Deck 5: States of Matter- Gases, Liquids, and Solids
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/75
العب
ملء الشاشة (f)
Deck 5: States of Matter- Gases, Liquids, and Solids
1
What law predicts the expansion of a balloon when helium is added?
A)Boyle's Law
B)Charles's Law
C)Avogadro's Law
D)Ideal Gas Law
E)None of the choices are correct.
A)Boyle's Law
B)Charles's Law
C)Avogadro's Law
D)Ideal Gas Law
E)None of the choices are correct.
Avogadro's Law
2
Gas pressure is a result of which of the following?
A)collisions of gas particles with each other
B)expansion of gas particles in an open container
C)the resistance of gas particles to flow
D)collisions of gas particles with the walls of the container
E)compressibility of gas particles
A)collisions of gas particles with each other
B)expansion of gas particles in an open container
C)the resistance of gas particles to flow
D)collisions of gas particles with the walls of the container
E)compressibility of gas particles
collisions of gas particles with the walls of the container
3
Which of the following is(are) NOT a type of crystalline solid?
A)covalent solid
B)molecular solid
C)amorphous solid
D)ionic solid
E)metallic solid
A)covalent solid
B)molecular solid
C)amorphous solid
D)ionic solid
E)metallic solid
amorphous solid
4
Calculate the density of nitrogen gas (N2) at STP, in g/L.[Molar mass: N2, 28.0 g/mol]
A)0.645 g/L
B)0.800 g/L
C)1.25 g/L
D)2.39 g/L
E)3.58 g/L
A)0.645 g/L
B)0.800 g/L
C)1.25 g/L
D)2.39 g/L
E)3.58 g/L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
5
At 100°C, which gas sample exerts the greatest pressure? 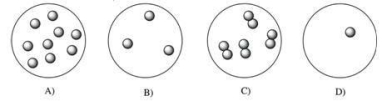
A)A
B)B
C)C
D)D
E)They would all exert the same pressure.

A)A
B)B
C)C
D)D
E)They would all exert the same pressure.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
6
Dalton?s Law of Partial Pressures states which of the following?
A)The total pressure exerted by a mixture of gases in a container is equal to the highest partial pressure exerted by any of the gases in the container.
B)The total pressure exerted by a mixture of gases in a container is the sum of the pressures that each gas would exert if present alone under similar conditions.
C)The total pressure exerted by a mixture of gases in a container is the average of the pressures that each gas would exert if present alone under similar conditions.
D)The total pressure exerted by a mixture of gases in a container is inversely proportional to the pressure that each gas would exert if present alone under similar conditions.
E)The total pressure exerted by a mixture of gases in a container is equal to the range of partial pressures of each gas in the container.
A)The total pressure exerted by a mixture of gases in a container is equal to the highest partial pressure exerted by any of the gases in the container.
B)The total pressure exerted by a mixture of gases in a container is the sum of the pressures that each gas would exert if present alone under similar conditions.
C)The total pressure exerted by a mixture of gases in a container is the average of the pressures that each gas would exert if present alone under similar conditions.
D)The total pressure exerted by a mixture of gases in a container is inversely proportional to the pressure that each gas would exert if present alone under similar conditions.
E)The total pressure exerted by a mixture of gases in a container is equal to the range of partial pressures of each gas in the container.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following statements correctly describes Charles's Law?
A)The volume of a gas is directly proportional to the number of moles of the gas, if the pressure and temperature remain constant.
B)The volume of a gas is directly proportional to the Kelvin temperature of the gas, if the pressure and amount of gas remain constant.
C)The pressure of a gas is inversely proportional to the volume of the gas, if the temperature and amount of gas remain constant.
D)The pressure of a gas is directly proportional to the Kelvin temperature of the gas, if the volume and amount of gas remain constant.
E)The total pressure exerted by a mixture of gases in a container is the sum of the partial pressures that each gas would exert alone.
A)The volume of a gas is directly proportional to the number of moles of the gas, if the pressure and temperature remain constant.
B)The volume of a gas is directly proportional to the Kelvin temperature of the gas, if the pressure and amount of gas remain constant.
C)The pressure of a gas is inversely proportional to the volume of the gas, if the temperature and amount of gas remain constant.
D)The pressure of a gas is directly proportional to the Kelvin temperature of the gas, if the volume and amount of gas remain constant.
E)The total pressure exerted by a mixture of gases in a container is the sum of the partial pressures that each gas would exert alone.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which of the following statements correctly describes Avogadro's Law?
A)Equal volumes of any ideal gas contain the same number of moles, if measured at the same temperature and pressure.
B)The volume of a gas is directly proportional to the Kelvin temperature of the gas, if the pressure and amount of gas remain constant.
C)The pressure of a gas is inversely proportional to the volume of the gas, if the temperature and amount of gas remain constant.
D)The pressure of a gas is directly proportional to the Kelvin temperature of the gas, if the volume and amount of gas remain constant.
E)The total pressure exerted by a mixture of gases in a container is the sum of the partial pressures that each gas would exert alone.
A)Equal volumes of any ideal gas contain the same number of moles, if measured at the same temperature and pressure.
B)The volume of a gas is directly proportional to the Kelvin temperature of the gas, if the pressure and amount of gas remain constant.
C)The pressure of a gas is inversely proportional to the volume of the gas, if the temperature and amount of gas remain constant.
D)The pressure of a gas is directly proportional to the Kelvin temperature of the gas, if the volume and amount of gas remain constant.
E)The total pressure exerted by a mixture of gases in a container is the sum of the partial pressures that each gas would exert alone.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
9
What state(s) of matter is most compressible?
A)solid
B)liquid
C)gas
D)solid and liquid
E)liquid and gas
A)solid
B)liquid
C)gas
D)solid and liquid
E)liquid and gas

