Deck 9: Acids and Bases, Ph, and Buffers
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/83
العب
ملء الشاشة (f)
Deck 9: Acids and Bases, Ph, and Buffers
1
Which solution has the highest concentration of [OH-]?
A) a solution with a pH of 4
B) a solution with a pH of 10
C) a solution with a [H3O+] of 6.8 × 10-5 M
D) a solution with a [H3O+] of 1 × 10-13 M
E) a solution with a [OH-] of 1 × 10-2 M
A) a solution with a pH of 4
B) a solution with a pH of 10
C) a solution with a [H3O+] of 6.8 × 10-5 M
D) a solution with a [H3O+] of 1 × 10-13 M
E) a solution with a [OH-] of 1 × 10-2 M
a solution with a [H3O+] of 1 × 10-13 M
2
A weak acid is also a _________ because it produces a low concentration of ions in solution.
A) weak electrolyte
B) strong electrolyte
C) nonelectrolyte
D) weak base
E) strong acid
A) weak electrolyte
B) strong electrolyte
C) nonelectrolyte
D) weak base
E) strong acid
weak electrolyte
3
What mole ratio of NaOH to H2SO4 is needed in a neutralization reaction?
A) 1:1
B) 1:2
C) 1:3
D) 2:1
E) 3:1
A) 1:1
B) 1:2
C) 1:3
D) 2:1
E) 3:1
2:1
4
Which would be the BEST treatment of metabolic acidosis?
A) the IV administration of an acid
B) the IV administration of a weak base
C) breathing into a paper bag
D) the IV administration of HCl
E) reducing breathing rate
A) the IV administration of an acid
B) the IV administration of a weak base
C) breathing into a paper bag
D) the IV administration of HCl
E) reducing breathing rate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which molecule is amphoteric?
A) CH3NH2
B) CH3OH
C) CH3COOH
D) H2SO4
E) NaOH
A) CH3NH2
B) CH3OH
C) CH3COOH
D) H2SO4
E) NaOH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which statement about neutralization reactions is FALSE?
A) Neutralization reactions occur between an acid and a base.
B) Neutralization reactions are equilibrium reactions.
C) Neutralization reactions produce water.
D) Neutralization reactions sometimes produce carbon dioxide.
E) Neutralization reactions always go to completion.
A) Neutralization reactions occur between an acid and a base.
B) Neutralization reactions are equilibrium reactions.
C) Neutralization reactions produce water.
D) Neutralization reactions sometimes produce carbon dioxide.
E) Neutralization reactions always go to completion.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following is a buffer system?
A) H2CO3 and HCO3-
B) NaCl and NaOH
C) HCl and NaOH
D) H2O and HCl
E) NaCl and NaNO3
A) H2CO3 and HCO3-
B) NaCl and NaOH
C) HCl and NaOH
D) H2O and HCl
E) NaCl and NaNO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which statement BEST describe a neutralization reaction?
A) An acid and base react to give a salt and sometimes water.
B) Two aqueous molecules react to give a liquid.
C) A positively charged ion reacts to give a neutral molecule.
D) An anion and a cation react to give a neutral molecule.
E) Two neutral molecules react to give a cation and an anion.
A) An acid and base react to give a salt and sometimes water.
B) Two aqueous molecules react to give a liquid.
C) A positively charged ion reacts to give a neutral molecule.
D) An anion and a cation react to give a neutral molecule.
E) Two neutral molecules react to give a cation and an anion.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which statement does NOT correctly describe pH?
A) pH is a measure of acidity of a solution.
B) pH is a measure of hydronium concentration in a solution.
C) The higher the pH, the more acidic a solution.
D) A pH of 7 is a neutral solution.
E) pH = -log[H3O+].
A) pH is a measure of acidity of a solution.
B) pH is a measure of hydronium concentration in a solution.
C) The higher the pH, the more acidic a solution.
D) A pH of 7 is a neutral solution.
E) pH = -log[H3O+].

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
10
Generally, strong bases are hydroxide salts of
A) transition metals.
B) the halogens.
C) alkali and alkaline earth metals.
D) the noble gases.
E) any element.
A) transition metals.
B) the halogens.
C) alkali and alkaline earth metals.
D) the noble gases.
E) any element.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which of the following types of molecules and ions is NOT a base?
A) an anion
B) a cation
C) a neutral molecule containing a nonbonding pair of electrons
D) a tertiary nitrogen
E) All of these are bases.
A) an anion
B) a cation
C) a neutral molecule containing a nonbonding pair of electrons
D) a tertiary nitrogen
E) All of these are bases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
12
A blood sample has a pH of 7.42.What is the concentration of hydronium in the sample?
A) 0.87 M
B) 2.6 × 107 M
C) -2.6 × 107 M
D) 3.8 × 108 M
E) 3.8 × 10-8 M
A) 0.87 M
B) 2.6 × 107 M
C) -2.6 × 107 M
D) 3.8 × 108 M
E) 3.8 × 10-8 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
13
The following figure illustrates the action of a HF and F- buffer where the sizes of the boxes are proportional to the concentrations of HF and F- in solution.According to this figure, what happens when H3O+ is added to the HF/F- buffer? 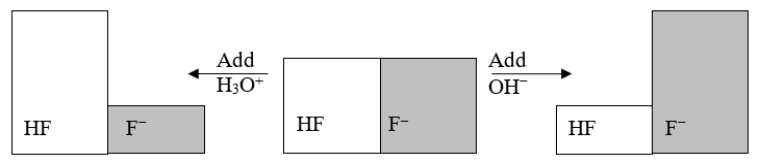
A) The pH decreases.
B) The pH increases.
C) The concentration of HF increases.
D) The concentration of F- increases.
E) Nothing happens.

