covalent bond.
B)The attraction between oppositely charged ions results in an ionic bond.
C)The electrons in covalent bonds may be shared equally or unequally between the atoms.
D)A bond dipole is the separation of charge that results when atoms sharing electrons have different electronegativity values.
E)A nonpolar covalent bond is one in which electrons are not shared equally between the atoms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
12
What is the formula of the compound sulfur trioxide?
A)S3O
B)SO3
C)S(O2)3
D)S3O2
E)S3O2?
A)S3O
B)SO3
C)S(O2)3
D)S3O2
E)S3O2?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
13
What is the formula of the ionic compound formed when ions of calcium and nitrogen combine?
A)CaN
B)CaN2
C)Ca2N
D)Ca3N2
E)Ca2N3
A)CaN
B)CaN2
C)Ca2N
D)Ca3N2
E)Ca2N3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
14
What is the name of Fe2+ in the Stock system?
A)iron 2 ion
B)iron(II)ion
C)ferric(II)ion
D)ferrous 2+ ion
E)iron cation
A)iron 2 ion
B)iron(II)ion
C)ferric(II)ion
D)ferrous 2+ ion
E)iron cation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which combination of atoms is most likely to form an ionic compound if they are allowed to react with each other?
A)metal and metal
B)metal and nonmetal
C)nonmetal and nonmetal
D)metalloid and metalloid
E)All combinations are likely to form an ionic compound.
A)metal and metal
B)metal and nonmetal
C)nonmetal and nonmetal
D)metalloid and metalloid
E)All combinations are likely to form an ionic compound.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
16
What is the name of the ion NH4+?
A)nitrogen tetrahydrate
B)nitrogen hydrate
C)mononitrogen tetrahydrogen
D)ninhydrin
E)ammonium
A)nitrogen tetrahydrate
B)nitrogen hydrate
C)mononitrogen tetrahydrogen
D)ninhydrin
E)ammonium

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
17
What is the Lewis symbol of the fluoride ion?
A)
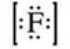
B)
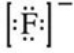
C)
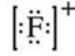
D)
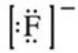
E)
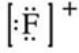
A)
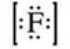
B)
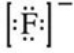
C)
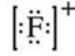
D)
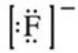
E)
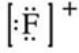

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
18
What is the three-dimensional arrangement of positive and negative ions in an ionic solid called?
A)dipole
B)polyatomic structure
C)crystal lattice
D)cubic ionic structure
E)intermolecular ionic forces
A)dipole
B)polyatomic structure
C)crystal lattice
D)cubic ionic structure
E)intermolecular ionic forces

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
19
What do the dots in a Lewis symbol represent?
A)protons
B)valence electrons
C)innermost (core)electrons
D)the nucleus
E)mass number
A)protons
B)valence electrons
C)innermost (core)electrons
D)the nucleus
E)mass number

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
20
What kind of bonding exists in substances that consist of discrete molecules?
A)ionic
B)covalent
C)hydrogen bonding
D)intermolecular
E)polar
A)ionic
B)covalent
C)hydrogen bonding
D)intermolecular
E)polar

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which properly represents the Lewis structure of hydrogen sulfide,H2S?
A)
B)
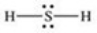
C)
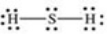
D)
E)
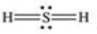
A)
B)
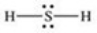
C)
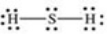
D)
E)
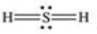

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
22
What is the formula of the ionic compound sodium carbonate?
A)NaCO2
B)Na2CO2
C)Na2CO3
D)Na(CO3)2
E)Na3CO3
A)NaCO2
B)Na2CO2
C)Na2CO3
D)Na(CO3)2
E)Na3CO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
23
What is wrong with the Lewis structure shown for sulfur trioxide,SO3? 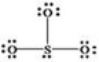
A)Oxygen,not sulfur,should be the central atom.
B)Sulfur should have 10 electrons around it instead of 8,to show its expanded octet.
C)The structure shows 26 valence electrons,but there should only be 24.
D)There are too many bonding electrons shown.
E)The structure should show each oxygen atom with a double bond to the sulfur atom.