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
10
A gas sample is prepared in which the components have the following partial pressures: nitrogen, 555 mmHg; oxygen, 149 mmHg; water vapor, 13 mmHg; argon, 7 mmHg.What is the total pressure of this mixture?
A)760 mmHg
B)724 mmHg
C)1480 mmHg
D)386 mmHg
E)614 mmHg
A)760 mmHg
B)724 mmHg
C)1480 mmHg
D)386 mmHg
E)614 mmHg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
11
What process is responsible for the formation of dew on the grass early in the morning?
A)sublimation
B)evaporation
C)condensation
D)freezing
E)deposition
A)sublimation
B)evaporation
C)condensation
D)freezing
E)deposition

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
12
Two molecules of ethane experience what type of attractive forces? 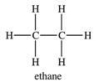
A)London forces
B)dipole-dipole interactions
C)hydrogen bonding
D)ionic bonding
E)covalent bonding

A)London forces
B)dipole-dipole interactions
C)hydrogen bonding
D)ionic bonding
E)covalent bonding

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which of the following represent STP (Standard Temperature and Pressure) conditions?
A)T = 0 K and P = 1 atm
B)T = 273 K and P = 0 atm
C)T = 1 K and P = 1 atm
D)T = 273 K and P = 1 atm
E)T = 0°C and P = 760 atm
A)T = 0 K and P = 1 atm
B)T = 273 K and P = 0 atm
C)T = 1 K and P = 1 atm
D)T = 273 K and P = 1 atm
E)T = 0°C and P = 760 atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of the following statements correctly describes Boyle's Law?
A)The volume of a gas is directly proportional to the number of moles of the gas, if the pressure and temperature remain constant.
B)The volume of a gas is directly proportional to the Kelvin temperature of the gas, if the pressure and amount of gas remain constant.
C)The pressure of a gas is inversely proportional to the volume of the gas, if the temperature and amount of gas remain constant.
D)The pressure of a gas is directly proportional to the Kelvin temperature of the gas, if the volume and amount of gas remain constant.
E)The total pressure exerted by a mixture of gases in a container is the sum of the partial pressures that each gas would exert alone.
A)The volume of a gas is directly proportional to the number of moles of the gas, if the pressure and temperature remain constant.
B)The volume of a gas is directly proportional to the Kelvin temperature of the gas, if the pressure and amount of gas remain constant.
C)The pressure of a gas is inversely proportional to the volume of the gas, if the temperature and amount of gas remain constant.
D)The pressure of a gas is directly proportional to the Kelvin temperature of the gas, if the volume and amount of gas remain constant.
E)The total pressure exerted by a mixture of gases in a container is the sum of the partial pressures that each gas would exert alone.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which gas under the specified conditions would come closest to exhibiting ideal gas behavior?
A)H2 at low pressure and high temperature
B)NH3 at low pressure and high temperature
C)H2 at high pressure and low temperature
D)NH3 at low pressure and low temperature
E)NH3 at high pressure and low temperature
A)H2 at low pressure and high temperature
B)NH3 at low pressure and high temperature
C)H2 at high pressure and low temperature
