Deck 21: Nuclear Chemistry: Applications to Energy and Medicine
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/144
العب
ملء الشاشة (f)
Deck 21: Nuclear Chemistry: Applications to Energy and Medicine
1
All elements with Z > 83 are ________
A) synthetic.
B) produced by nuclear fission.
C) radioactive.
D) not found in nature.
E) unreactive.
A) synthetic.
B) produced by nuclear fission.
C) radioactive.
D) not found in nature.
E) unreactive.
radioactive.
2
Which element has stable isotopes with the largest ratio of neutrons to protons?
A) H
B) C
C) I
D) Sr
E) Ca
A) H
B) C
C) I
D) Sr
E) Ca
I
3
Which one of the following statements is not correct?
A) Carbon-10 is unstable because it has too few neutrons.
B) All nuclides with Z > 83 decay into nuclides with smaller Z values.
C) Generally, the number of neutrons in a nuclide is equal to or less than the atomic number.
D) As the atomic number increases, the ratio of neutrons to protons in a nuclide increases.
E) It is very unusual to find a nuclide with an odd number of protons and an odd number of neutrons.
A) Carbon-10 is unstable because it has too few neutrons.
B) All nuclides with Z > 83 decay into nuclides with smaller Z values.
C) Generally, the number of neutrons in a nuclide is equal to or less than the atomic number.
D) As the atomic number increases, the ratio of neutrons to protons in a nuclide increases.
E) It is very unusual to find a nuclide with an odd number of protons and an odd number of neutrons.
Generally, the number of neutrons in a nuclide is equal to or less than the atomic number.
4
Which process converts a proton into a neutron?
A) beta emission
B) positron emission
C) alpha emission
D) gamma emission
E) neutron emission
A) beta emission
B) positron emission
C) alpha emission
D) gamma emission
E) neutron emission

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
5
The peak in nuclear binding energy/nucleon occurs for an isotope of ________
A) helium.
B) iron.
C) uranium.
D) carbon.
E) lead.
A) helium.
B) iron.
C) uranium.
D) carbon.
E) lead.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
6
The mass of a particular isotope is given by ________
A) the mass number.
B) adding the masses of protons, neutrons, and electrons.
C) measuring it.
D) dividing the mass number by 6.02 * 1023.
E) dividing the molar mass of the element by 6.02 * 1023.
A) the mass number.
B) adding the masses of protons, neutrons, and electrons.
C) measuring it.
D) dividing the mass number by 6.02 * 1023.
E) dividing the molar mass of the element by 6.02 * 1023.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
7
The most naturally abundant nuclides are those that ________
A) have an odd number of protons and an odd number of neutrons.
B) have an even number of protons and an even number of neutrons.
C) have noble gas configurations of electrons.
D) can be produced by fission reactions.
E) can be produced by fusion reactions.
A) have an odd number of protons and an odd number of neutrons.
B) have an even number of protons and an even number of neutrons.
C) have noble gas configurations of electrons.
D) can be produced by fission reactions.
E) can be produced by fusion reactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
8
The isotope "belt of stability" is an area in a plot of ________
A) nuclear binding energy versus atomic number.
B) isotopic strength versus isotopic weakness.
C) nuclear mass versus mass number.
D) neutron number versus atomic number.
E) mass number versus atomic number.
A) nuclear binding energy versus atomic number.
B) isotopic strength versus isotopic weakness.
C) nuclear mass versus mass number.
D) neutron number versus atomic number.
E) mass number versus atomic number.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
9
The first step in the disintegration of uranium is . What particle is emitted in this reaction?
A) ( particle)
B) neutron
C) proton
D) electron
E) (X- ray)
A) ( particle)
B) neutron
C) proton
D) electron
E) (X- ray)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
10
What repulsive forces must be overcome for any element other than hydrogen to exist?
A) The repulsion between neutrons and other neutrons.
B) The repulsion between protons and other protons.
C) The repulsion between protons and neutrons.
D) The repulsion between positrons and electrons.
E) The repulsion between neutrons and electrons.
A) The repulsion between neutrons and other neutrons.
B) The repulsion between protons and other protons.
C) The repulsion between protons and neutrons.
D) The repulsion between positrons and electrons.
E) The repulsion between neutrons and electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which of the following nuclides are most likely to be unstable because they have too many neutrons?
I. carbon-14
II. sodium-24
III. silicon-26
IV. aluminum-27
V. phosphorus-31
A) I only
B) I and II
C) II and III
D) III, IV, and V
E) all of these
I. carbon-14
II. sodium-24
III. silicon-26
IV. aluminum-27
V. phosphorus-31
A) I only
B) I and II
C) II and III
D) III, IV, and V
E) all of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
12
Light elements with Z < 20 generally have a neutron/proton ratio about equal to ________
A) 0.5.
B) 0.8.
C) 1.0.
D) 1.3.
E) 1.5.
A) 0.5.
B) 0.8.
C) 1.0.
D) 1.3.
E) 1.5.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which graph below illustrates decay? 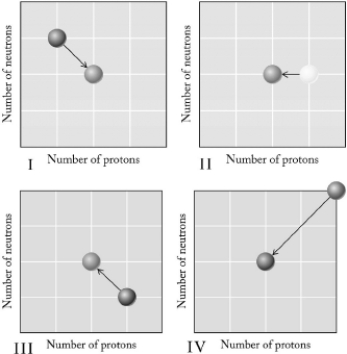
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
14
The heaviest elements will generally have a neutron/proton ratio about equal to ________
A) 0.5.
B) 0.8.
C) 1.0.
D) 1.3.
E) 1.5.
A) 0.5.
B) 0.8.
C) 1.0.
D) 1.3.
E) 1.5.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which one of the following statements is not correct?
A) Oxygen-15 is unstable because it has too few neutrons.
B) Nucleons are held together in a nuclide by the electromagnetic force.
C) All nuclides with Z > 83 decay into more stable nuclides with smaller Z values.
D) As the atomic number increases, the ratio of neutrons to protons in a nuclide increases.
E) Generally, the number of neutrons in a nuclide equals the number of protons, or nearly so, when the atomic number is small, i.e., Z < 18.
A) Oxygen-15 is unstable because it has too few neutrons.
B) Nucleons are held together in a nuclide by the electromagnetic force.
C) All nuclides with Z > 83 decay into more stable nuclides with smaller Z values.
D) As the atomic number increases, the ratio of neutrons to protons in a nuclide increases.
E) Generally, the number of neutrons in a nuclide equals the number of protons, or nearly so, when the atomic number is small, i.e., Z < 18.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which process converts a neutron into a proton?
A) beta emission
B) positron emission
C) electron capture
D) alpha emission
E) gamma emission
A) beta emission
B) positron emission
C) electron capture
D) alpha emission
E) gamma emission