A) The pH decreases.
B) The pH increases.
C) The concentration of HF increases.
D) The concentration of F- increases.
E) Nothing happens.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
14
What is the concentration of H3O+ and OH- in pure water at room temperature?
A) 1.0 × 10-14 M
B) 1.0 × 10-7 M
C) 1.0 M
D) 1.0 × 107 M
E) 1.0 × 1014 M
A) 1.0 × 10-14 M
B) 1.0 × 10-7 M
C) 1.0 M
D) 1.0 × 107 M
E) 1.0 × 1014 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which solution has the highest concentration of [OH-]?
A) a solution with a [H3O+] of 1 × 10-5 M
B) a solution with a [H3O+] of 1 × 10-13 M
C) a solution with a [OH-] of 1 × 10-5 M
D) a solution with a [OH-] of 1 × 10-13 M
E) a solution with a [OH-] of 1 × 10-2 M
A) a solution with a [H3O+] of 1 × 10-5 M
B) a solution with a [H3O+] of 1 × 10-13 M
C) a solution with a [OH-] of 1 × 10-5 M
D) a solution with a [OH-] of 1 × 10-13 M
E) a solution with a [OH-] of 1 × 10-2 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
16
The boxed species in the following reaction is 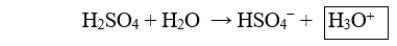
A) a hydroxide.
B) a hydronium.
C) a proton.
D) the acid.
E) the conjugate base.
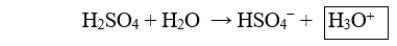
A) a hydroxide.
B) a hydronium.
C) a proton.
D) the acid.
E) the conjugate base.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
17
The following figure illustrates the action of a HF and F- buffer where the sizes of the boxes are proportional to the concentrations of HF and F- in solution.What will happen when a small amount of base (OH-)is added to the HF/F- buffer? 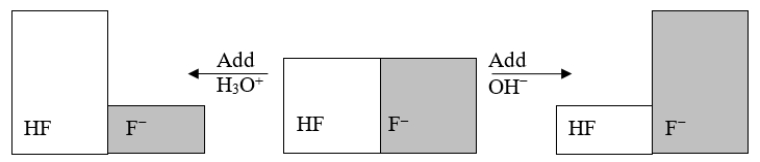
A) The concentration of OH- will increase.
B) The concentration of H3O+ will increase.
C) The concentration of HF will increase.
D) The concentration of F- will increase.
E) The pH of the solution will increase.

A) The concentration of OH- will increase.
B) The concentration of H3O+ will increase.
C) The concentration of HF will increase.
D) The concentration of F- will increase.
E) The pH of the solution will increase.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
18
A conjugate acid-base pair is
A) the reactants in an acid-base reaction.
B) the products of an acid-base reaction.
C) two species that differ only by a proton.
D) two species that differ only by a hydroxyl group.
E) a single molecule that can act as both an acid and a base.
A) the reactants in an acid-base reaction.
B) the products of an acid-base reaction.
C) two species that differ only by a proton.
D) two species that differ only by a hydroxyl group.
E) a single molecule that can act as both an acid and a base.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which of the following statements does NOT describe a pH buffer?
A) A common buffer is a solution of acetic acid and acetate.
B) A buffer is a weak acid and the acid's conjugate base in equal concentrations.
C) A buffer is a physical barrier that blocks the addition of acid or base to a solution.
D) A buffer is a solution that resists change of pH upon addition of small amounts of acid or base.
E) Phosphates and bicarbonate are buffering systems in the body.
A) A common buffer is a solution of acetic acid and acetate.
B) A buffer is a weak acid and the acid's conjugate base in equal concentrations.
C) A buffer is a physical barrier that blocks the addition of acid or base to a solution.
D) A buffer is a solution that resists change of pH upon addition of small amounts of acid or base.
E) Phosphates and bicarbonate are buffering systems in the body.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
20
Each circle is a sample of an aqueous acidic or basic solution.Which solution contains a strong acid? 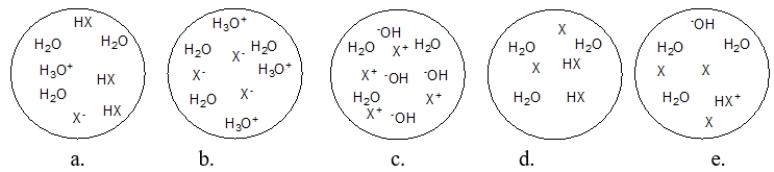
A) figure a
B) figure b
C) figure c
D) figure d
E) figure e