D)NH3 at low pressure and low temperature
E)NH3 at high pressure and low temperature

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
16
Sarin is a nerve gas that was previously used as a chemical weapon.Considering the physical properties of sarin shown in the table, which statement about sarin is FALSE? 












A)A sample of sarin at 0°C exists as a liquid.
B)A sample of sarin at room temperature (22°C) exists as a liquid.
C)A sample of sarin at -100°C exists as a solid.
D)A sample of sarin at 100°C exists as a gas.
E)A sample of sarin at 170°C exists as a gas.













A)A sample of sarin at 0°C exists as a liquid.
B)A sample of sarin at room temperature (22°C) exists as a liquid.
C)A sample of sarin at -100°C exists as a solid.
D)A sample of sarin at 100°C exists as a gas.
E)A sample of sarin at 170°C exists as a gas.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which statement INCORRECTLY describes what happens to a sample of gas when it experiences an increase in temperature?
A)The volume of the gas increases, as long as the pressure remains constant.
B)The pressure of the gas increases, if the volume remains constant.
C)The kinetic energy of the gas particles increases.
D)The number of gas particles increases.
E)The gas particles move with increased speed.
A)The volume of the gas increases, as long as the pressure remains constant.
B)The pressure of the gas increases, if the volume remains constant.
C)The kinetic energy of the gas particles increases.
D)The number of gas particles increases.
E)The gas particles move with increased speed.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
18
What is the volume occupied by a mole of an ideal gas, if the pressure is 626 mmHg and the temperature is 25.0°C?
A)29.7 L
B)3.28 × 10-3 L
C)2.49 L
D)3.91 × 10-2 L
E)5.66 L
A)29.7 L
B)3.28 × 10-3 L
C)2.49 L
D)3.91 × 10-2 L
E)5.66 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
19
Consider 1.00 L of air in a patient's lungs at 37.0°C and 1.00 atm pressure.What volume would this air occupy if it were at 25.0°C under a pressure of 5.00 × 102 atm (a typical pressure in a compressed air cylinder)?
A)1.35 × 10-3 L
B)480 L
C)2.08 × 10-3 L
D)1.92 × 10-3 L
E)374 L
A)1.35 × 10-3 L
B)480 L
C)2.08 × 10-3 L
D)1.92 × 10-3 L
E)374 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which of the following is NOT a unit for measuring pressure?
A)Pa
B)kJ
C)atm
D)mm Hg
E)torr
A)Pa
B)kJ
C)atm
D)mm Hg
E)torr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
21
One standard atmosphere of pressure (1 atm) is equivalent to what pressure expressed in units of mm Hg?
A)14.7 mm Hg
B)380 mm Hg
C)760 mm Hg
D)0.333 mm Hg
E)30 mm Hg
A)14.7 mm Hg
B)380 mm Hg
C)760 mm Hg
D)0.333 mm Hg
E)30 mm Hg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
22
A helium-filled weather balloon is launched from the ground where the pressure is 752 mmHg and the temperature is 21°C.Under these conditions its volume is 75.0 L.When it has climbed to an altitude where the pressure is 89 mmHg and the temperature is 0°C, what is its volume?
A)0.00 L
B)8.24 L
C)9.56 L
D)588 L
E)682 L
A)0.00 L
B)8.24 L
C)9.56 L
D)588 L
E)682 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
23
Who discovered the gas law represented in the figure shown? 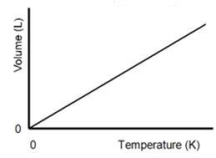
A)Boyle
B)Charles
C)Dalton
D)Avogadro
E)Torricelli