A)Oxygen,not sulfur,should be the central atom.
B)Sulfur should have 10 electrons around it instead of 8,to show its expanded octet.
C)The structure shows 26 valence electrons,but there should only be 24.
D)There are too many bonding electrons shown.
E)The structure should show each oxygen atom with a double bond to the sulfur atom.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
24
The drug "lithium" is often prescribed to treat mental illness.It is not the element lithium,but the ionic compound Li2CO3,that is actually administered.What is the name of this compound?
A)dilithium carbon trioxide
B)lithium carbide
C)lithium carboxide
D)lithium carbonate
E)lithium tricarbonate
A)dilithium carbon trioxide
B)lithium carbide
C)lithium carboxide
D)lithium carbonate
E)lithium tricarbonate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
25
Baking soda consists of the ionic compound sodium bicarbonate.What is the formula of this compound?
A)Na(CO3)2
B)Na2CO3
C)NaBiCO3
D)NaHCO3
E)Na2HCO3
A)Na(CO3)2
B)Na2CO3
C)NaBiCO3
D)NaHCO3
E)Na2HCO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
26
Vehicle airbags inflate when the ionic compound sodium azide rapidly decomposes to the elements sodium and nitrogen.If the azide ion is a polyatomic ion with the formula N3−,what is the formula of sodium azide?
A)NaN3
B)Na3N3
C)Na3(N3)3
D)Na3N
E)Na(N3)3
A)NaN3
B)Na3N3
C)Na3(N3)3
D)Na3N
E)Na(N3)3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
27
Predict the formula of the compound formed when ions of sodium and sulfur combine.
A)Na2S
B)NaS2
C)NaS
D)Na2S2
E)Na3S
A)Na2S
B)NaS2
C)NaS
D)Na2S2
E)Na3S

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
28
Formaldehyde is a covalent compound used as a preservative for biological specimens.Which statement concerning the Lewis structure of formaldehyde,shown below,is FALSE? 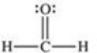
A)Four electrons are being shared between the carbon and oxygen atoms.
B)The oxygen atom has four valence electrons that are not being shared.
C)The oxygen and carbon atoms each have an octet of electrons in their valence shells.
D)The hydrogen atoms have incomplete valence shells.
E)There are a total of 12 valence electrons in formaldehyde.
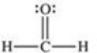
A)Four electrons are being shared between the carbon and oxygen atoms.
B)The oxygen atom has four valence electrons that are not being shared.
C)The oxygen and carbon atoms each have an octet of electrons in their valence shells.
D)The hydrogen atoms have incomplete valence shells.
E)There are a total of 12 valence electrons in formaldehyde.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
29
What is the formula of the sulfate ion?
A)SO4-
B)SO3-
C)SO42-
D)SO32-
E)SO22-
A)SO4-
B)SO3-
C)SO42-
D)SO32-
E)SO22-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
30
What is the Lewis structure of methanethiol,CH3SH?
A)
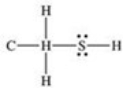
B)
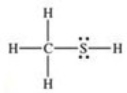
C)
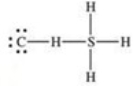
D)
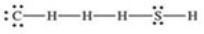
E)
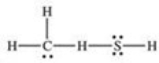
A)

B)

C)

D)
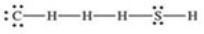
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
31
What is the name of the ion whose formula is NO3-?
A)nitrogen trioxide ion
B)nitrate ion
C)ammonium ion
D)sodium oxide ion
E)nitride ion
A)nitrogen trioxide ion
B)nitrate ion
C)ammonium ion
D)sodium oxide ion
E)nitride ion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
32
The polyatomic ion known as the diphosphate ion is an important intermediate formed in metabolic processes.If the ionic compound calcium diphosphate has the formula Ca2P2O7,which of the following correctly represents the symbol for the diphosphate ion?
A)P2O74−
B)P2O714-
C)PO4+
D)P2O72−
E)PO2−
A)P2O74−
B)P2O714-
C)PO4+
D)P2O72−
E)PO2−