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which process converts a proton into a neutron?
A) beta emission
B) electron capture
C) alpha emission
D) gamma emission
E) neutron emission
A) beta emission
B) electron capture
C) alpha emission
D) gamma emission
E) neutron emission

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
18
Calculate the nuclear binding energy per nucleon for the beryllium-8 isotope. Here are some helpful data:
A) 1.2035 * 10-6 J/nucleon
B) 1.08479 * 10-9 J/nucleon
C) 1.1317 * 10-12 J/nucleon
D) 8.7266* 10-12 J/nucleon
E) 1.08479 * 10-15 J/nucleon
A) 1.2035 * 10-6 J/nucleon
B) 1.08479 * 10-9 J/nucleon
C) 1.1317 * 10-12 J/nucleon
D) 8.7266* 10-12 J/nucleon
E) 1.08479 * 10-15 J/nucleon

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
19
The mass of an atomic nucleus is ________ the sum of the masses of its constituent nucleons (protons and neutrons).
A) always greater than
B) always less than
C) always the same as
D) sometimes greater than and sometimes smaller than
E) never smaller than
A) always greater than
B) always less than
C) always the same as
D) sometimes greater than and sometimes smaller than
E) never smaller than

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
20
The heaviest stable nucleus is an isotope of ________
A) platinum.
B) gold.
C) lead.
D) bismuth.
E) xenon.
A) platinum.
B) gold.
C) lead.
D) bismuth.
E) xenon.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
21
When a positron and an electron collide, they ________
A) form a neutron.
B) strongly repel one another and recoil.
C) annihilate each other and produce gamma rays.
D) form a proton.
E) form a neutron and emit gamma rays.
A) form a neutron.
B) strongly repel one another and recoil.
C) annihilate each other and produce gamma rays.
D) form a proton.
E) form a neutron and emit gamma rays.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
22
When a nucleus undergoes radioactive decay, the new atomic number ________
I. can be greater than the original atomic number.
II. can be the same as the original atomic number.
III. can be less than the original atomic number.
IV. must be the same as the original atomic number.
A) I only
B) II only
C) III only
D) IV only
E) I, II, and III only
I. can be greater than the original atomic number.
II. can be the same as the original atomic number.
III. can be less than the original atomic number.
IV. must be the same as the original atomic number.
A) I only
B) II only
C) III only
D) IV only
E) I, II, and III only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
23
When a nucleus undergoes radioactive decay, the new mass number is ________
A) always less than the original mass number.
B) always the same as the original mass number.
C) always more than the original mass number.
D) never less than the original mass number.
E) never more than the original mass number.
A) always less than the original mass number.
B) always the same as the original mass number.
C) always more than the original mass number.
D) never less than the original mass number.
E) never more than the original mass number.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
24
Which graph below illustrates neutron capture followed by decay? 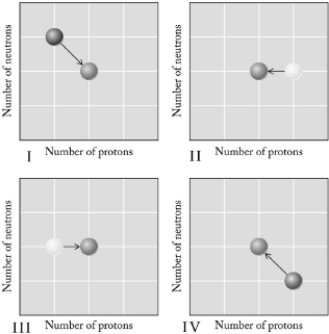
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
25
Cobalt-60 decays to nickel-60. What particle is emitted?
A) proton
B) neutron
C) electron
D) positron
E) alpha
A) proton
B) neutron
C) electron
D) positron
E) alpha

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which graph below illustrates electron capture? 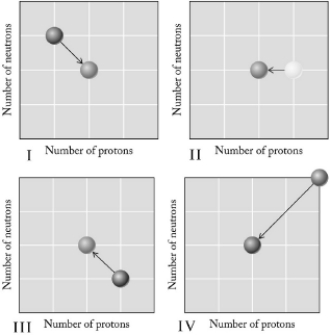
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
27
Plutonium-238 is an emitter and a compact heat source. Coupled with a PbTe thermoelectric device, it was once used as a very reliable electrical energy source for cardiac pacemakers. What is the product of the radioactive decay of plutonium-238?
A) thorium-230
B) uranium-234
C) curium-242
D) californium-246
E) plutonium-234
A) thorium-230
B) uranium-234
C) curium-242
D) californium-246
E) plutonium-234

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
28
K-capture is associated with ________
A) conversion of a neutron to a proton.
B) conversion of a proton to a neutron.
C) increase in mass number.
D) decrease in mass number.
E) emission of rays.
A) conversion of a neutron to a proton.
B) conversion of a proton to a neutron.
C) increase in mass number.
D) decrease in mass number.
E) emission of rays.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
29
Radon-220 decays to polonium-216. What particle is emitted?
A) beta
B) positron
C) neutron
D) alpha
E) gamma
A) beta
B) positron
C) neutron
D) alpha
E) gamma

