Deck 10: Carboxylic Acids and Their Derivatives
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/36
العب
ملء الشاشة (f)
Deck 10: Carboxylic Acids and Their Derivatives
1
The IUPAC name for succinic acid is butanedioic acid.What is the structure of succinic acid?
A) CH3CH2CH2CO2H
B) CH3CH=CHCO2H
C) HO2CCH2CH2CO2H
D) CH3CH2CH2CO3H
E) HOCH2CH2CH2CO2H
A) CH3CH2CH2CO2H
B) CH3CH=CHCO2H
C) HO2CCH2CH2CO2H
D) CH3CH2CH2CO3H
E) HOCH2CH2CH2CO2H
HO2CCH2CH2CO2H
2
The boiling point of propanoic acid is higher than that of 1-butanol because:
A) propanoic acid has a higher molecular weight than 1-butanol.
B) propanoic acid is more soluble in water than 1-butanol.
C) propanoic acid is a better hydrogen bond donor than 1-butanol.
D) propanoic acid forms hydrogen bonded dimers and 1-butanol does not.
E) 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
A) propanoic acid has a higher molecular weight than 1-butanol.
B) propanoic acid is more soluble in water than 1-butanol.
C) propanoic acid is a better hydrogen bond donor than 1-butanol.
D) propanoic acid forms hydrogen bonded dimers and 1-butanol does not.
E) 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
propanoic acid forms hydrogen bonded dimers and 1-butanol does not.
3
The formula for butanoic anhydride is
A)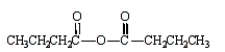
B)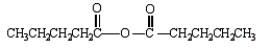
C)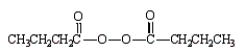
D)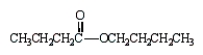
E)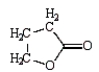
A)

B)

C)
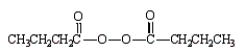
D)

E)


4
What is a correct name for the following structure? 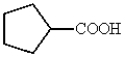
A) pentanoic acid
B) cyclopentanecarboxylic acid
C) cyclopentanoic acid
D) cyclopentylacetic acid
E) formyl cyclopentane

A) pentanoic acid
B) cyclopentanecarboxylic acid
C) cyclopentanoic acid
D) cyclopentylacetic acid
E) formyl cyclopentane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which structure best describes the double bond character of the amide bond in acetamide? 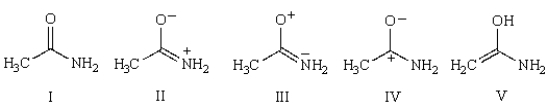
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which of the following carboxylic acids is terephthalic acid? 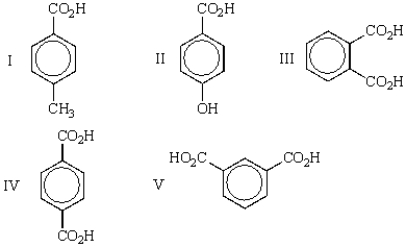
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following molecules would have the highest boiling point?
A) CH3CO2H
B) CH3CH2OH
C) CH3CHO
D) CH3CH=CH2
E) HCO2H
A) CH3CO2H
B) CH3CH2OH
C) CH3CHO
D) CH3CH=CH2
E) HCO2H

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
8
The IUPAC name for 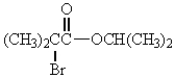 is
is
A) 2-bromoisopropyl isopropanoate.
B) isopropyl-2-bromoisobutanoate.
C) isopropyl-2-bromo-2-methylpropanoate.
D) 2-bromoisobutanoyl-2-propanoate.
E) isopropyl-2-bromo-3-methylbutanoate.
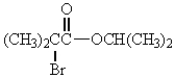 is
isA) 2-bromoisopropyl isopropanoate.
B) isopropyl-2-bromoisobutanoate.
C) isopropyl-2-bromo-2-methylpropanoate.
D) 2-bromoisobutanoyl-2-propanoate.
E) isopropyl-2-bromo-3-methylbutanoate.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
9
What is the IUPAC name of the following molecule? 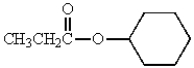
A) cyclohexyl propanoate
B) cyclohexyl acetate
C) ethyl cyclohexanoate
D) propyl cyclohexanoate
E) propanoyl cyclohexyl ether

A) cyclohexyl propanoate
B) cyclohexyl acetate
C) ethyl cyclohexanoate
D) propyl cyclohexanoate
E) propanoyl cyclohexyl ether

