Deck 2: Atomic Structure and Nuclear Radiation
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/135
العب
ملء الشاشة (f)
Deck 2: Atomic Structure and Nuclear Radiation
1
Which pair does NOT correctly match an element symbol to its full name?
A) C - carbon
B) O - oxygen
C) H - helium
D) N- nitrogen
E) Cl - chlorine
A) C - carbon
B) O - oxygen
C) H - helium
D) N- nitrogen
E) Cl - chlorine
H - helium
2
Which isotope in Zirconium is the lightest? 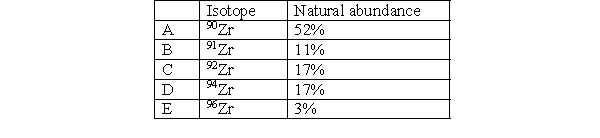
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E
A
3
The identity of an element is determined by its number of _____.
A) protons
B) neutrons
C) electrons
D) protons and neutrons
E) protons, neutrons, and electrons
A) protons
B) neutrons
C) electrons
D) protons and neutrons
E) protons, neutrons, and electrons
protons
4
Which of the following is NOT a symptom of iron deficiency?
A) dizziness
B) shortness of breath
C) frequent urination
D) headaches
E) fatigue
A) dizziness
B) shortness of breath
C) frequent urination
D) headaches
E) fatigue

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which isotope in Zirconium has the fewest number of neutrons? 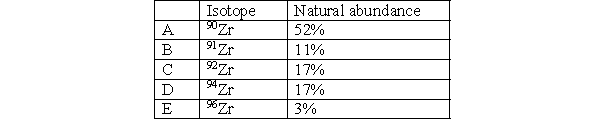
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
6
_____ are the subatomic particles that have the smallest mass.
A) Protons
B) Neutrons
C) Electrons
D) Protons and neutrons
E) Protons, neutrons, and electrons
A) Protons
B) Neutrons
C) Electrons
D) Protons and neutrons
E) Protons, neutrons, and electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following statements about the model of the atom is true?
A) The nucleus is much less dense than the surrounding electrons.
B) Electrons orbit the nucleus like planets around the Sun.
C) This is the first model of the atom.
D) The atom is mostly empty space.
E) The model of the atom was developed by looking directly at an atom.
A) The nucleus is much less dense than the surrounding electrons.
B) Electrons orbit the nucleus like planets around the Sun.
C) This is the first model of the atom.
D) The atom is mostly empty space.
E) The model of the atom was developed by looking directly at an atom.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
8
According to the current model of the atom, the part of the diagram labeled B is made up of 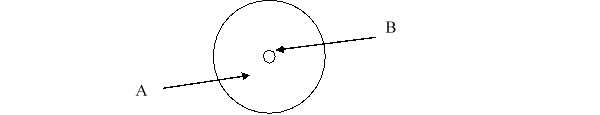
A) protons.
B) neutrons.
C) electrons.
D) protons and neutrons.
E) protons, neutrons, and electrons.

A) protons.
B) neutrons.
C) electrons.
D) protons and neutrons.
E) protons, neutrons, and electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
9
The nucleus is composed of _____.
A) protons
B) neutrons
C) electrons
D) protons and neutrons
E) protons, neutrons, and electrons
A) protons
B) neutrons
C) electrons
D) protons and neutrons
E) protons, neutrons, and electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which of the following statements describes how our model of the atom has changed?
A) The model of the atom has never changed.
B) Electrons are now commonly directly observed.
C) Electrons are now known to orbit the nucleus like a planet orbits the Sun.
D) The path of a single electron can now be followed exactly.
E) We now determine the probabality of finding an electron in a region of space.
A) The model of the atom has never changed.
B) Electrons are now commonly directly observed.
C) Electrons are now known to orbit the nucleus like a planet orbits the Sun.
D) The path of a single electron can now be followed exactly.
E) We now determine the probabality of finding an electron in a region of space.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
11
_____ have a positive charge.
A) Protons
B) Neutrons
C) Electrons
D) Protons and neutrons
E) Protons, neutrons, and electrons
A) Protons
B) Neutrons
C) Electrons
D) Protons and neutrons
E) Protons, neutrons, and electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
12
_____ are neutral.
A) Protons
B) Neutrons
C) Electrons
D) Protons and neutrons
E) Protons, neutrons, and electrons
A) Protons
B) Neutrons
C) Electrons
D) Protons and neutrons
E) Protons, neutrons, and electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which isotope in Zirconium is the least abundant? 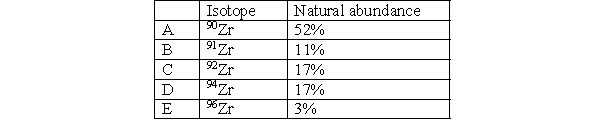
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of the following diagrams of an atom best represents the scale of the nucleus and electrons?
A)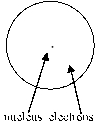
B)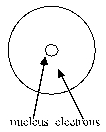
C)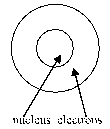
D)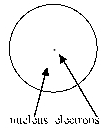
E)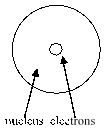
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
15
_____ have a negative charge.
A) Protons
B) Neutrons
C) Electrons
D) Protons and neutrons
E) Protons, neutrons, and electrons
A) Protons
B) Neutrons
C) Electrons
D) Protons and neutrons
E) Protons, neutrons, and electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
16
_____ have a mass of approximately 1 amu.
A) Protons
B) Neutrons
C) Electrons
D) Protons and neutrons
E) Protons, neutrons, and electrons
A) Protons
B) Neutrons
C) Electrons
D) Protons and neutrons
E) Protons, neutrons, and electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which pair correctly matches an element to its atomic number?
A) 9.012 - Be
B) 12.01 - C
C) 39.94 - Ar
D) 9 - F
E) 133 - Cs
A) 9.012 - Be
B) 12.01 - C
C) 39.94 - Ar
D) 9 - F
E) 133 - Cs