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
30
Strontium-90 is most likely to decay by ________
A) gamma emission.
B) beta emission.
C) positron emission.
D) alpha emission.
E) electron capture.
A) gamma emission.
B) beta emission.
C) positron emission.
D) alpha emission.
E) electron capture.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
31
Cobalt-59 is a stable isotope. Cobalt-60 is used as a radioactive source approved by the U.S. Food and Drug Administration for irradiation of food. This process kills microbes and insects and can delay ripening. What decay pathway is likely for cobalt-60?
A) ( emission)
B) ( emission)
C) positron emission
D) ( emission)
E) X-ray emission
A) ( emission)
B) ( emission)
C) positron emission
D) ( emission)
E) X-ray emission

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
32
Which graph below illustrates decay? 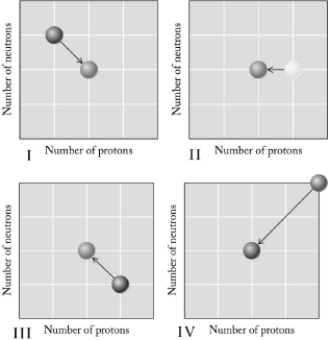
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
33
Nitrogen-13 decays by positron emission to produce ________
A) carbon-13.
B) oxygen-17.
C) boron-11.
D) carbon-14.
E) boron-13.
A) carbon-13.
B) oxygen-17.
C) boron-11.
D) carbon-14.
E) boron-13.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which graph below illustrates positron emission? 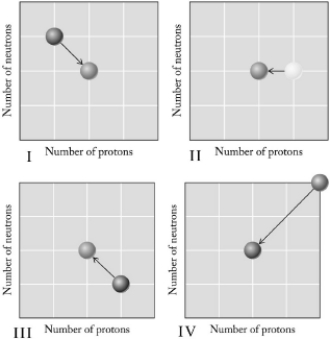
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
35
Tritium (3H) is used in glowing "EXIT" signs conveniently located where there is no electricity for light bulbs. What decay route is likely for tritium?
A)( emission)
B)( emission)
C)(positron emission)
D)( emission)
E) (X-ray emission)
A)( emission)
B)( emission)
C)(positron emission)
D)( emission)
E) (X-ray emission)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
36
Cobalt-56 decays by emitting a positron. What is the product?
A) cobalt-55
B) cobalt-56
C) nickel-56
D) iron-56
E) iron-55
A) cobalt-55
B) cobalt-56
C) nickel-56
D) iron-56
E) iron-55

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
37
Strontium-88 is the most abundant stable isotope. Strontium-90 is a particularly hazardous radioactive isotope because, as an alkali earth metal, it will substitute for calcium in bones and teeth. Predict its decay pathway.
A) ( emission)
B) ( emission)
C) positron emission
D) ( emission)
E) (X-ray emission)
A) ( emission)
B) ( emission)
C) positron emission
D) ( emission)
E) (X-ray emission)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
38
Positron emission is associated with ________
A) conversion of a neutron to a proton.
B) conversion of a proton to a neutron.
C) increase in mass number.
D) decrease in mass number.
E) emission of rays.
A) conversion of a neutron to a proton.
B) conversion of a proton to a neutron.
C) increase in mass number.
D) decrease in mass number.
E) emission of rays.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
39
Rhenium-185 is a stable isotope. Rhenium-186 is a radioisotope used in the treatment of cancer. What type of emission is likely for this isotope?
A) alpha
B) beta
C) positron
D) gamma ray
E) X-ray
A) alpha
B) beta
C) positron
D) gamma ray
E) X-ray

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
40
Beta emission is associated with ________
A) conversion of a neutron to a proton.
B) conversion of a proton to a neutron.
C) increase in mass number.
D) decrease in mass number.
E) emission of rays.
A) conversion of a neutron to a proton.
B) conversion of a proton to a neutron.
C) increase in mass number.
D) decrease in mass number.
E) emission of rays.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
41
What fraction of a sample's radioactive atoms remain after four half-lives?
A) 1/2
B) 1/4
C) 1/8
D) 1/10
E) 1/16
A) 1/2
B) 1/4
C) 1/8
D) 1/10
E) 1/16