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which of the following statements regarding 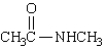 is false?
is false?
A) Rotation around the C-N bond is restricted.
B) The carbonyl group, NH group, and both methyl carbons lie in a plane.
C) The nitrogen is strongly basic.
D) The C-N bond is shorter than the C-N bond in amines.
E) The compound is named Nmethylacetamide.
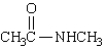 is false?
is false?A) Rotation around the C-N bond is restricted.
B) The carbonyl group, NH group, and both methyl carbons lie in a plane.
C) The nitrogen is strongly basic.
D) The C-N bond is shorter than the C-N bond in amines.
E) The compound is named Nmethylacetamide.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which of the following represents p-bromobenzoic acid?
A)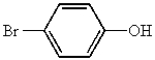
B)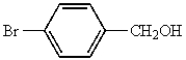
C)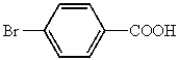
D)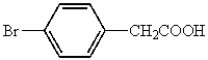
E)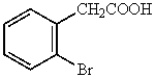
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which of the following molecules is not an anhydride?
A)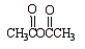
B)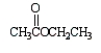
C)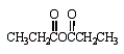
D)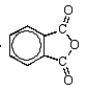
E)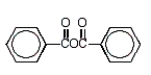
A)
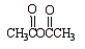
B)
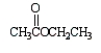
C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which of the following compounds undergoes hydrolysis at the fastest rate upon reaction with sodium hydroxide in water? 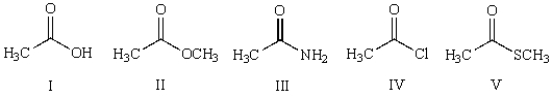
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
14
Rank the following according to their relative acidities 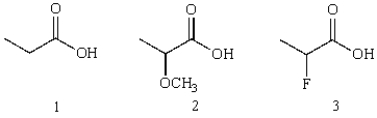
A) 3>2>1
B) 1>2>3
C) 2>3>1
D) 3>1>2
E) 2>1>3

A) 3>2>1
B) 1>2>3
C) 2>3>1
D) 3>1>2
E) 2>1>3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
15
The IUPAC name for 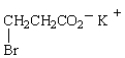 is
is
A) potassium 3-bromopropanoate.
B) potassium 2-bromopropanoate.
C) potassium 3-bromopropionate.
D) potassium -bromopropionate.
E) potassium -bromopropanoate.
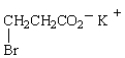 is
isA) potassium 3-bromopropanoate.
B) potassium 2-bromopropanoate.
C) potassium 3-bromopropionate.
D) potassium -bromopropionate.
E) potassium -bromopropanoate.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which of the following molecules is the weakest acid?
A) CH3CH2CO2H
B) CH3CH2CH3
C) CH3CH2OH
D) CH3CH2NH2
E) H2O
A) CH3CH2CO2H
B) CH3CH2CH3
C) CH3CH2OH
D) CH3CH2NH2
E) H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which of the following molecules has the most acidic -hydrogen?
A)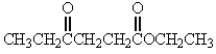
B)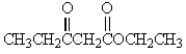
C)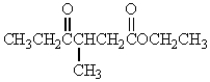
D)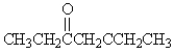
E)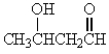
A)
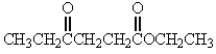
B)
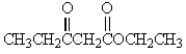
C)

D)
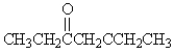
E)
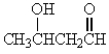

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
18
What is the IUPAC name of CH3CO2CH(CH3)2?
A) ethyl acetate
B) propyl acetate
C) isopropyl ethanoate
D) ethyl propanoate
E) dimethyl acetate
A) ethyl acetate
B) propyl acetate
C) isopropyl ethanoate
D) ethyl propanoate
E) dimethyl acetate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
19
What is the name of the following molecule, (CH3)2CHCONH2?
A) 2-methylpropanamide
B) 3-methylpropanamide
C) butyramide
D) ( -methylbutyramide)
E) methylethanamide
A) 2-methylpropanamide
B) 3-methylpropanamide
C) butyramide
D) ( -methylbutyramide)
E) methylethanamide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
20
What is the common name for CH3COOH?
A) formic acid
B) acetic acid
C) propionic acid
D) oxalic acid
E) malonic acid
A) formic acid
B) acetic acid
C) propionic acid
D) oxalic acid
E) malonic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
21
The following structure is an intermediate in the reaction of 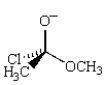
A)CH3CO2CH3 with HCl.
B)CH3COCl with CH3O- Na+.
C)CH3CO2CH3 with HOCl.
D)CH3CH(OCH3)2 with Cl2.
E)CH3Cl with (CH3)2C=O.