A)Boyle
B)Charles
C)Dalton
D)Avogadro
E)Torricelli

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
24
Hydrogen cyanide (HCN) is a poisonous gas that has been used to execute prisoners in the gas chamber.How many moles of HCN are present in a 67.0 L cylinder at 25.0°C that is registering a pressure of 742 mm Hg?
A)2.24 mol
B)2.67 mol
C)2.81 mol
D)31.9 mol
E)1.71 × 103 mol
A)2.24 mol
B)2.67 mol
C)2.81 mol
D)31.9 mol
E)1.71 × 103 mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
25
Who formulated the relationship between the volume and the pressure of a gas?
A)Boyle
B)Charles
C)Dalton
D)Gay-Lussac
E)Torricelli
A)Boyle
B)Charles
C)Dalton
D)Gay-Lussac
E)Torricelli

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
26
In the graph shown, which line is the best representation of Boyle's Law regarding behavior of a gas? 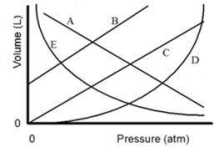
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
27
A sample of oxygen gas occupies 3.0 L at a pressure of 1.5 atm.If the volume of this sample increases without changing the temperature or the amount of gas, what can be said about the pressure?
A)The pressure of oxygen will remain at 1.5 atm.
B)The pressure of oxygen will be less than 1.5 atm.
C)The pressure of oxygen will be greater than 1.5 atm.
D)The pressure of oxygen will be 4.5 atm.
E)It is impossible to predict.
A)The pressure of oxygen will remain at 1.5 atm.
B)The pressure of oxygen will be less than 1.5 atm.
C)The pressure of oxygen will be greater than 1.5 atm.
D)The pressure of oxygen will be 4.5 atm.
E)It is impossible to predict.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
28
A sample of oxygen occupies 1.00 L.If the temperature remains constant, and the pressure on the oxygen is decreased to one third the original pressure, what is the new volume?
A)3.00 L
B)1.50 L
C)0.667 L
D)0.500 L
E)0.333 L
A)3.00 L
B)1.50 L
C)0.667 L
D)0.500 L
E)0.333 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
29
What property of liquids explains the formation of spherical water droplets on green leaves?
A)viscosity
B)surface tension
C)adhesion
D)vapor pressure
E)None of the choices are correct.
A)viscosity
B)surface tension
C)adhesion
D)vapor pressure
E)None of the choices are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which of the following statements conflicts with the kinetic molecular theory of gases?
A)There are no forces between gas particles.
B)Gas particles occupy a negligible volume compared with the volume of their container.
C)The average kinetic energy of the gas particles is proportional to the absolute temperature.
D)Gas particles lose energy only when they collide with the walls of the container.
E)Gas particles are in constant, random motion.
A)There are no forces between gas particles.
B)Gas particles occupy a negligible volume compared with the volume of their container.
C)The average kinetic energy of the gas particles is proportional to the absolute temperature.
D)Gas particles lose energy only when they collide with the walls of the container.
E)Gas particles are in constant, random motion.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
31
Carbon dioxide acts as a greenhouse gas by
A)absorbing visible radiation.
B)absorbing ultraviolet radiation.
C)absorbing infrared radiation.
D)storing solar energy.
E)trapping sunlight during photosynthesis.
A)absorbing visible radiation.
B)absorbing ultraviolet radiation.
C)absorbing infrared radiation.
D)storing solar energy.
E)trapping sunlight during photosynthesis.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
32
To which elements must hydrogen be bonded in order to display hydrogen bonding?
A)fluorine
B)oxygen
C)nitrogen
D)All of the choices are correct.
E)Both oxygen and nitrogen are correct.
A)fluorine
B)oxygen
C)nitrogen
D)All of the choices are correct.
E)Both oxygen and nitrogen are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
33
How can pure water be made to boil at a temperature below 100°C?
A)increase the volume of water
B)decrease the volume of water
C)increase the pressure above 1 atm
D)decrease the pressure below 1 atm
E)Both decrease the volume of water and increase the pressure above 1 atm are correct.
A)increase the volume of water
B)decrease the volume of water
C)increase the pressure above 1 atm
D)decrease the pressure below 1 atm
E)Both decrease the volume of water and increase the pressure above 1 atm are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
34
Aircraft cabins are pressurized for passenger comfort and safety when cruising altitudes are greater than about 12,000 feet.If the inside of an aircraft cabin is pressurized to 10.9 psi, what is the equivalent pressure in atm?
A)75 atm
B)1.3 atm
C)1.1 atm
D)0.0009 atm
E)0.74 atm
A)75 atm
B)1.3 atm
C)1.1 atm
D)0.0009 atm
E)0.74 atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
35
What quantity is directly proportional to the kinetic energy of the particles in a gas?
A)distance between molecules
B)absolute temperature
C)atomic mass
D)formula mass
E)volume of the individual particles
A)distance between molecules
B)absolute temperature
C)atomic mass
D)formula mass
E)volume of the individual particles