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
42
A 10.00 g sample of wood from an archaeological site produced 3,072 particles in a 10-hour measurement owing to the presence of carbon-14, while a 10.00 g sample of new wood produced 9,216 particles in the same period of time. The half-life of carbon-14 is 5,730 years. How old is the wood from the archaeological site?
A) 5,730 years
B) 2,865 years
C) 4,040 years
D) 9,080 years
E) The correct answer differs by more than 100 years from the values given in A-D.
A) 5,730 years
B) 2,865 years
C) 4,040 years
D) 9,080 years
E) The correct answer differs by more than 100 years from the values given in A-D.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
43
Which graph below describes the decay of two radioactive nuclides, P and Q, when the half-life of Q is longer than the half-life of P? In these graphs ln[N(0)/N(t)] is plotted on the y-axis, with time (t) on the x-axis. N(0) = number of radioactive nuclides present at time = 0, and
N(t) = number present at time = t.
A)![<strong>Which graph below describes the decay of two radioactive nuclides, P and Q, when the half-life of Q is longer than the half-life of P? In these graphs ln[N(0)/N(t)] is plotted on the y-axis, with time (t) on the x-axis. N(0) = number of radioactive nuclides present at time = 0, and N(t) = number present at time = t.</strong> A) B) C) D)](https://d2lvgg3v3hfg70.cloudfront.net/TB3834/11eb0df4_170e_d038_9431_e516922d6f6a_TB3834_00.jpg)
B)![<strong>Which graph below describes the decay of two radioactive nuclides, P and Q, when the half-life of Q is longer than the half-life of P? In these graphs ln[N(0)/N(t)] is plotted on the y-axis, with time (t) on the x-axis. N(0) = number of radioactive nuclides present at time = 0, and N(t) = number present at time = t.</strong> A) B) C) D)](https://d2lvgg3v3hfg70.cloudfront.net/TB3834/11eb0df4_170e_f749_9431_93e277b29259_TB3834_00.jpg)
C)![<strong>Which graph below describes the decay of two radioactive nuclides, P and Q, when the half-life of Q is longer than the half-life of P? In these graphs ln[N(0)/N(t)] is plotted on the y-axis, with time (t) on the x-axis. N(0) = number of radioactive nuclides present at time = 0, and N(t) = number present at time = t.</strong> A) B) C) D)](https://d2lvgg3v3hfg70.cloudfront.net/TB3834/11eb0df4_170e_f74a_9431_15b0bc59bb0a_TB3834_00.jpg)
D)![<strong>Which graph below describes the decay of two radioactive nuclides, P and Q, when the half-life of Q is longer than the half-life of P? In these graphs ln[N(0)/N(t)] is plotted on the y-axis, with time (t) on the x-axis. N(0) = number of radioactive nuclides present at time = 0, and N(t) = number present at time = t.</strong> A) B) C) D)](https://d2lvgg3v3hfg70.cloudfront.net/TB3834/11eb0df4_170e_f74b_9431_9902c030b412_TB3834_00.jpg)
N(t) = number present at time = t.
A)
![<strong>Which graph below describes the decay of two radioactive nuclides, P and Q, when the half-life of Q is longer than the half-life of P? In these graphs ln[N(0)/N(t)] is plotted on the y-axis, with time (t) on the x-axis. N(0) = number of radioactive nuclides present at time = 0, and N(t) = number present at time = t.</strong> A) B) C) D)](https://d2lvgg3v3hfg70.cloudfront.net/TB3834/11eb0df4_170e_d038_9431_e516922d6f6a_TB3834_00.jpg)
B)
![<strong>Which graph below describes the decay of two radioactive nuclides, P and Q, when the half-life of Q is longer than the half-life of P? In these graphs ln[N(0)/N(t)] is plotted on the y-axis, with time (t) on the x-axis. N(0) = number of radioactive nuclides present at time = 0, and N(t) = number present at time = t.</strong> A) B) C) D)](https://d2lvgg3v3hfg70.cloudfront.net/TB3834/11eb0df4_170e_f749_9431_93e277b29259_TB3834_00.jpg)
C)
![<strong>Which graph below describes the decay of two radioactive nuclides, P and Q, when the half-life of Q is longer than the half-life of P? In these graphs ln[N(0)/N(t)] is plotted on the y-axis, with time (t) on the x-axis. N(0) = number of radioactive nuclides present at time = 0, and N(t) = number present at time = t.</strong> A) B) C) D)](https://d2lvgg3v3hfg70.cloudfront.net/TB3834/11eb0df4_170e_f74a_9431_15b0bc59bb0a_TB3834_00.jpg)
D)
![<strong>Which graph below describes the decay of two radioactive nuclides, P and Q, when the half-life of Q is longer than the half-life of P? In these graphs ln[N(0)/N(t)] is plotted on the y-axis, with time (t) on the x-axis. N(0) = number of radioactive nuclides present at time = 0, and N(t) = number present at time = t.</strong> A) B) C) D)](https://d2lvgg3v3hfg70.cloudfront.net/TB3834/11eb0df4_170e_f74b_9431_9902c030b412_TB3834_00.jpg)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
44
Which graph below describes the decay of two radioactive nuclides, P and Q, when the half-life of Q is shorter than the half-life of P? In these graphs ln[N(0)/N(t)] is plotted on the y-axis, with time (t) on the x-axis. N(0) = number of radioactive nuclides present at time = 0, and N(t) = number present at time = t.
A)![<strong>Which graph below describes the decay of two radioactive nuclides, P and Q, when the half-life of Q is shorter than the half-life of P? In these graphs ln[N(0)/N(t)] is plotted on the y-axis, with time (t) on the x-axis. N(0) = number of radioactive nuclides present at time = 0, and N(t) = number present at time = t.</strong> A) B) C) D)](https://d2lvgg3v3hfg70.cloudfront.net/TB3834/11eb0df4_170e_f74c_9431_b3e94cb80aa7_TB3834_00.jpg)
B)![<strong>Which graph below describes the decay of two radioactive nuclides, P and Q, when the half-life of Q is shorter than the half-life of P? In these graphs ln[N(0)/N(t)] is plotted on the y-axis, with time (t) on the x-axis. N(0) = number of radioactive nuclides present at time = 0, and N(t) = number present at time = t.</strong> A) B) C) D)](https://d2lvgg3v3hfg70.cloudfront.net/TB3834/11eb0df4_170e_f74d_9431_b7d0b9c351a7_TB3834_00.jpg)
C)![<strong>Which graph below describes the decay of two radioactive nuclides, P and Q, when the half-life of Q is shorter than the half-life of P? In these graphs ln[N(0)/N(t)] is plotted on the y-axis, with time (t) on the x-axis. N(0) = number of radioactive nuclides present at time = 0, and N(t) = number present at time = t.</strong> A) B) C) D)](https://d2lvgg3v3hfg70.cloudfront.net/TB3834/11eb0df4_170e_f74e_9431_97b273183208_TB3834_00.jpg)
D)![<strong>Which graph below describes the decay of two radioactive nuclides, P and Q, when the half-life of Q is shorter than the half-life of P? In these graphs ln[N(0)/N(t)] is plotted on the y-axis, with time (t) on the x-axis. N(0) = number of radioactive nuclides present at time = 0, and N(t) = number present at time = t.</strong> A) B) C) D)](https://d2lvgg3v3hfg70.cloudfront.net/TB3834/11eb0df4_170f_1e5f_9431_336f88e2ab64_TB3834_00.jpg)
A)
![<strong>Which graph below describes the decay of two radioactive nuclides, P and Q, when the half-life of Q is shorter than the half-life of P? In these graphs ln[N(0)/N(t)] is plotted on the y-axis, with time (t) on the x-axis. N(0) = number of radioactive nuclides present at time = 0, and N(t) = number present at time = t.</strong> A) B) C) D)](https://d2lvgg3v3hfg70.cloudfront.net/TB3834/11eb0df4_170e_f74c_9431_b3e94cb80aa7_TB3834_00.jpg)
B)
![<strong>Which graph below describes the decay of two radioactive nuclides, P and Q, when the half-life of Q is shorter than the half-life of P? In these graphs ln[N(0)/N(t)] is plotted on the y-axis, with time (t) on the x-axis. N(0) = number of radioactive nuclides present at time = 0, and N(t) = number present at time = t.</strong> A) B) C) D)](https://d2lvgg3v3hfg70.cloudfront.net/TB3834/11eb0df4_170e_f74d_9431_b7d0b9c351a7_TB3834_00.jpg)
C)
![<strong>Which graph below describes the decay of two radioactive nuclides, P and Q, when the half-life of Q is shorter than the half-life of P? In these graphs ln[N(0)/N(t)] is plotted on the y-axis, with time (t) on the x-axis. N(0) = number of radioactive nuclides present at time = 0, and N(t) = number present at time = t.</strong> A) B) C) D)](https://d2lvgg3v3hfg70.cloudfront.net/TB3834/11eb0df4_170e_f74e_9431_97b273183208_TB3834_00.jpg)
D)
![<strong>Which graph below describes the decay of two radioactive nuclides, P and Q, when the half-life of Q is shorter than the half-life of P? In these graphs ln[N(0)/N(t)] is plotted on the y-axis, with time (t) on the x-axis. N(0) = number of radioactive nuclides present at time = 0, and N(t) = number present at time = t.</strong> A) B) C) D)](https://d2lvgg3v3hfg70.cloudfront.net/TB3834/11eb0df4_170f_1e5f_9431_336f88e2ab64_TB3834_00.jpg)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
45
Radiation seed therapy is a common method for treating some prostate cancers and brain tumors. Palladium-103 is one isotope used for this treatment. Palladium-103 decays with a half-life of 17 days by emitting X-rays with a photon energy of 23,000 eV. What mass of palladium-103 would have to be embedded in a 25 g tumor to produce a radiation dose of 125 Gy in 2 months' time (60 days)? Note: 1 Gy = 1 J/kg, and 1 eV = 1.60 * 10-19 J.
A) 0.37 g
B) 0.31 g
C) 0.10 g
D) 0.23 g
E) 0.16 g
A) 0.37 g
B) 0.31 g
C) 0.10 g
D) 0.23 g
E) 0.16 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
46
Radiation seed therapy is a common method for treating some prostate cancers and brain tumors. Palladium-103, with a half-life of 17 days, is one isotope used for this treatment. How long does it take for the activity of palladium-103 to decrease to 1.0% of its initial value and essentially cease to be effective?
A) 3.7 mo
B) 13 mo
C) 2.1 mo
D) 7.4 mo
E) 4.2 mo
A) 3.7 mo
B) 13 mo
C) 2.1 mo
D) 7.4 mo
E) 4.2 mo