A)CH3CO2CH3 with HCl.
B)CH3COCl with CH3O- Na+.
C)CH3CO2CH3 with HOCl.
D)CH3CH(OCH3)2 with Cl2.
E)CH3Cl with (CH3)2C=O.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
22
Ethyl propanoate can be prepared by the reaction of
A) ethanoic acid with propanol.
B) ethanoic acid with isopropanol.
C) ethanoyl chloride with propanol.
D) propanoyl chloride with ethanol.
E) acetic anhydride with propanol.
A) ethanoic acid with propanol.
B) ethanoic acid with isopropanol.
C) ethanoyl chloride with propanol.
D) propanoyl chloride with ethanol.
E) acetic anhydride with propanol.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which structure represents the tetrahedral intermediate in the following reaction? 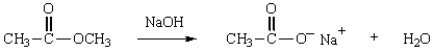
A)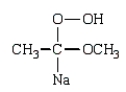
B)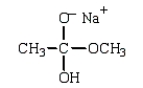
C)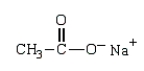
D)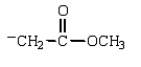
E)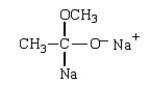
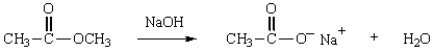
A)

B)

C)
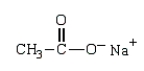
D)
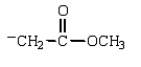
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
24
Oxidation of 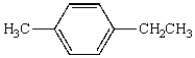 with KMnO4
with KMnO4
A)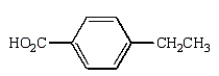
B)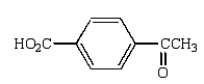
C)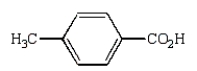
D)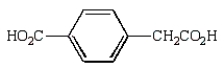
E)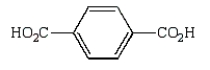
 with KMnO4
with KMnO4A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
25
The mechanism of saponification is
A) nucleophilic aromatic substitution.
B) nucleophilic acyl substitution.
C) electrophilic aromatic substitution.
D) SN2.
E) SN1.
A) nucleophilic aromatic substitution.
B) nucleophilic acyl substitution.
C) electrophilic aromatic substitution.
D) SN2.
E) SN1.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
26
Treatment of 1 mole of CH3CH2CO2CH2CH3 with 2 moles of Na+ -OCH2CH3 followed by the addition of an aqueous solution of acid gives
A)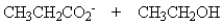
B)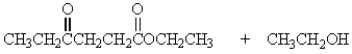
C)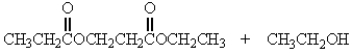
D)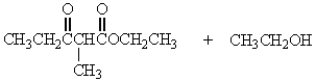
E) no reaction
A)
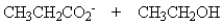
B)
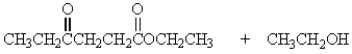
C)
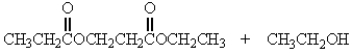
D)
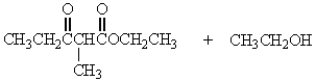
E) no reaction

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
27
What is the product of the following reaction sequence? 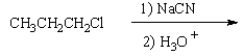
A)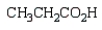
B)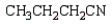
C)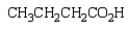
D)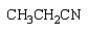
E)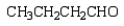
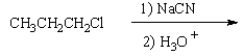
A)
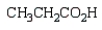
B)
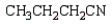
C)
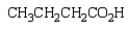
D)
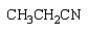
E)
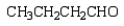

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
28
The product(s) from 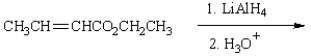 are:
are:
A)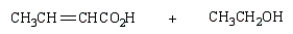
B)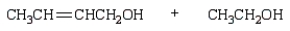
C)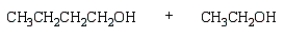
D)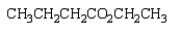
E)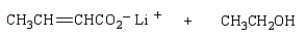
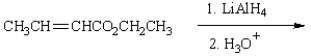 are:
are:A)
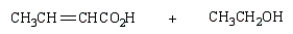
B)
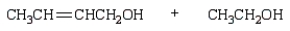
C)
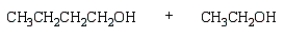
D)
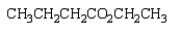
E)
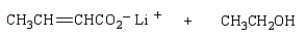

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
29
The expected product of the reaction 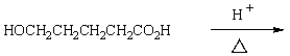 is:
is:
A)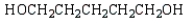
B)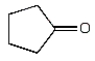
C)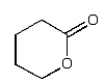
D)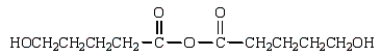
E)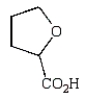
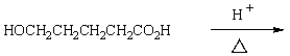 is:
is:A)
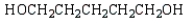
B)