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
33
What statement about the ammonia molecule,NH3,is FALSE?
A)The molecule contains three polar bonds.
B)The molecule itself is not polar.
C)The molecule has a trigonal pyramidal shape.
D)The nitrogen atom has one nonbonding pair of electrons.
E)The pull of the electrons in the N-H bonds is toward the nitrogen atom.
A)The molecule contains three polar bonds.
B)The molecule itself is not polar.
C)The molecule has a trigonal pyramidal shape.
D)The nitrogen atom has one nonbonding pair of electrons.
E)The pull of the electrons in the N-H bonds is toward the nitrogen atom.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
34
At what temperature is a liquid converted into a gas?
A)the boiling point of the liquid
B)the combustion point of the liquid
C)the flash point of the liquid
D)the condensation point of the liquid
E)100°C
A)the boiling point of the liquid
B)the combustion point of the liquid
C)the flash point of the liquid
D)the condensation point of the liquid
E)100°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
35
What is the name of the compound whose formula is CCl4?
A)carbon tetrachloride
B)carbon(IV)chloride
C)carbon chloride
D)carbotetrachloride
E)carbonate tetrachloride
A)carbon tetrachloride
B)carbon(IV)chloride
C)carbon chloride
D)carbotetrachloride
E)carbonate tetrachloride

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which formula represents ammonia?
A)Al3
B)AlH3
C)NH4+
D)NH3
E)AN4
A)Al3
B)AlH3
C)NH4+
D)NH3
E)AN4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
37
What is the name of CuF2 in the Stock system?
A)copper(I)fluoride
B)copper(II)fluorate
C)copper difluoride
D)copper fluoride
E)copper(II)fluoride
A)copper(I)fluoride
B)copper(II)fluorate
C)copper difluoride
D)copper fluoride
E)copper(II)fluoride

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
38
The ionic compound iron(II)sulfate is used in iron-containing supplements.What does the (II)in the name of this compound specifically indicate?
A)There are two iron ions in this compound.
B)The charge on the iron ion is 2+.
C)The charge on the sulfate ion is 2−.
D)Iron has two valence electrons.
E)There are two sulfate ions in this compound.
A)There are two iron ions in this compound.
B)The charge on the iron ion is 2+.
C)The charge on the sulfate ion is 2−.
D)Iron has two valence electrons.
E)There are two sulfate ions in this compound.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
39
How many dots are present in the Lewis symbol for the fluorine atom?
A)5
B)6
C)7
D)8
E)10
A)5
B)6
C)7
D)8
E)10

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which statement about general bonding characteristics is FALSE?
A)Bond energy is the amount of energy required to break a bond holding two atoms together.
B)Bond length is the distance of separation of two nuclei in a covalent bond.
C)Triple bonds have higher bond energies than double bonds.
D)Single bonds are easier to break than double bonds.
E)A triple bond is longer than a single bond.
A)Bond energy is the amount of energy required to break a bond holding two atoms together.
B)Bond length is the distance of separation of two nuclei in a covalent bond.
C)Triple bonds have higher bond energies than double bonds.
D)Single bonds are easier to break than double bonds.
E)A triple bond is longer than a single bond.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
41
In the compound shown below,how can the bond between carbon and chlorine be described? 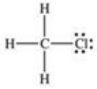
A)intermolecular
B)ionic
C)nonpolar covalent
D)polar covalent
E)nonpolar ionic

A)intermolecular
B)ionic
C)nonpolar covalent
D)polar covalent
E)nonpolar ionic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
42
How many bonding electrons are present in the Lewis structure for the bicarbonate ion,shown below? 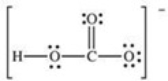
A)4
B)5
C)8
D)10
E)24