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
47
A nerdy Scotch-drinking physicist, Nicola, suspected that the 50-year-old Scotch she purchased was misrepresented. Reasoning that water must contain the radioactive hydrogen isotope, tritium, because this isotope is produced in the atmosphere by cosmic rays, she decided she could use the tritium concentration in the Scotch to determine when it was bottled. She found that the tritium concentration in the Scotch was only 64% that of the tritium concentration in the water on the Isle of Islay where the Scotch was bottled. How old, really, was this 50-year-old Scotch? The half-life of tritium is 12.3 years.
A) 6 years
B) 8 years
C) 12 years
D) 24 years
E) It really was 50 years old.
A) 6 years
B) 8 years
C) 12 years
D) 24 years
E) It really was 50 years old.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
48
Radiation seed therapy is a common method for treating some prostate cancers and brain tumors. Iodine-125 is one isotope used for this treatment. Iodine-125 decays with a half-life of 60 days by emitting X-rays with a photon energy of 27,000 eV. What mass of iodine-125 would have to be embedded in a 25 g tumor to produce a radiation dose of 125 Gy in 3 months' time (90 days)? Note: 1 Gy = 1 J/kg, and 1 eV = 1.60 * 10-19 J.
A) 0.37 g
B) 0.31 g
C) 0.10 g
D) 0.23 g
E) 0.16 g
A) 0.37 g
B) 0.31 g
C) 0.10 g
D) 0.23 g
E) 0.16 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
49
Uranium-238 ( ) decays to form thorium-234 (
) with a half-life of 4.5 *109 years. How many years will it take for 75% of the uranium-238 to decay?
A) 9.0 * 1010 years
B) 4.5 * 109 years
C) 4.5 * 1010 years
D) 9.0 *109 years
E) 3.8* 109 years
) with a half-life of 4.5 *109 years. How many years will it take for 75% of the uranium-238 to decay?
A) 9.0 * 1010 years
B) 4.5 * 109 years
C) 4.5 * 1010 years
D) 9.0 *109 years
E) 3.8* 109 years