A) figure a
B) figure b
C) figure c
D) figure d
E) figure e

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
21
The concentration of H3O+ in a solution is 1 × 10-4 M.Which statement describes how the concentration of OH- in the solution could be determined?
A) It is not possible to determine [OH-].
B) Calculate pH.
C) Measure the pH with a pH probe or dipstick.
D) Solve for [OH-] using the ion-product constant equation.
E) The [OH-] is unchanged when an acid is added to a solution.
A) It is not possible to determine [OH-].
B) Calculate pH.
C) Measure the pH with a pH probe or dipstick.
D) Solve for [OH-] using the ion-product constant equation.
E) The [OH-] is unchanged when an acid is added to a solution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which equation BEST describes what will happen to formic acid when it reacts with water?
A) HCOOH (aq)+ 2 H2O (l)→ CO22- (aq)+ 2 H3O+ (aq)
B) HCOOH (aq)+ H2O (l)→ HCOOH2+ (aq)+ OH- (aq)
C) HCOOH (aq)+ H2O (l)→ HCOO- (aq)+ H3O+ (aq)
D) HCOOH (aq)+ H2O (l)→ H2COOH (aq)+ OH- (aq)
E) HCOOH (aq)+ H2O (l)→ HCOO- (aq)+ HO- (aq)
A) HCOOH (aq)+ 2 H2O (l)→ CO22- (aq)+ 2 H3O+ (aq)
B) HCOOH (aq)+ H2O (l)→ HCOOH2+ (aq)+ OH- (aq)
C) HCOOH (aq)+ H2O (l)→ HCOO- (aq)+ H3O+ (aq)
D) HCOOH (aq)+ H2O (l)→ H2COOH (aq)+ OH- (aq)
E) HCOOH (aq)+ H2O (l)→ HCOO- (aq)+ HO- (aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
23
Select the choice that correctly states whether the substance is an acid or a base.
A) Vinegar is basic.
B) Amines are basic.
C) Gastric juice is basic.
D) Sodium hydroxide is acidic.
E) Soap is acidic.
A) Vinegar is basic.
B) Amines are basic.
C) Gastric juice is basic.
D) Sodium hydroxide is acidic.
E) Soap is acidic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
24
What is the pH of a solution with a [OH- ] of 1.0 × 10-10 M?
A) 1.0 × 10-10
B) 10.0
C) 4.00
D) -10.0
E) -4.00
A) 1.0 × 10-10
B) 10.0
C) 4.00
D) -10.0
E) -4.00

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
25
The boxed species in the following reaction is 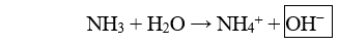
A) a hydroxide ion.
B) a hydronium ion.
C) a proton.
D) the acid.
E) the conjugate acid.
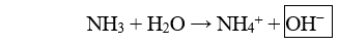
A) a hydroxide ion.
B) a hydronium ion.
C) a proton.
D) the acid.
E) the conjugate acid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
26
If the concentration of hydronium ion in water is 1 × 10-6 M, the concentration of hydroxide ion is _____ and there is more _____ in the solution.
A) 1 × 10-8 M; hydroxide
B) 1 × 10-8 M; hydronium
C) 1 × 106 M; hydroxide
D) 1 × 106 M; hydronium
A) 1 × 10-8 M; hydroxide
B) 1 × 10-8 M; hydronium
C) 1 × 106 M; hydroxide
D) 1 × 106 M; hydronium

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
27
How do strong and weak acids differ?
A) Strong acids ionize completely in aqueous solution and weak acids don't.
B) Only strong acids produce hydronium in aqueous solution.
C) Strong acids donate protons and weak acids do not.
D) Strong acids increase the concentration of OH- in solution.
E) Weak acids degrade less readily than strong acids.
A) Strong acids ionize completely in aqueous solution and weak acids don't.
B) Only strong acids produce hydronium in aqueous solution.
C) Strong acids donate protons and weak acids do not.
D) Strong acids increase the concentration of OH- in solution.
E) Weak acids degrade less readily than strong acids.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which of the following statements about acids is FALSE?
A) An example of an acid is HBr.
B) An acid increases the concentration of hydroxide in solution.
C) An acid increases the concentration of protons in solution.
D) An acid increases the concentration of hydronium in solution.
E) An acid is a proton donor.
A) An example of an acid is HBr.
B) An acid increases the concentration of hydroxide in solution.
C) An acid increases the concentration of protons in solution.
D) An acid increases the concentration of hydronium in solution.
E) An acid is a proton donor.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
29
The concentration of H3O+ in a solution is 1 × 10-4 M.What is the concentration of hydroxide ion in this solution?
A) 1 × 10-10 M
B) 1 × 10-8 M
C) 1 × 10-12 M
D) 1 × 10-7 M
E) 1 × 1010 M
A) 1 × 10-10 M
B) 1 × 10-8 M
C) 1 × 10-12 M
D) 1 × 10-7 M
E) 1 × 1010 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
30
What is the pH of a solution with a [H3O+] of 1.0 × 10-5 M?
A) 9.00
B) 1.0 × 10-5 M
C) -5.00
D) -9.00
E) 5.00
A) 9.00
B) 1.0 × 10-5 M
C) -5.00
D) -9.00
E) 5.00