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
18
The most common nutritional deficiency in the world is_____.
A) scurvy
B) anemia
C) kwashiorkor
D) rickets
E) pellagra
A) scurvy
B) anemia
C) kwashiorkor
D) rickets
E) pellagra

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
19
According to the current model of the atom, the part of the diagram labeled A is made up of 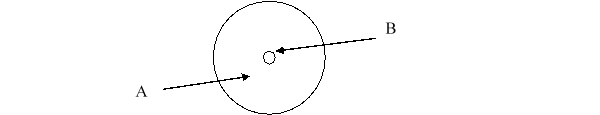
A) protons.
B) neutrons.
C) electrons.
D) protons and neutrons.
E) protons, neutrons, and electrons.

A) protons.
B) neutrons.
C) electrons.
D) protons and neutrons.
E) protons, neutrons, and electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
20
_____ determine the physical and chemical characteristics of an atom.
A) Protons
B) Neutrons
C) Electrons
D) Protons and neutrons
E) Protons, neutrons, and electrons
A) Protons
B) Neutrons
C) Electrons
D) Protons and neutrons
E) Protons, neutrons, and electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
21
According to the periodic table, the atomic mass of potassium (K) is ______. ?
A) 4
B) 19
C) 39.10
D) K
E) 2
A) 4
B) 19
C) 39.10
D) K
E) 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
22
_____ make up the majority of compounds found in living organisms.
A) Building-block elements
B) Macronutrients
C) Micronutrients
D) Metals
E) Metalloids
A) Building-block elements
B) Macronutrients
C) Micronutrients
D) Metals
E) Metalloids

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
23
The average atomic mass of zirconium is 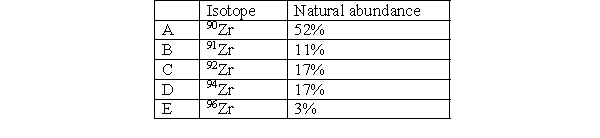
A) less than 90 because the atomic mass only depends on the number of protons in the atom.
B) 90 because 90Zn has the highest natural abundance.
C) greater than 90 but less than 96 because the atomic mass takes into account the abundance of all naturally occurring isotopes.
D) 96 because the atomic mass is the mass of the highest naturally occurring isotope.
E) greater than 96 because the atomic mass is the sum of masses of the naturally occurring isotopes.

A) less than 90 because the atomic mass only depends on the number of protons in the atom.
B) 90 because 90Zn has the highest natural abundance.
C) greater than 90 but less than 96 because the atomic mass takes into account the abundance of all naturally occurring isotopes.
D) 96 because the atomic mass is the mass of the highest naturally occurring isotope.
E) greater than 96 because the atomic mass is the sum of masses of the naturally occurring isotopes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
24
According to the periodic table, which element is found in period 2, group 5A?
A) nitrogen
B) vanadium
C) strontium
D) boron
E) cadmium
A) nitrogen
B) vanadium
C) strontium
D) boron
E) cadmium

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
25
According to the periodic table, which element is in period 4, group 6A?
A) Cr
B) La
C) Ga
D) Se
E) Al
A) Cr
B) La
C) Ga
D) Se
E) Al