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
36
A balloon filled with helium has a volume of 1.00 × 103 L at 20°C.What would be the balloon's volume at 30°C, if the pressure surrounding the balloon remains constant?
A)6.7 × 102 L
B)9.70 × 102 L
C)1.03 × 103 L
D)1.11 × 103 L
E)1.50 × 103 L
A)6.7 × 102 L
B)9.70 × 102 L
C)1.03 × 103 L
D)1.11 × 103 L
E)1.50 × 103 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
37
A given mass of oxygen at room temperature occupies a volume of 500.0 mL at 1.50 atm pressure.What pressure must be applied to compress the gas to a volume of only 150.0 mL?
A)500 atm
B)150 atm
C)5.00 atm
D)1.50 atm
E)0.500 atm
A)500 atm
B)150 atm
C)5.00 atm
D)1.50 atm
E)0.500 atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
38
What is the volume occupied by one mole of helium at 0°C and 1 atm pressure?
A)1.0 L
B)22.4 L
C)4.0 L
D)40.0 L
E)12.2 L
A)1.0 L
B)22.4 L
C)4.0 L
D)40.0 L
E)12.2 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
39
Which of the following statements concerning gases is FALSE?
A)Gases are made up of tiny atoms or molecules that are in constant, random motion.
B)Gases expand to fill the shape and volume of their container because the rapidly moving particles experience negligible attractive forces.
C)Gas pressure is the result of particles of a gas colliding with each other.
D)As the temperature of a gas increases, the kinetic energy of the particles increases.
E)Gases are highly compressible because most of the volume occupied by a gas is empty space.
A)Gases are made up of tiny atoms or molecules that are in constant, random motion.
B)Gases expand to fill the shape and volume of their container because the rapidly moving particles experience negligible attractive forces.
C)Gas pressure is the result of particles of a gas colliding with each other.
D)As the temperature of a gas increases, the kinetic energy of the particles increases.
E)Gases are highly compressible because most of the volume occupied by a gas is empty space.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
40
A barometer measures
A)the pressure of a gas sample in a container.
B)the difference in pressure between two gas samples.
C)the difference in pressure between a gas sample and atmospheric pressure.
D)atmospheric pressure.
E)the pressure of an ideal gas.
A)the pressure of a gas sample in a container.
B)the difference in pressure between two gas samples.
C)the difference in pressure between a gas sample and atmospheric pressure.
D)atmospheric pressure.
E)the pressure of an ideal gas.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
41
What mass of helium is contained in a 2.0 L balloon at 30.0oC and 735 mm Hg?
A)26.5 g
B)0.477 g
C)514 g
D)66.1 g
E)0.312 g
A)26.5 g
B)0.477 g
C)514 g
D)66.1 g
E)0.312 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
42
Which substance is expected to have the highest boiling point?
A)CO2
B)HCl
C)CH3OH
D)CH4
E)BF3
A)CO2
B)HCl
C)CH3OH
D)CH4
E)BF3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
43
Which statement is true concerning an ideal gas?
A)Gases behave more ideally at low temperatures than at high temperatures.
B)Polar molecules are more ideal than nonpolar molecules.
C)Gases behave more ideally at low pressure than at high pressure.
D)Both the statements Gases behave more ideally at low temperatures than at high temperatures and Polar molecules are more ideal than nonpolar molecules are correct.
E)Both the statements Polar molecules are more ideal than nonpolar molecules and Gases behave more ideally at low pressure than at high pressure are true.
A)Gases behave more ideally at low temperatures than at high temperatures.
B)Polar molecules are more ideal than nonpolar molecules.
C)Gases behave more ideally at low pressure than at high pressure.
D)Both the statements Gases behave more ideally at low temperatures than at high temperatures and Polar molecules are more ideal than nonpolar molecules are correct.
E)Both the statements Polar molecules are more ideal than nonpolar molecules and Gases behave more ideally at low pressure than at high pressure are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
44
Which statement about a crystalline solid is always TRUE?
A)A crystalline solid has a regular repeating structure of the atoms, ions, or molecules that make up the solid.
B)A crystalline solid will always have a high melting point.
C)A crystalline solid will always have a low melting point.
D)A crystalline solid contains only ionic bonds.
E)A crystalline solid is another name for an amorphous solid.
A)A crystalline solid has a regular repeating structure of the atoms, ions, or molecules that make up the solid.
B)A crystalline solid will always have a high melting point.
C)A crystalline solid will always have a low melting point.
D)A crystalline solid contains only ionic bonds.
E)A crystalline solid is another name for an amorphous solid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
45
How are the volume and temperature of a gas related?
A)They are directly related when the pressure and amount of gas remain constant.
B)They are inversely related when the pressure and amount of gas remain constant.
C)They are equal to each other when the pressure and amount of gas remain constant.
D)There is no apparent relation between the two.
E)The amount and type of gas must be specified in order to determine their relationship.
A)They are directly related when the pressure and amount of gas remain constant.
B)They are inversely related when the pressure and amount of gas remain constant.
C)They are equal to each other when the pressure and amount of gas remain constant.
D)There is no apparent relation between the two.
E)The amount and type of gas must be specified in order to determine their relationship.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
46
Which property is typical for molecular solids?
A)consist of only metal atoms
B)contain ionic and covalent bonds
C)have low melting points
D)have no organized structure
E)will boil at very high temperature
A)consist of only metal atoms
B)contain ionic and covalent bonds
C)have low melting points
D)have no organized structure
E)will boil at very high temperature