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which of the following are conjugate acid-base pairs in the acid-base reaction between ethanamine and water? CH3CH2NH2 (aq)+ H2O (l)⇌ CH3CH2NH3+ (aq)+ OH- (aq)
I.H2O and CH3CH2NH2
II.H2O and OH-
III.CH3CH2NH2 and CH3CH2NH3+
IV.CH3CH2NH3+ and OH-
A) I only
B) I and IV
C) II only
D) II and IV
E) II and III
I.H2O and CH3CH2NH2
II.H2O and OH-
III.CH3CH2NH2 and CH3CH2NH3+
IV.CH3CH2NH3+ and OH-
A) I only
B) I and IV
C) II only
D) II and IV
E) II and III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
32
Which statement does NOT correctly describe buffer capacity?
A) Buffer capacity is determined only by the identity of the buffer.
B) The addition of amounts of acid or base exceeding the buffer capacity will change the pH of the solution.
C) The higher the concentration of weak acid and conjugate base in solution, the higher the buffer capacity.
D) The greatest amount of acid or base that a buffer can accept while maintaining pH is called the buffer capacity.
E) Once the buffer capacity is exceeded in cells, key functions of the body can be disrupted.
A) Buffer capacity is determined only by the identity of the buffer.
B) The addition of amounts of acid or base exceeding the buffer capacity will change the pH of the solution.
C) The higher the concentration of weak acid and conjugate base in solution, the higher the buffer capacity.
D) The greatest amount of acid or base that a buffer can accept while maintaining pH is called the buffer capacity.
E) Once the buffer capacity is exceeded in cells, key functions of the body can be disrupted.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which condition could cause respiratory acidosis?
A) hyperventilation
B) asthma
C) kidney failure
D) excessive vomiting
E) starvation
A) hyperventilation
B) asthma
C) kidney failure
D) excessive vomiting
E) starvation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
34
What is the pH of a urine sample with a hydronium concentration of 7.9 × 10-8?
A) -7.10
B) 7.10
C) 6.90
D) -6.90
E) 7.00
A) -7.10
B) 7.10
C) 6.90
D) -6.90
E) 7.00

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
35
Which change in blood pH would you expect to observe in a hyperventilating patient, and what are those changes called?
A) a drop in pH, alkalosis
B) a drop in pH, acidosis
C) an increase in pH, alkalosis
D) an increase in pH, acidosis
E) There will not be any change in blood pH.
A) a drop in pH, alkalosis
B) a drop in pH, acidosis
C) an increase in pH, alkalosis
D) an increase in pH, acidosis
E) There will not be any change in blood pH.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which of the following reactions illustrate the reaction of a base? I.NH3 + H2O → NH4+ + OH-
II)HCl + H2O → H3O+ + Cl-
III)NaOH → Na+ + OH-
IV)NH4+ + H2O → H3O+ + NH3
A) I only
B) II only
C) I and III
D) I, III, and IV
E) All of the above are reactions of a base.
II)HCl + H2O → H3O+ + Cl-
III)NaOH → Na+ + OH-
IV)NH4+ + H2O → H3O+ + NH3
A) I only
B) II only
C) I and III
D) I, III, and IV
E) All of the above are reactions of a base.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
37
What is the [H3O+] in a solution that has a [OH-] of 3.2 × 10-4 M?
A) 3.2 × 10-10 M
B) 3.2 × 1010 M
C) 3.1 × 10-11 M
D) 3.1 × 1011 M
E) 1.0 × 10-7 M
A) 3.2 × 10-10 M
B) 3.2 × 1010 M
C) 3.1 × 10-11 M
D) 3.1 × 1011 M
E) 1.0 × 10-7 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which of the following statements BEST describes what happens when an acid reacts with water? I.The acid donates a proton.
II.Water donates a proton.
III.Water acts as a base.
IV.Hydronium is formed.
V.Hydroxide is formed.
A) I only
B) II only
C) I, III, and V
D) I, III, and IV
E) II and V
II.Water donates a proton.
III.Water acts as a base.
IV.Hydronium is formed.
V.Hydroxide is formed.
A) I only
B) II only
C) I, III, and V
D) I, III, and IV
E) II and V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
39
The reaction between acetic acid and water is given below, followed by a list of changes that could be made to the reaction solution.Which change will result in the equilibrium shifting to the left? CH3COOH + H2O ⇌ CH3COO- + H3O+
Changes that could be made to the solution
I.Adding more CH3COOH
II.Removing H2O
III.Removing H3O+
IV.Adding more CH3COO-
A) All of these changes will result in the equilibrium shifting to the left.
B) Only I will result in the equilibrium shifting to the left.
C) Only IV will result in the equilibrium shifting to the left.
D) I and III will result in the equilibrium shifting to the left.
E) II and IV will result in the equilibrium shifting to the left.
Changes that could be made to the solution
I.Adding more CH3COOH
II.Removing H2O
III.Removing H3O+
IV.Adding more CH3COO-
A) All of these changes will result in the equilibrium shifting to the left.
B) Only I will result in the equilibrium shifting to the left.
C) Only IV will result in the equilibrium shifting to the left.
D) I and III will result in the equilibrium shifting to the left.
E) II and IV will result in the equilibrium shifting to the left.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
40
If the concentration of hydronium ion in water is 1 × 10-12 M, the concentration of hydroxide ion is _____ and there is more _____ in the solution.
A) 1 × 10-2 M; hydroxide
B) 1 × 10-2 M; hydronium
C) 1 × 1012 M; hydroxide
D) 1 × 1012 M; hydronium
A) 1 × 10-2 M; hydroxide
B) 1 × 10-2 M; hydronium
C) 1 × 1012 M; hydroxide
D) 1 × 1012 M; hydronium