A)4
B)5
C)8
D)10
E)24

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
43
In the molecule AX2,the central atom A has two lone pairs of electrons in addition to the two bond pairs in the A-X bonds.What is the shape of this molecule?
A)bent,bond angle close to 109.5°
B)bent,bond angle close to 120°
C)linear
D)trigonal planar
E)trigonal pyramidal
A)bent,bond angle close to 109.5°
B)bent,bond angle close to 120°
C)linear
D)trigonal planar
E)trigonal pyramidal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
44
If the shape of a molecule is tetrahedral,what are the values of the bond angles?
A)90°
B)109.5°
C)120°
D)180°
E)90° and 120°
A)90°
B)109.5°
C)120°
D)180°
E)90° and 120°

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
45
Which element has the greatest electronegativity?
A)Si
B)P
C)Cl
D)Ar
E)Br
A)Si
B)P
C)Cl
D)Ar
E)Br

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
46
What is the Stock name of Cu+?
A)cupric ion
B)cuprous ion
C)copper(I)ion
D)copper(II)ion
E)cuprate ion
A)cupric ion
B)cuprous ion
C)copper(I)ion
D)copper(II)ion
E)cuprate ion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
47
The ionic compound magnesium hydroxide can be found in antacid tablets.What is the formula of magnesium hydroxide?
A)Mg2OH
B)MgOH2
C)MgO2H2
D)Mg(OH)2
E)MgOH
A)Mg2OH
B)MgOH2
C)MgO2H2
D)Mg(OH)2
E)MgOH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
48
How many nonbonding electrons are in CH4?
A)0
B)1
C)2
D)3
E)8
A)0
B)1
C)2
D)3
E)8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
49
What kind of bond results when electron transfer occurs between atoms of two different elements?
A)ionic
B)covalent
C)nonpolar
D)single
E)double
A)ionic
B)covalent
C)nonpolar
D)single
E)double

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
50
What term describes the temperature at which a solid is converted into a liquid?
A)critical point
B)flash point
C)sublimation point
D)melting point
E)boiling point
A)critical point
B)flash point
C)sublimation point
D)melting point
E)boiling point

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
51
How many bonding electrons are in CO2?
A)1
B)2
C)3
D)4
E)8
A)1
B)2
C)3
D)4
E)8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
52
Assuming reactions between the following pairs of elements,which pair is most likely to form an ionic compound?
A)copper and tin
B)chlorine and oxygen
C)cesium and iodine
D)carbon and chlorine
E)fluorine and iodine
A)copper and tin
B)chlorine and oxygen
C)cesium and iodine
D)carbon and chlorine
E)fluorine and iodine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
53
A double bond between two atoms,A and B,________.
A)is longer than a single bond between the same two atoms.
B)has a lower bond energy than a single bond between the same two atoms.
C)arises when two electrons are transferred from A to B.
D)consists of two electrons shared between A and B.
E)consists of four electrons shared between A and B.
A)is longer than a single bond between the same two atoms.
B)has a lower bond energy than a single bond between the same two atoms.
C)arises when two electrons are transferred from A to B.
D)consists of two electrons shared between A and B.
E)consists of four electrons shared between A and B.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
54
What term describes a solution of a compound in water that does not conduct an electric current?
A)amorphous solution
B)an electrolyte solution
C)a nonelectrolyte solution
D)superconducting solution
E)isoelectric solution
A)amorphous solution
B)an electrolyte solution
C)a nonelectrolyte solution
D)superconducting solution
E)isoelectric solution

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
55
What is the name of Fe2(SO4)3 in the Stock system?
A)iron monosulfuric acid
B)iron(II)sulfate
C)iron(III)sulfate
D)iron trisulfate
E)iron(II)trisulfate
A)iron monosulfuric acid
B)iron(II)sulfate
C)iron(III)sulfate
D)iron trisulfate
E)iron(II)trisulfate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
56
Which one of the following is NOT true about elements that form cations?
A)The atoms lose electrons in forming ions.
B)The elements are metals.
C)They are located to the left of the periodic table.
D)They have low ionization energies.
E)They have high electron affinities.
A)The atoms lose electrons in forming ions.
B)The elements are metals.
C)They are located to the left of the periodic table.
D)They have low ionization energies.
E)They have high electron affinities.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
57
Which element has the greatest electronegativity?
A)H
B)Cl
C)O
D)F
E)Na
A)H
B)Cl
C)O
D)F
E)Na