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
47
Surface tension
A)increases with increasing temperature.
B)is unaffected by temperature.
C)is higher for nonpolar substances than for polar ones.
D)is lowered by surfactants.
E)is the same as viscosity.
A)increases with increasing temperature.
B)is unaffected by temperature.
C)is higher for nonpolar substances than for polar ones.
D)is lowered by surfactants.
E)is the same as viscosity.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
48
Glycerol is a very polar compound.Dimethyl ether is only slightly polar.Which statement is TRUE? 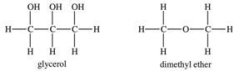
A)Glycerol is less viscous than dimethyl ether.
B)Glycerol will have a greater surface tension than dimethyl ether.
C)Dimethyl ether has a lower vapor pressure than glycerol.
D)The viscosity of both substances will increase with increasing temperature.
E)Glycerol will evaporate at a faster rate than dimethyl ether.

A)Glycerol is less viscous than dimethyl ether.
B)Glycerol will have a greater surface tension than dimethyl ether.
C)Dimethyl ether has a lower vapor pressure than glycerol.
D)The viscosity of both substances will increase with increasing temperature.
E)Glycerol will evaporate at a faster rate than dimethyl ether.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
49
What is the term that describes a liquid changing to a vapor at a temperature less than its boiling point?
A)evaporation
B)sublimation
C)dissociation
D)condensation
E)supercooling
A)evaporation
B)sublimation
C)dissociation
D)condensation
E)supercooling

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
50
A gas with pressure of 5.0 atm is compressed at constant temperature from 10.0 L to 2.0 L.What is the pressure of the gas after it is compressed?
A)4.0 L
B)1.0 × 102 L
C)1.0 L
D)0.40 L
E)25 L
A)4.0 L
B)1.0 × 102 L
C)1.0 L
D)0.40 L
E)25 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
51
Of the following gases, which will behave most like an ideal gas?
A)N2
B)HF
C)NH3
D)CH3Cl
E)CO
A)N2
B)HF
C)NH3
D)CH3Cl
E)CO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which of the following is a statement of Dalton's law of partial pressure?
A)The total pressure of a mixture of gases is the sum of the partial pressure of each gas that makes up the mixture.
B)The partial pressure of a gas is equal to the total pressure of the mixture.
C)Gases can exist as mixtures at STP only.
D)The total pressure of a mixture of gases is the product of the partial pressures of each gas in the mixture.
E)In a mixture of two gases, the pressure of each gas is ½ the total pressure.
A)The total pressure of a mixture of gases is the sum of the partial pressure of each gas that makes up the mixture.
B)The partial pressure of a gas is equal to the total pressure of the mixture.
C)Gases can exist as mixtures at STP only.
D)The total pressure of a mixture of gases is the product of the partial pressures of each gas in the mixture.
E)In a mixture of two gases, the pressure of each gas is ½ the total pressure.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
53
Which of the following is TRUE for a liquid that is placed in a sealed container?
A)Evaporation of the liquid cannot occur.
B)Once equilibrium is established between the liquid and the vapor, the rate of the evaporation process will equal the rate of condensation.