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
41
Which species in the following neutralization reaction are spectator ions? NaOH + HCl → H2O + NaCl
A) H3O+
B) OH-
C) H2O
D) NaCl
E) H+
A) H3O+
B) OH-
C) H2O
D) NaCl
E) H+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
42
What is the pH of a solution with a [OH-] of 4.1 × 10-3 M?
A) -2.39
B) 2.39
C) 11.6
D) -11.6
E) 7.00
A) -2.39
B) 2.39
C) 11.6
D) -11.6
E) 7.00

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
43
The following figure illustrates the action of a HF and F- buffer where the sizes of the boxes are proportional to the concentrations of HF and F- in solution.Which of the following chemical equations represents the reaction that occurs when OH- is added to the HF/F- buffer? 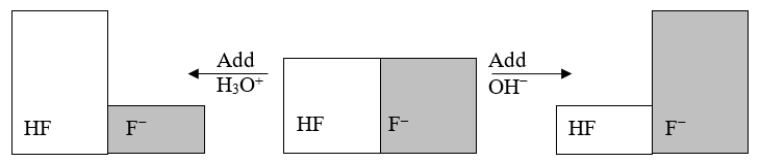
A) HF + H2O ⇌ F- + H3O+
B) F- + H2O ⇌ F- + OH-
C) HF + OH- ⇌ F- + H2O
D) F- + 2 OH- ⇌ HF + O2
E) F- + OH- ⇌ HOF

A) HF + H2O ⇌ F- + H3O+
B) F- + H2O ⇌ F- + OH-
C) HF + OH- ⇌ F- + H2O
D) F- + 2 OH- ⇌ HF + O2
E) F- + OH- ⇌ HOF

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
44
Which of the following atomic diagrams best represents H+? 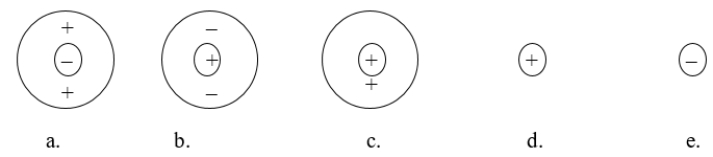
A) figure a
B) figure b
C) figure c
D) figure d
E) figure e

A) figure a
B) figure b
C) figure c
D) figure d
E) figure e

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
45
Water can react as both an acid and a base, depending on its environment.Because of this characteristic, water is a(n)_________ molecule.
A) amphoteric
B) autonomous
C) complex
D) reactive
E) conjugated
A) amphoteric
B) autonomous
C) complex
D) reactive
E) conjugated

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
46
Consider a buffer solution containing CH3COO-Na+ and CH3COOH.If you add hydronium (H3O+)until all of the CH3COO- is converted into CH3COOH and then add a little more hydronium, what do you expect to observe?
A) The pH increases.
B) The pH decreases.
C) The pH stays the same.
D) The solution will be neutralized.
E) The pH changes, but it is not possible to determine how it will change.
A) The pH increases.
B) The pH decreases.
C) The pH stays the same.
D) The solution will be neutralized.
E) The pH changes, but it is not possible to determine how it will change.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
47
What happens to pH when the buffer capacity of a solution is exceeded?
A) The pH increases.
B) The pH decreases.
C) The solution will become unstable.
D) The pH will either increase or decrease, depending on whether acid or base is added to the solution.
E) The pH will not be changed.
A) The pH increases.
B) The pH decreases.
C) The solution will become unstable.
D) The pH will either increase or decrease, depending on whether acid or base is added to the solution.
E) The pH will not be changed.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
48
The neutralization reaction of potassium hydrogen carbonate (KHCO3)and HI produces what spectator ion(s)?
A) CO32-
B) K+ and I-
C) HCO3-
D) H3O+ and OH-
E) H+
A) CO32-
B) K+ and I-
C) HCO3-
D) H3O+ and OH-
E) H+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
49
Which of the following compounds is a Brønsted-Lowry base but not an Arrhenius base?
A) HCl
B) NaOH
C) NH3
D) Ca(OH)2
E) CH3COOH
A) HCl
B) NaOH
C) NH3
D) Ca(OH)2
E) CH3COOH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
50
The neutralization reaction of potassium hydrogen carbonate (KHCO3)and HI produces what gas?
A) CO2
B) O2
C) H2CO3
D) H2O
E) H+
A) CO2
B) O2
C) H2CO3
D) H2O
E) H+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
51
Which of the following statements about bases is FALSE?
A) A base decreases the concentration of hydronium in solution.
B) An example of a base is Ca(OH)2.
C) A base increases the concentration of hydroxide in solution.
D) A base is a proton acceptor.
E) A base is always negatively charged.
A) A base decreases the concentration of hydronium in solution.
B) An example of a base is Ca(OH)2.
C) A base increases the concentration of hydroxide in solution.
D) A base is a proton acceptor.
E) A base is always negatively charged.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
52
The boxed species in the following reaction is 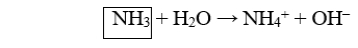
A) a hydroxide.
B) a hydronium.
C) a proton.
D) the acid.
E) the base.
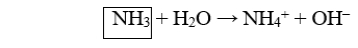
A) a hydroxide.
B) a hydronium.
C) a proton.
D) the acid.
E) the base.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
53
Each circle is a sample of an aqueous acidic or basic solution.Which solution contains a weak base? 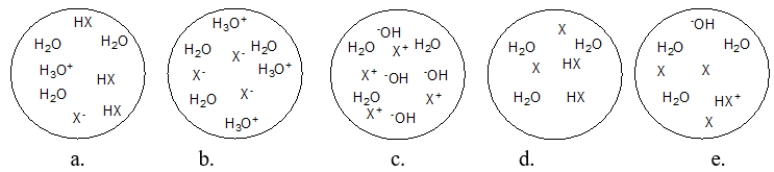
A) figure a
B) figure b
C) figure c
D) figure d
E) figure e