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
50
The activity of a sample of gas obtained from a basement containing radon-222 was found to be 8 pCi/L. This isotope has a half-life of 3.8 days. If no additional radon-222 entered the basement, how long would it take for the activity to decline to 1 pCi/L?
A) about 4 days
B) a bit more than 10 days
C) about 1 day
D) a bit less than 10 days
E) about 20 days
A) about 4 days
B) a bit more than 10 days
C) about 1 day
D) a bit less than 10 days
E) about 20 days

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
51
Radiation seed therapy is a common method for treating some prostate cancers and brain tumors. Cesium-131 is a new isotope being proposed for this treatment. Cesium-131 decays with a half-life of 9.7 days by emitting X-rays with a photon energy of 29,000 eV. What mass of cesium-131 would have to be embedded in a 15 g tumor to produce a radiation dose of 115 Gy in 3 weeks' time (21 days)? Note: 1 Gy = 1 J/kg, and 1 eV = 1.60 *10-19 J.
A) 0.37 g
B) 0.31 g
C) 0.10 g
D) 0.23 g
E) 0.16 g
A) 0.37 g
B) 0.31 g
C) 0.10 g
D) 0.23 g
E) 0.16 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
52
Radiation seed therapy is a common method for treating some prostate cancers and brain tumors. Iodine-125, with a half-life of 60 days, is one isotope used for this treatment. How long does it take for the activity of iodine-125 to decrease to 1.0% of its initial value and essentially cease to be effective?
A) 3.7 mo
B) 13 mo
C) 2.1 mo
D) 7.4 mo
E) 4.2 mo
A) 3.7 mo
B) 13 mo
C) 2.1 mo
D) 7.4 mo
E) 4.2 mo

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
53
Tritium (3H) is used in glowing "EXIT" signs located where there is no electricity for light bulbs. If the half-life of tritium is 12.26 years, what percentage of the original quantity of the isotope is left in the sign after 18.5 years?
A) 0.632%
B) 63.2%
C) 35.1%
D) 1.51%
E) 25.0%
A) 0.632%
B) 63.2%
C) 35.1%
D) 1.51%
E) 25.0%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
54
Radiation seed therapy is a common method for treating some prostate cancers and brain tumors. Cesium-131, with a half-life of 9.7 days, is a new isotope proposed for this treatment. How long does it take for the activity of cesium-131 to decrease to 1.0% of its initial value and essentially cease to be effective?
A) 3.7 mo
B) 13 mo
C) 2.1 mo
D) 7.4 mo
E) 4.2 mo
A) 3.7 mo
B) 13 mo
C) 2.1 mo
D) 7.4 mo
E) 4.2 mo

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
55
Phosphorus-32 is a radioactive isotope used as a tracer in the liver. How much phosphorus-32 was originally used if there is only 3.50 mg left in a sample after 288 h? (The half-life of phosphorus-32 is 14.3 days.)
A) 1.96 mg
B) 6.26 mg
C) 4.17 mg
D) 7.00 mg
E) 17.9 mg
A) 1.96 mg
B) 6.26 mg
C) 4.17 mg
D) 7.00 mg
E) 17.9 mg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
56
A half-life is ________
A) the life that a nuclear chemist leads.
B) half of the lifetime of an unstable nucleus.
C) the time for one-half of the unstable nuclei to decay.
D) constantly changing.
E) independent of the rate constant for decay.
A) the life that a nuclear chemist leads.
B) half of the lifetime of an unstable nucleus.
C) the time for one-half of the unstable nuclei to decay.
D) constantly changing.
E) independent of the rate constant for decay.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
57
Prozac (fluoxetine hydrochloride) is a selective serotonin reuptake inhibitor (SSRI) used to treat depression. Its function in the brain can be studied by using radiolabeled 18F instead of natural abundance fluorine in its structure. The positron emission is detected by a PET scan.
The half-life of 18F is 109.7 minutes. If radiolabeled Prozac were administered to a patient for a PET scan at 8:00 A.M. on Monday, at what time would its activity reach 10% of the original activity?
A) 9:49 A.M., Monday
B) 9:07 P.M., Friday
C) 10:42 A.M., Tuesday
D) 2:04 P.M., Monday
E) 6:07 P.M., Monday
The half-life of 18F is 109.7 minutes. If radiolabeled Prozac were administered to a patient for a PET scan at 8:00 A.M. on Monday, at what time would its activity reach 10% of the original activity?
A) 9:49 A.M., Monday
B) 9:07 P.M., Friday
C) 10:42 A.M., Tuesday
D) 2:04 P.M., Monday
E) 6:07 P.M., Monday

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
58
Plutonium-238 is an emitter and a compact heat source. Coupled with a PbTe thermoelectric device, it is used as a very reliable electrical energy source for cardiac pacemakers. It has a half-life of 86 years and an atomic weight of 238.0495 g/mol. One mol is equal to 6.02 * 1023 atoms. If 100.0 grams of 238Pu were used in a pacemaker, how many particles
Would it produce in 10 years?
A) 6.02 * 1023
B) 3.01* 1023
C) 3.01 * 1022
D) 1.96 * 1022
E) 2.33 *1023
Would it produce in 10 years?
A) 6.02 * 1023
B) 3.01* 1023
C) 3.01 * 1022
D) 1.96 * 1022
E) 2.33 *1023

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
59
Iodine-131 has a half-life of 8.1 days and is used as a tracer for the thyroid gland. If a patient drinks a sodium iodide (NaI) solution containing iodine-131 on a Tuesday, how many days will it take for the concentration of iodine-131 to drop to 5.0% of its initial concentration?
A) 19 days
B) 0.81 day
C) 8.1 days
D) 35 days
E) 4.3 days
A) 19 days
B) 0.81 day
C) 8.1 days
D) 35 days
E) 4.3 days