C)All molecules stay in the liquid state.
D)Condensation of the evaporated molecules in the jar can only occur once all of the liquid molecules are in the vapor (or gaseous) state.
E)A set vapor pressure will be reached, which is the same at all temperatures.
A)Evaporation of the liquid cannot occur.
B)Once equilibrium is established between the liquid and the vapor, the rate of the evaporation process will equal the rate of condensation.
C)All molecules stay in the liquid state.
D)Condensation of the evaporated molecules in the jar can only occur once all of the liquid molecules are in the vapor (or gaseous) state.
E)A set vapor pressure will be reached, which is the same at all temperatures.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
54
According to the kinetic molecular theory, which statement concerning gases is TRUE?
A)Gases are mostly empty space.
B)The average kinetic energy of the molecules increases with decreasing temperature.
C)Gas molecules are strongly attracted to each other.
D)When gas molecules collide they lose energy.
E)Gas molecules are very closely packed together.
A)Gases are mostly empty space.
B)The average kinetic energy of the molecules increases with decreasing temperature.
C)Gas molecules are strongly attracted to each other.
D)When gas molecules collide they lose energy.
E)Gas molecules are very closely packed together.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
55
A balloon containing helium at constant pressure and temperature has the volume doubled by adding a more helium.Which statement(s) is(are) TRUE?
A)The number of helium atoms doubled.
B)The number of moles of helium doubled.
C)The mass of helium in the balloon doubled.
D)All of the statements are true.
E)All of the statements are false.
A)The number of helium atoms doubled.
B)The number of moles of helium doubled.
C)The mass of helium in the balloon doubled.
D)All of the statements are true.
E)All of the statements are false.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
56
An aerosol spray paint can with a volume of 350 mL registers a pressure of 4.5 atm at room temperature.What can be said about the pressure inside the can if it is stored outside during the winter months?
A)The pressure of the gas will be greater than 4.5 atm.
B)The pressure of the gas will be less than 4.5 atm.
C)The pressure of the gas will remain at 4.5 atm.
D)The pressure of the gas will be 1575 atm.
E)It is impossible to say anything about the pressure without additional information.
A)The pressure of the gas will be greater than 4.5 atm.
B)The pressure of the gas will be less than 4.5 atm.
C)The pressure of the gas will remain at 4.5 atm.
D)The pressure of the gas will be 1575 atm.
E)It is impossible to say anything about the pressure without additional information.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
57
A balloon at 25oC has 1.00 L of air.The balloon is heated to 40oC at constant pressure.What is the new volume?
A)1.00 × 103 L
B)1.05 L
C)1.60 L
D)15.0 L
E)2.25 L
A)1.00 × 103 L
B)1.05 L
C)1.60 L
D)15.0 L
E)2.25 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
58
What is the experimental quantity that serves as a measure of resistance to flow of a liquid?
A)vapor pressure
B)surface tension
C)resistivity
D)viscosity
E)compressibility
A)vapor pressure
B)surface tension
C)resistivity
D)viscosity
E)compressibility