A) figure a
B) figure b
C) figure c
D) figure d
E) figure e

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
54
The boxed species in the following reaction is 
A) a hydroxide.
B) a hydronium.
C) a proton.
D) the acid.
E) the base.

A) a hydroxide.
B) a hydronium.
C) a proton.
D) the acid.
E) the base.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
55
The following figure illustrates the action of a HF and F- buffer where the sizes of the boxes are proportional to the concentrations of HF and F- in solution.If you add hydronium until all of the F- is converted into HF and then add a little more hydronium, what is observed? 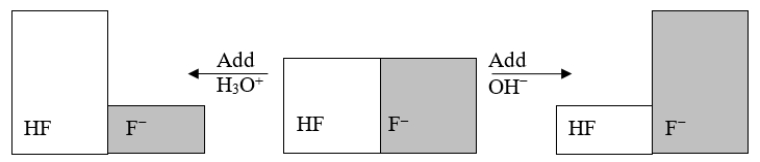
A) The pH increases.
B) The pH decreases.
C) The pH stays the same.
D) The solution will be neutralized.
E) The pH changes, but it is not possible to determine how it will change.

A) The pH increases.
B) The pH decreases.
C) The pH stays the same.
D) The solution will be neutralized.
E) The pH changes, but it is not possible to determine how it will change.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
56
What is the role of the kidney in maintaining acid-base homeostasis in the blood?
A) removing excess CO2
B) adding extra H+ as needed
C) adding/removing water
D) adding/removing hydrogen carbonate ion
E) All of the above are roles of the kidney.
A) removing excess CO2
B) adding extra H+ as needed
C) adding/removing water
D) adding/removing hydrogen carbonate ion
E) All of the above are roles of the kidney.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
57
Which reaction BEST illustrates the reaction of an acid in aqueous solution?
A) HCl + H2O → Cl- + H3O+
B) NH3 + H2O → +NH4 + H3O+
C) HCl + H2O → HCl + H3O+
D) NH3 + H2O → +NH4 + HO-
E) HCl + H2O → Cl- + HO-
A) HCl + H2O → Cl- + H3O+
B) NH3 + H2O → +NH4 + H3O+
C) HCl + H2O → HCl + H3O+
D) NH3 + H2O → +NH4 + HO-
E) HCl + H2O → Cl- + HO-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
58
What do all bases have in common?
A) They contain an OH- group.
B) They contain a nitrogen.
C) They contain an oxygen.
D) They are salts.
E) They contain a nonbonding pair of electrons.
A) They contain an OH- group.
B) They contain a nitrogen.
C) They contain an oxygen.
D) They are salts.
E) They contain a nonbonding pair of electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
59
Each circle is a sample of an aqueous acidic or basic solution.Which solution contains a weak acid? 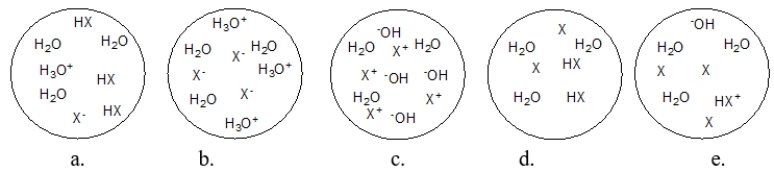
A) figure a
B) figure b
C) figure c
D) figure d
E) figure e