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
58
What is the correct formula of diphosphorus pentoxide?
A)P5O2
B)PO2
C)P2O4
D)P2O5
E)P5O
A)P5O2
B)PO2
C)P2O4
D)P2O5
E)P5O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
59
Assuming reactions between the following pairs of elements,which pair is most likely to form a covalent compound?
A)lithium and iodine
B)sodium and oxygen
C)calcium and chlorine
D)copper and tin
E)carbon and oxygen
A)lithium and iodine
B)sodium and oxygen
C)calcium and chlorine
D)copper and tin
E)carbon and oxygen

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
60
Which Lewis symbol for an ion is incorrect?
A)Na+
B)Ca2+
C)Sn4+
D)
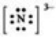
E)
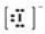
A)Na+
B)Ca2+
C)Sn4+
D)
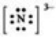
E)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
61
The common name of iron(III)chloride is ferric chloride.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
62
Which of the following bonds would be the most polar?
A)P-F
B)N-F
C)S-Cl
D)Br-Br
E)F-F
A)P-F
B)N-F
C)S-Cl
D)Br-Br
E)F-F

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
63
A covalent bond is formed when
A)two metals share a pair of electrons.
B)a metal and a nonmetal share a pair of electrons.
C)a nonmetal donates an electron to a metal.
D)cations and anions join as an ion pair.
E)two nonmetals share a pair of electrons.
A)two metals share a pair of electrons.
B)a metal and a nonmetal share a pair of electrons.
C)a nonmetal donates an electron to a metal.
D)cations and anions join as an ion pair.
E)two nonmetals share a pair of electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
64
What is the proper name for Mg(CN)2?
A)manganese carbon nitride
B)manganese dicyanide
C)magnesium dicarbon dinitride
D)magnesium(II)dicyanide
E)magnesium cyanide
A)manganese carbon nitride
B)manganese dicyanide
C)magnesium dicarbon dinitride
D)magnesium(II)dicyanide
E)magnesium cyanide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
65
Which compound contains a central atom with an exception to the octet rule known as an expanded octet?
A)BeH2
B)NO
C)PF5
D)CH4
E)BF3
A)BeH2
B)NO
C)PF5
D)CH4
E)BF3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
66
Which of the following Lewis structures has a possible resonance structure? 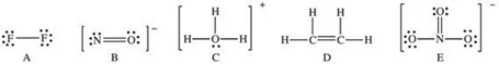
A)A
B)B
C)C
D)D
E)E
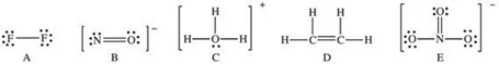
A)A
B)B
C)C
D)D
E)E

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
67
According to the VSEPR theory,what is the geometry (shape)around the phosphorus atom in the Lewis structure shown below? 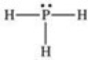
A)linear
B)bent (angular)
C)trigonal planar
D)trigonal pyramidal
E)tetrahedral