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
26
According to the periodic table, which of the following sets of terms accurately describes chlorine? 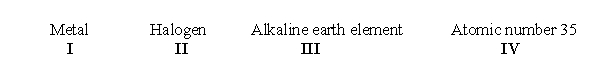
A) I only
B) II only
C) I and II
D) II and IV
E) III and IV
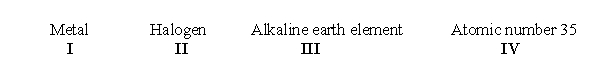
A) I only
B) II only
C) I and II
D) II and IV
E) III and IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
27
What is the identity of element X? 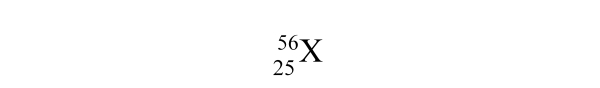
A) xenon
B) manganese
C) gold
D) copper
E) iron
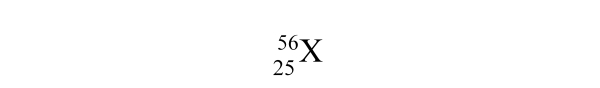
A) xenon
B) manganese
C) gold
D) copper
E) iron

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
28
An element is a solid at room temperature and a shiny, metallic grey. However, it is a poor conductor of electricity and temperature and it is also brittle. Which of the following elements fits this description?
A) oxygen
B) lithium
C) helium
D) antimony
E) iron
A) oxygen
B) lithium
C) helium
D) antimony
E) iron

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
29
According to the periodic table, the atomic number of potassium (K) is ______. ?
A) 4
B) 19
C) 39.10
D) K
E) 2
A) 4
B) 19
C) 39.10
D) K
E) 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
30
What is the mass number of element X? 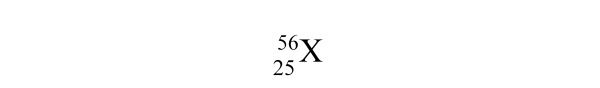
A) 25
B) 56
C) 81
D) 31
E) None of the above values is the mass number.
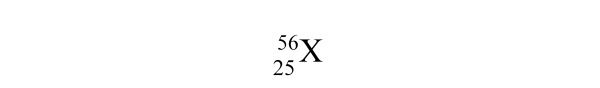
A) 25
B) 56
C) 81
D) 31
E) None of the above values is the mass number.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
31
The elements numbered 21-30 are examples of _______..
A) transition metals
B) noble gases
C) alkali earth metals
D) alkali metals
E) halogens
A) transition metals
B) noble gases
C) alkali earth metals
D) alkali metals
E) halogens

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
32
According to the periodic table, what types of elements are in group 7A?
A) transition metals
B) noble gases
C) alkali earth metals
D) alkali metals
E) halogens
A) transition metals
B) noble gases
C) alkali earth metals
D) alkali metals
E) halogens

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following statements about isotopes is false?
A) Isotopes are atoms with same number of protons but different numbers of neutrons.
B) Most elements naturally have more than one isotope.
C) Isotopes are atoms with the same atomic number but different mass numbers.
D) An isotope with more neutrons will have a greater mass than an isotope with fewer neutrons.
E) All of the above are true.
A) Isotopes are atoms with same number of protons but different numbers of neutrons.
B) Most elements naturally have more than one isotope.
C) Isotopes are atoms with the same atomic number but different mass numbers.
D) An isotope with more neutrons will have a greater mass than an isotope with fewer neutrons.
E) All of the above are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
34
The periods are the _____ of the periodic table.
A) transition metals
B) halogens
C) rows
D) columns
E) numbers
A) transition metals
B) halogens
C) rows
D) columns
E) numbers

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
35
The number of protons is equal to the _____ in a neutral atom.
A) number of neutrons
B) number of electrons
C) mass number
D) average atomic mass
E) group number
A) number of neutrons
B) number of electrons
C) mass number
D) average atomic mass
E) group number

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which of the following terms is NOT a characteristic of a metal?
A) malleable
B) a good conductor of heat
C) a good conductor of electricity
D) shiny
E) a gas at room temperature
A) malleable
B) a good conductor of heat
C) a good conductor of electricity
D) shiny
E) a gas at room temperature