A) figure a
B) figure b
C) figure c
D) figure d
E) figure e

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
60
Which statement BEST describes the following reaction? HCOOH + H2O ⇌ HCOO- + H3O+
A) This is the reaction of a strong acid.
B) This is the reaction of a weak acid.
C) This reaction is the dissociation of a strong base.
D) This is the reaction of a weak base.
E) This is not an acid-base reaction.
A) This is the reaction of a strong acid.
B) This is the reaction of a weak acid.
C) This reaction is the dissociation of a strong base.
D) This is the reaction of a weak base.
E) This is not an acid-base reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
61
Which of the following is a balanced equation for a neutralization reaction?
A) Al(OH)3 (s)+ HCl (aq)→ 3 H2O (l)+ AlCl3 (aq)
B) Mg(OH)2 (s)+ HNO3 (aq)→ 2 H2O (l)+ Mg(NO3)2 (aq)
C) Ca(OH)2 (s)+ HNO3 (aq)→ 2 H2O (l)+ Ca(NO3)2 (aq)
D) KOH (s)+ HNO3 (aq)→ H2O (l)+ KNO3 (aq)
E) 2 Al(OH)3 (s)+ HCl (aq)→ 3 H2O (l)+ AlCl3 (aq)
A) Al(OH)3 (s)+ HCl (aq)→ 3 H2O (l)+ AlCl3 (aq)
B) Mg(OH)2 (s)+ HNO3 (aq)→ 2 H2O (l)+ Mg(NO3)2 (aq)
C) Ca(OH)2 (s)+ HNO3 (aq)→ 2 H2O (l)+ Ca(NO3)2 (aq)
D) KOH (s)+ HNO3 (aq)→ H2O (l)+ KNO3 (aq)
E) 2 Al(OH)3 (s)+ HCl (aq)→ 3 H2O (l)+ AlCl3 (aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
62
The boxed species in the following reaction is 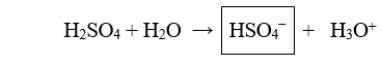
A) a hydroxide.
B) a hydronium.
C) a proton.
D) the acid.
E) the conjugate base.
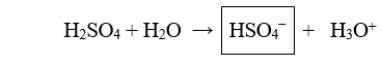
A) a hydroxide.
B) a hydronium.
C) a proton.
D) the acid.
E) the conjugate base.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
63
Which molecule is a base? 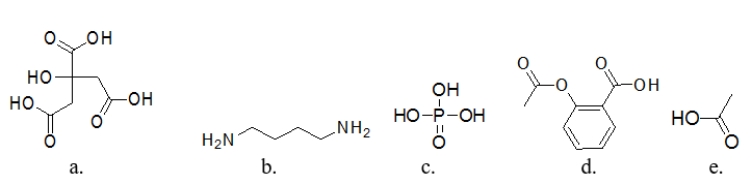
A) molecule a
B) molecule b
C) molecule c
D) molecule d
E) molecule e

A) molecule a
B) molecule b
C) molecule c
D) molecule d
E) molecule e

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
64
Which base is a strong base?
A) LiOH
B) CH3CH2NH2
C) NH2CH2COOH
D) NH2CH2CH2CH2NH2
E) All of the above molecules are strong bases.
A) LiOH
B) CH3CH2NH2
C) NH2CH2COOH
D) NH2CH2CH2CH2NH2
E) All of the above molecules are strong bases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
65
Which strong acid is found in the body?
A) HNO3
B) HCl
C) H2SO4
D) HClO4
E) HBr
A) HNO3
B) HCl
C) H2SO4
D) HClO4
E) HBr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
66
Which acid is NOT a strong acid?
A) HCl
B) HI
C) HNO3
D) CH3COOH
E) H2SO4
A) HCl
B) HI
C) HNO3
D) CH3COOH
E) H2SO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
67
Which buffer system is the primary buffering system in the blood?
A) proteins
B) DNA and RNA
C) H2PO4-/HPO42-
D) H2CO3/HCO3-
E) CH3COOH/CH3COO-
A) proteins
B) DNA and RNA
C) H2PO4-/HPO42-
D) H2CO3/HCO3-
E) CH3COOH/CH3COO-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
68
The following reaction is a reversible reaction.Which statement BEST describes what it means for this reaction to be reversible? HCOOH + H2O ⇌ HCOO- + H3O+
A) This reaction only occurs in the reverse direction as written above.
B) All of the reactant molecules react to make product and then all of the product molecules react to make reactants again.
C) Forward and reverse reactions proceed at the same rate.
D) Forward and reverse reactions occur simultaneously.
E) The rate of the reverse reaction is must faster than the rate of the forward reaction.
A) This reaction only occurs in the reverse direction as written above.
B) All of the reactant molecules react to make product and then all of the product molecules react to make reactants again.
C) Forward and reverse reactions proceed at the same rate.
D) Forward and reverse reactions occur simultaneously.
E) The rate of the reverse reaction is must faster than the rate of the forward reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
69
A sample of gastric juice has a pH of 1.20.What is the concentration of hydroxide in this sample?
A) 1.6 × 10-13 M
B) 6.3 × 10-2 M
C) 1.0 × 10-7 M
D) 6.3 × 1012 M
E) 6.3 × 10-16 M
A) 1.6 × 10-13 M
B) 6.3 × 10-2 M
C) 1.0 × 10-7 M
D) 6.3 × 1012 M
E) 6.3 × 10-16 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
70
What is the pH of a solution with a [H3O+] of 7.9 × 10-11 M?
A) 10.1
B) -10.1
C) 3.90
D) -3.90
E) 11.9
A) 10.1
B) -10.1
C) 3.90
D) -3.90
E) 11.9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
71
Which statement BEST describes the role of water in the reaction of ethanamine and water? CH3CH2NH2 (aq)+ H2O (l)⇌ CH3CH2NH3+ (aq)+ OH- (aq)
A) Water is the acid.
B) Water is the base.
C) Water is the conjugate acid.
D) Water is the conjugate base.
E) Water is both an acid and a base.
A) Water is the acid.
B) Water is the base.
C) Water is the conjugate acid.
D) Water is the conjugate base.
E) Water is both an acid and a base.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
72
The [H3O+] of a solution with a pH of 2 is ______ the [H3O+] of a solution with a pH of 3.
A) one-tenth
B) ten times
C) one-half
D) twice
E) the same as
A) one-tenth
B) ten times
C) one-half
D) twice
E) the same as