A)linear
B)bent (angular)
C)trigonal planar
D)trigonal pyramidal
E)tetrahedral

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
68
The name of SnO2 is tin(II)oxide.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
69
Which of the following would NOT describe the Lewis structure of PF3?
A)The Lewis structure has a double bond between phosphorus and one of the fluorine atoms.
B)No resonance structures can be drawn.
C)The molecule has a lone pair (nonbonding pair)on the central atom.
D)The Lewis structure has 26 valence electrons.
E)The Lewis structure is not an exception to the octet rule.
A)The Lewis structure has a double bond between phosphorus and one of the fluorine atoms.
B)No resonance structures can be drawn.
C)The molecule has a lone pair (nonbonding pair)on the central atom.
D)The Lewis structure has 26 valence electrons.
E)The Lewis structure is not an exception to the octet rule.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
70
There are three atoms of iodine represented in the formula NaIO3.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
71
In Lewis symbols,the chemical symbol of an element represents both the nucleus and the lower energy (nonvalence)electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
72
Draw the Lewis structures of F2,O2 and N2.Which statement is true?
A)All three molecules have a single bond between the atoms.
B)B.Both O2 and N2 have double bonds connecting the atoms.
C)Fluorine has the shortest bond between the two atoms.
D)The bond between the nitrogen atoms in N2 is stronger than the bond in F2 and O2.
E)All three molecules have triple bonds between the atoms.
A)All three molecules have a single bond between the atoms.
B)B.Both O2 and N2 have double bonds connecting the atoms.
C)Fluorine has the shortest bond between the two atoms.
D)The bond between the nitrogen atoms in N2 is stronger than the bond in F2 and O2.
E)All three molecules have triple bonds between the atoms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
73
A certain compound has a very high melting point,and when it dissolves in water the solution conducts electricity.Which one of the following compounds would have these properties?
A)O2
B)Cr
C)CCl4
D)Na2SO4
E)He
A)O2
B)Cr
C)CCl4
D)Na2SO4
E)He

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
74
What is the formula of iron(III)bromide?
A)Fe(Br2)3
B)Fe3Br
C)Fe3Br3
D)FeBr3
E)Fe2Br3
A)Fe(Br2)3
B)Fe3Br
C)Fe3Br3
D)FeBr3
E)Fe2Br3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
75
What is the proper name for N2O5?
A)nitrogen(IV)oxide
B)dinitrogen pentoxide
C)nitrogen oxide
D)nitrate
E)nitrite
A)nitrogen(IV)oxide
B)dinitrogen pentoxide
C)nitrogen oxide
D)nitrate
E)nitrite

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
76
Which statement is TRUE concerning the solubility of a substance in H2O?
A)Polar and nonpolar substances are equally soluble in water.
B)A nonpolar substance is soluble in water.
C)All substances are soluble in water.
D)Solubility of a substance in water has no relationship to the polarity of the substance being dissolved.
E)A polar substance is soluble in water.
A)Polar and nonpolar substances are equally soluble in water.
B)A nonpolar substance is soluble in water.
C)All substances are soluble in water.
D)Solubility of a substance in water has no relationship to the polarity of the substance being dissolved.
E)A polar substance is soluble in water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
77
How many total valence electrons are in SO42- ?
A)2
B)64
C)32
D)12
E)16
A)2
B)64
C)32
D)12
E)16

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
78
Which of the following is an essential feature of ionic bonding?
A)Elements with high ionization energy form bonds with elements with high electron affinity.
B)Elements with high ionization energy form bonds with elements with low electron affinity.
C)Elements with low ionization energy form bonds with elements with low electron affinity.
D)Elements with low ionization energy form bonds with elements with high electron affinity.
E)The ionization and electron affinity of elements does not determine bonding.
A)Elements with high ionization energy form bonds with elements with high electron affinity.
B)Elements with high ionization energy form bonds with elements with low electron affinity.
C)Elements with low ionization energy form bonds with elements with low electron affinity.
D)Elements with low ionization energy form bonds with elements with high electron affinity.
E)The ionization and electron affinity of elements does not determine bonding.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
79
What is the molecular geometry of CO2?
A)linear
B)trigonal planar
C)angular
D)trigonal pyramidal
E)tetrahedral
A)linear
B)trigonal planar
C)angular
D)trigonal pyramidal
E)tetrahedral

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck
80
Which of the following molecules is polar?
A)
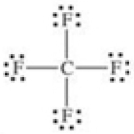
B)
C)
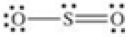
D)

E)
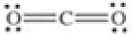
A)

B)
C)
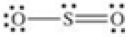
D)

E)
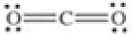

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 93 في هذه المجموعة.
فتح الحزمة
k this deck