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
59
Liquids and solids are both highly compressible.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
60
Which one of the following substances, with their structures shown, will NOT display hydrogen bonding? 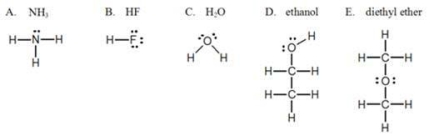
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
61
Polar compounds generally have higher boiling points than nonpolar compounds of similar molecular weight.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
62
Glycerol has a lower viscosity than ethanol.
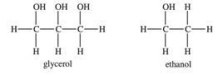


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
63
All compounds containing both oxygen and hydrogen will exhibit hydrogen bonding.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
64
The boiling point of a liquid is dependent on the atmospheric pressure.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
65
As temperature increases, viscosity decreases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
66
Metals conduct electricity well due to the mobility of the metal ions in the solid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
67
Ionic compounds tend to have higher melting points than molecular compounds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
68
The attractive forces between acetone molecules are stronger than the attractive forces between water molecules.
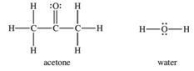


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
69
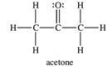
When acetone (a component of nail polish remover) evaporates, hydrogen bonds between acetone molecules must be broken up.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
70
Polar gases exhibit more ideal behavior than nonpolar ones.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
71
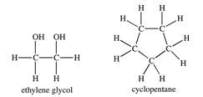
Ethylene glycol molecules have a greater attraction for cyclopentane molecules than for each other.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
72
Dalton's Law states that the volume of a gas varies directly with the absolute temperature.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
73
The density of hydrogen gas is lower than that of uranium hexafluoride (UF6) gas if both are measured at STP.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
74
Detergents and soaps decrease the surface tension of water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck
75
The temperature in which water boils inside a pressure cooker is lower than in an open pan at sea level.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 75 في هذه المجموعة.
فتح الحزمة
k this deck