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
60
Carbon-14 measurements on the linen wrappings from the Book of Isaiah on the Dead Sea Scrolls indicated that the scrolls contained about 79.5% of the carbon-14 found in living tissue. Approximately how old are these scrolls? The half-life of carbon-14 is 5,730 years.
A) 570 years
B) 820 years
C) 1,300 years
D) 1,900 years
E) 4,600 years
A) 570 years
B) 820 years
C) 1,300 years
D) 1,900 years
E) 4,600 years

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
61
Which type of radiation does the most tissue damage, but only when the emitter is internally ingested?
A) alpha
B) beta
C) gamma
D) neutron
E) positron
A) alpha
B) beta
C) gamma
D) neutron
E) positron

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
62
Radiation seed therapy is a common method for treating some prostate cancers and brain tumors. Cesium-131, which decays by electron capture and gamma ray emission with a half-life of 9.69 days, is a new isotope proposed for such treatment. If a tumor were seeded with 1.00 g of cesium-131 (130.91 g/mol), what would the initial radioactivity level be?
Note: 1 Ci = 3.70*1010/s.
A) 5.84 mCi
B) 78.3 mCi
C) 103 mCi
D) 254 mCi
E) 39.4 mCi
Note: 1 Ci = 3.70*1010/s.
A) 5.84 mCi
B) 78.3 mCi
C) 103 mCi
D) 254 mCi
E) 39.4 mCi

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
63
Small traces of radioactive substances, mainly from the food we eat, are present in the human body. The most abundant radioisotope in the body is potassium-40. A person weighing 70 kg contains about 150 g of potassium, and about 0.01% of this amount is potassium-40. The half-life of potassium-40 is 1.25 x109 years. Estimate the rate at which radiation is produced by the decay of potassium-40 in the body.
A) 4,000 decays/s
B) 40,000 decays/s
C) 400,000 decays/s
D) 400 decays/s
E) 4 decays/s
A) 4,000 decays/s
B) 40,000 decays/s
C) 400,000 decays/s
D) 400 decays/s
E) 4 decays/s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
64
Common activity levels of radon-222 are in the range of 1 picocurie/liter (pCi/L) at room temperature and pressure. How many atoms of radon decay each minute in a 1 L sample of gas with radiation at this level? Recall that 1 Bq = 1 decay/s and 1 Ci = 3.7 x1010 Bq.
A) 6 x10-4
B) 6 x 108
C) 2 x 1012
D) 2
E) 4 x10-2
A) 6 x10-4
B) 6 x 108
C) 2 x 1012
D) 2
E) 4 x10-2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
65
In 2002, a wooden beam from the Wupatki Indian ruin in Arizona was found to contain 235 mg of carbon-14. A sample of wood of the same mass cut sometime in 2002 contained 264 mg of carbon-14. When was the beam from Wupatki cut if the half-life of carbon-14 is 5,715 years?
A) 1974 A.D.
B) 750 A.D.
C) 959 A.D.
D) 1042 A.D.
E) 926 A.D.
A) 1974 A.D.
B) 750 A.D.
C) 959 A.D.
D) 1042 A.D.
E) 926 A.D.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
66
The half-life of a radioactive isotope is 1.0 minute. In an experiment, the number of decay events was monitored in 1-minute intervals over a 5-minute period. What would you predict for the most likely observations if 50 decay events were observed in the first minute? In the second minute, ________ events were observed, and in the last minute, ________ events were observed.
A) 50; 50
B) 25; 3
C) 25; 25
D) 50; 100
E) 25; 13
A) 50; 50
B) 25; 3
C) 25; 25
D) 50; 100
E) 25; 13

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
67
Which type of radiation has the greatest penetration ability?
A) alpha
B) beta
C) gamma
D) neutron
E) positron
A) alpha
B) beta
C) gamma
D) neutron
E) positron

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
68
The argon-40 method of radioisotope dating was developed in the Geology Department at Stony Brook University to determine the age of rocks brought back from the moon. This method assumes that no argon-40 gas was trapped when the molten rock solidified, and that no argon-40 can escape from the solid rock. One sample that was analyzed had a ratio of potassium-40 to argon-40 of 0.100 (NK/NAr = 0.100). Potassium-40 decays into argon-40 by positron emission with a half-life of 1.28 x109 years. How many years ago was this moon rock formed?
A) 2.6 x 108 years
B) 2.3 x 109 years
C) 4.4 x 109 years
D) 1.3 x 1010 years
E) 1.3 x108 years
A) 2.6 x 108 years
B) 2.3 x 109 years
C) 4.4 x 109 years
D) 1.3 x 1010 years
E) 1.3 x108 years