C)

D)
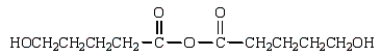
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
30
Saponification of 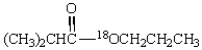 is expected to give:
is expected to give:
A)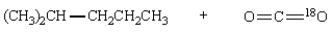
B)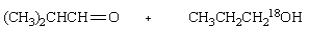
C)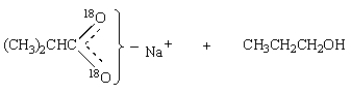
D)
E)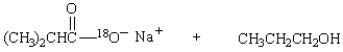
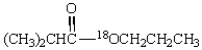 is expected to give:
is expected to give:A)
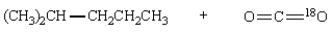
B)
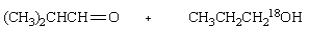
C)
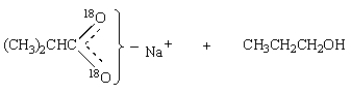
D)

E)
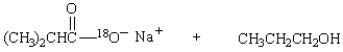

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
31
Benzoic acid cannot be prepared by which one of the following methods?
A)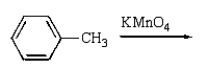
B)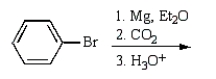
C)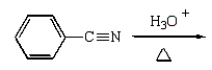
D)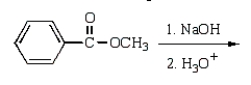
E)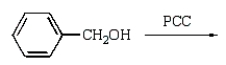
A)
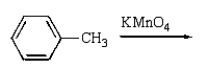
B)
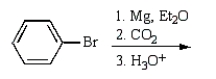
C)
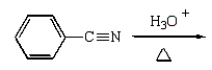
D)
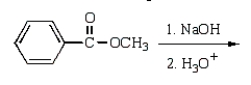
E)
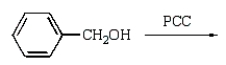

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
32
The product(s) in the reaction 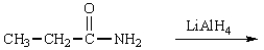 are:
are:
A)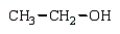
B)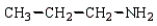
C)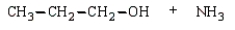
D)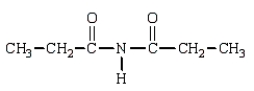
E)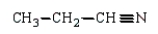
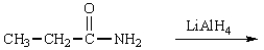 are:
are:A)
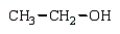
B)
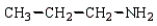
C)
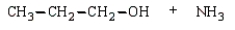
D)

E)
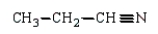

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following carbonyl compounds reacts fastest with water?
A) carboxylic acid
B) ester
C) ketone
D) acid chloride
E) amide
A) carboxylic acid
B) ester
C) ketone
D) acid chloride
E) amide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
34
(CH3)3CCO2H can best be prepared by:
A)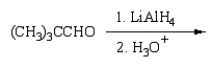
B)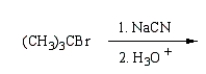
C)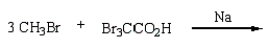
D)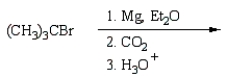
E)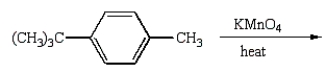
A)
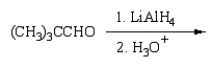
B)
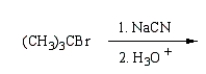
C)
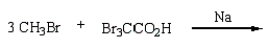
D)
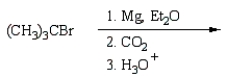
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
35
The product of the following reaction sequence is: 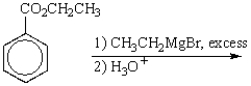
A)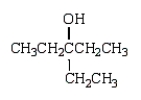
B)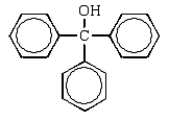
C)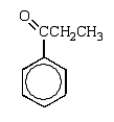
D)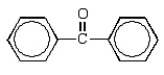
E)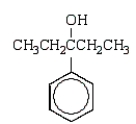
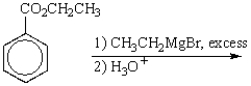
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck
36
Acid chlorides react with alcohols to give:
A) ketones
B) esters
C) acetals
D) amides
E) carboxylic acids
A) ketones
B) esters
C) acetals
D) amides
E) carboxylic acids

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 36 في هذه المجموعة.
فتح الحزمة
k this deck