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
73
When an acid is dissolved in water, it reacts to give _________ and ________.
A) hydroxide; hydronium
B) hydronium; the conjugate acid
C) hydroxide; the conjugate acid
D) hydronium; the conjugate base
E) hydroxide; the conjugate base
A) hydroxide; hydronium
B) hydronium; the conjugate acid
C) hydroxide; the conjugate acid
D) hydronium; the conjugate base
E) hydroxide; the conjugate base

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
74
Which statement BEST describes what it means for the acid-base reaction between acetic acid and water to be in equilibrium? CH3COOH + H2O ⇌ CH3COO- + H3O+
A) The mass of acetic acid and acetate is equal.
B) The number of moles of acetate equals the number of moles of acetic acid.
C) The number of moles of hydronium equals the number of moles of water.
D) The forward and reverse reactions proceed at the same rate.
E) The reaction is balanced.
A) The mass of acetic acid and acetate is equal.
B) The number of moles of acetate equals the number of moles of acetic acid.
C) The number of moles of hydronium equals the number of moles of water.
D) The forward and reverse reactions proceed at the same rate.
E) The reaction is balanced.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
75
Which of the following equations is the correctly balanced equation for the neutralization reaction of aluminum hydroxide and HCl?
A) Al(OH)3 (s)+ HCl (aq)→ 3 H2O (l)+ AlCl3 (aq)
B) 3 Al(OH)3 (s)+ HCl (aq)→ 3 H2O (l)+ AlCl3 (aq)
C) Al(OH)3 (s)+ 3 HCl (aq)→ 3 H2O (l)+ AlCl3 (aq)
D) Al(OH)3 (s)+ HCl (aq)→ H2O (l)+ AlCl3 (aq)
E) 3 Al(OH)3 (s)+ HCl (aq)→ 3 H2O (l)+ 3 AlCl3 (aq)
A) Al(OH)3 (s)+ HCl (aq)→ 3 H2O (l)+ AlCl3 (aq)
B) 3 Al(OH)3 (s)+ HCl (aq)→ 3 H2O (l)+ AlCl3 (aq)
C) Al(OH)3 (s)+ 3 HCl (aq)→ 3 H2O (l)+ AlCl3 (aq)
D) Al(OH)3 (s)+ HCl (aq)→ H2O (l)+ AlCl3 (aq)
E) 3 Al(OH)3 (s)+ HCl (aq)→ 3 H2O (l)+ 3 AlCl3 (aq)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
76
What is the [OH-] in a solution that has a [H3O+] of 1 × 10-6 M?
A) 1 × 10-20 M
B) 1 × 10-8 M
C) 1 × 10-6 M
D) 1 × 10-7 M
E) 1 × 107 M
A) 1 × 10-20 M
B) 1 × 10-8 M
C) 1 × 10-6 M
D) 1 × 10-7 M
E) 1 × 107 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
77
Which pH range is referred to as physiological pH?
A) 6-8
B) 7.35-7.45
C) 6.7-6.8
D) 6.5-7.5
E) 5-8
A) 6-8
B) 7.35-7.45
C) 6.7-6.8
D) 6.5-7.5
E) 5-8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
78
Which statement BEST describes the following reaction? 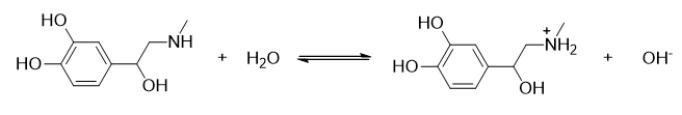
A) This is the reaction of a strong acid.
B) This is the reaction of a weak acid.
C) This reaction is the dissociation of a strong base.
D) This is the reaction of a weak base.
E) This is not an acid-base reaction.

A) This is the reaction of a strong acid.
B) This is the reaction of a weak acid.
C) This reaction is the dissociation of a strong base.
D) This is the reaction of a weak base.
E) This is not an acid-base reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
79
All acid-base reactions that we consider in this chapter occur in
A) acidic solution.
B) basic solution.
C) neutral solution.
D) aqueous solution.
E) nonpolar solvents.
A) acidic solution.
B) basic solution.
C) neutral solution.
D) aqueous solution.
E) nonpolar solvents.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
80
Each circle is a sample of an aqueous acidic or basic solution.Which solution contains a strong base? 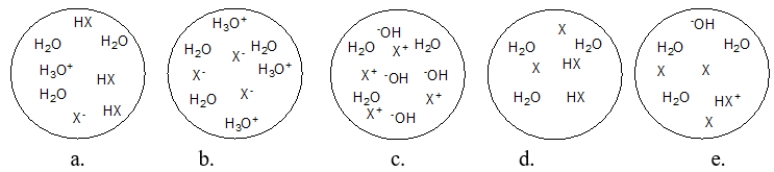
A) figure a
B) figure b
C) figure c
D) figure d
E) figure e

A) figure a
B) figure b
C) figure c
D) figure d
E) figure e

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck