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
69
In a Geiger counter, which is used to detect radioactivity, a particle produced by radioactive decay ________
A) ionizes an inert gas to produce a pulse of electrical current.
B) heats up an inert gas to produce a temperature pulse.
C) excites a phosphor to produce a pulse of visible light.
D) hits a metal plate to produce a voltage pulse.
E) hits a metal plate, vaporizing atoms, to produce a pressure pulse.
A) ionizes an inert gas to produce a pulse of electrical current.
B) heats up an inert gas to produce a temperature pulse.
C) excites a phosphor to produce a pulse of visible light.
D) hits a metal plate to produce a voltage pulse.
E) hits a metal plate, vaporizing atoms, to produce a pressure pulse.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
70
The dominant mechanism through which radiation damages biological tissue and living organisms is by ________
A) overheating the tissue.
B) making the tissue radioactive.
C) directly breaking bonds in DNA.
D) directly ionizing biological molecules.
E) ionizing water and producing hydroxy radicals.
A) overheating the tissue.
B) making the tissue radioactive.
C) directly breaking bonds in DNA.
D) directly ionizing biological molecules.
E) ionizing water and producing hydroxy radicals.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
71
Radiation seed therapy is a common method for treating some prostate cancers and brain tumors. Iodine-125, which decays by electron capture followed by gamma ray emission with a half-life of 59.4 days, is one isotope used for this treatment. If a tumor were seeded with 1.00 g of iodine-125 (124.90 g/mol), what would the initial radioactivity level be?
Note: 1 Ci = 3.70 *1010/s.
A) 17.6 mCi
B) 32.5 mCi
C) 96.7 mCi
D) 1.40 mCi
E) 58.6 mCi
Note: 1 Ci = 3.70 *1010/s.
A) 17.6 mCi
B) 32.5 mCi
C) 96.7 mCi
D) 1.40 mCi
E) 58.6 mCi

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
72
A rock from the moon was found to contain 1.0 * 10-5 g of uranium-238 and 4.0*10-6 g lead-206. All of the lead-206 came from the decay of uranium-238. The half-life for this decay is
4)5 * 109 years. How old is this rock?
A) 1 * 106 years
B) 2* 107 years
C) 4 * 108 years
D) 3 *109 years
E) 5 * 1010 years
4)5 * 109 years. How old is this rock?
A) 1 * 106 years
B) 2* 107 years
C) 4 * 108 years
D) 3 *109 years
E) 5 * 1010 years

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
73
Which of the following units is used to quantify the biological effects of radiation?
A) curies
B) sieverts
C) rads
D) becquerels
E) joules
A) curies
B) sieverts
C) rads
D) becquerels
E) joules

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
74
Radiation seed therapy is a common method for treating some prostate cancers and brain tumors. Palladium-103, which decays by electron capture and gamma ray emission with a half-life of 17.0 days, is one isotope used for this treatment. How much palladium-103 (102.91 g/mol) is needed to produce an initial radioactivity level of 1.30 mCi? Note: 1 Ci = 3.70 x1010/s.
A) 0.0174 g
B) 0.0251 g
C) 0.0316 g
D) 0.0995 g
E) 0.0594 g
A) 0.0174 g
B) 0.0251 g
C) 0.0316 g
D) 0.0995 g
E) 0.0594 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
75
The activity of a radioactive sample is the number of decay events per second. The SI unit of activity is the becquerel (1 Bq = 1/s). An older unit for activity is the curie (1 Ci = 3.70 x1010/s). What is the activity in Bq of a sample with an activity of 47 µCi of radiation?
A) 7.9 x102 Bq
B) 7.9 x 108 Bq
C) 1.7 x 1012 Bq
D) 1.7 x 106 Bq
E) 1.3 x 10-16 Bq
A) 7.9 x102 Bq
B) 7.9 x 108 Bq
C) 1.7 x 1012 Bq
D) 1.7 x 106 Bq
E) 1.3 x 10-16 Bq

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
76
Which of the following units is used to quantify the biological effects of radiation?
A) curies
B) rems
C) rads
D) becquerels
E) joules
A) curies
B) rems
C) rads
D) becquerels
E) joules

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
77
Radon-222 has a half-life of 3.8 days. A sample from a basement in Colorado was analyzed
5)0 days after it was collected and found to have an activity of 0.17 Bq. What was the original activity of this sample?
A) 0.22 Bq
B) 0.32 Bq
C) 0.42 Bq
D) 0.62 Bq
E) 0.52 Bq
5)0 days after it was collected and found to have an activity of 0.17 Bq. What was the original activity of this sample?
A) 0.22 Bq
B) 0.32 Bq
C) 0.42 Bq
D) 0.62 Bq
E) 0.52 Bq

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
78
Which statement, A-D, regarding the activity of a radioactive sample, is not correct? If all are correct, respond E.
A) The activity of a sample is the number of decay events per second.
B) The activity depends on a rate constant for the decay and the amount of the radioisotope in the sample.
C) Radioactive decay follows first-order reaction kinetics.
D) The activity will decrease to half its initial value in a time equal to ln(2)/k, where k is the rate constant.
E) A-D are all correct.
A) The activity of a sample is the number of decay events per second.
B) The activity depends on a rate constant for the decay and the amount of the radioisotope in the sample.
C) Radioactive decay follows first-order reaction kinetics.
D) The activity will decrease to half its initial value in a time equal to ln(2)/k, where k is the rate constant.
E) A-D are all correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
79
A person's body generates about 0.2 µCi of radioactivity! Determine the total radioactivity emitted by 300 students in a lecture hall. (1 Ci = 3.7 x 1010 Bq and 1 Bq = 1 decay/s)
A) 2 x106 decays/s
B) 9 x 1016 decays/s
C) 70 decays/s
D) 2 x107 decays/s
E) 7 x103 decays/s
A) 2 x106 decays/s
B) 9 x 1016 decays/s
C) 70 decays/s
D) 2 x107 decays/s
E) 7 x103 decays/s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck
80
Particles emitted in radioactive decay have different penetrating power. The penetration depths of two particles, labeled X and Y, in a column of water are shown below. The Y particle most likely is not ________ 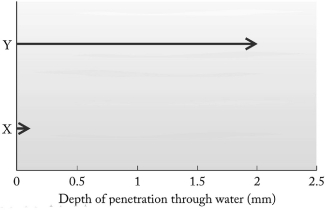
A) an particle.
B) a particle.
C) a positron.
D) a ray.

A) an particle.
B) a particle.
C) a positron.
D) a ray.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 144 في هذه المجموعة.
فتح الحزمة
k this deck