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
37
How many electrons are in a neutral atom of element X? 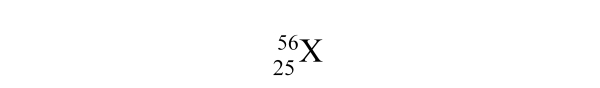
A) 25
B) 56
C) 81
D) 31
E) None of the above values is the number of electrons.
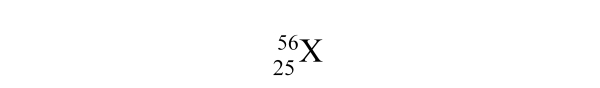
A) 25
B) 56
C) 81
D) 31
E) None of the above values is the number of electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
38
Most of the micronutrients are
A) transition metals.
B) metalloids.
C) nonmetals.
D) alkali earth metals.
E) noble gases.
A) transition metals.
B) metalloids.
C) nonmetals.
D) alkali earth metals.
E) noble gases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
39
What is the atomic number of element X? 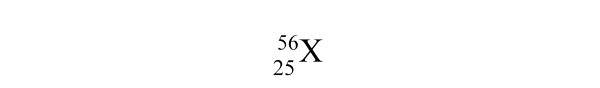
A) 25
B) 56
C) 81
D) 31
E) None of the above values is the atomic number.
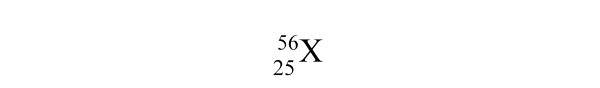
A) 25
B) 56
C) 81
D) 31
E) None of the above values is the atomic number.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
40
An atom of carbon containing 7 neutrons can be written which of the following ways? 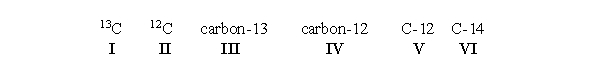 All of the choices are correct.
All of the choices are correct.
A) II, IV, or V
B) I, III
C) I, II
D) III, IV
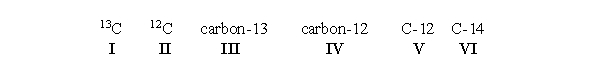 All of the choices are correct.
All of the choices are correct.A) II, IV, or V
B) I, III
C) I, II
D) III, IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
41
_____ is an important component of hemoglobin. Without this protein, tissues become starved of oxygen and fatigue and shortness of breath results.
A) Iodine
B) Flourine
C) Zinc
D) Iron
E) Oxygen
A) Iodine
B) Flourine
C) Zinc
D) Iron
E) Oxygen

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
42
_____ is most commonly ingested along with salt.
A) Iodine
B) Flourine
C) Zinc
D) Iron
E) Oxygen
A) Iodine
B) Flourine
C) Zinc
D) Iron
E) Oxygen

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
43
Which element is this? 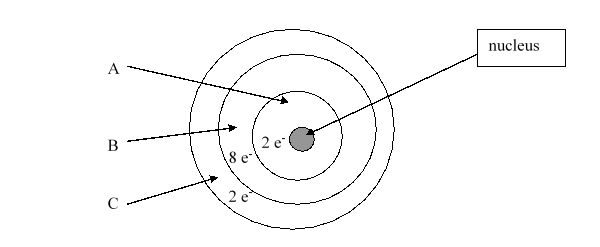
A) beryllium
B) magnesium
C) oxygen
D) neon
E) calcium

A) beryllium
B) magnesium
C) oxygen
D) neon
E) calcium

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
44
Which atom is in its ground state? 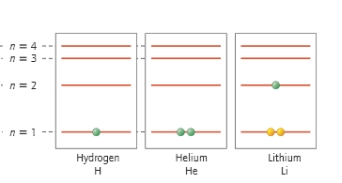
A) H
B) He
C) H and He
D) Li
E) All of the above

A) H
B) He
C) H and He
D) Li
E) All of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
45
How many electrons are core electrons? 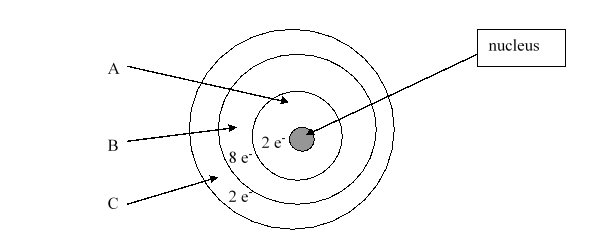
A) 12
B) 10
C) 8
D) 4
E) 2

A) 12
B) 10
C) 8
D) 4
E) 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
46
Which electron shell is closest to the nucleus of the atom? 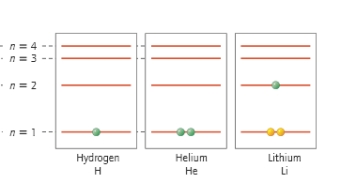
A) n =1
B) n =2
C) n =3
D) n =4
E) All electrons are equally close to the nucleus.

A) n =1
B) n =2
C) n =3
D) n =4
E) All electrons are equally close to the nucleus.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
47
_____ is an important component of the immune system as well as required by many enzymes.
A) Iodine
B) Flourine
C) Zinc
D) Iron
E) Oxygen
A) Iodine
B) Flourine
C) Zinc
D) Iron
E) Oxygen

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
48
Micro- and macronutrients are
A) equally distributed throughout the body.
B) all metals and metalloids.
C) obtained through the diet.
D) only found in the first three periods of the periodic table.
E) all required in quantities of more than 100 mg per day.
A) equally distributed throughout the body.
B) all metals and metalloids.
C) obtained through the diet.
D) only found in the first three periods of the periodic table.
E) all required in quantities of more than 100 mg per day.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
49
Which atom has the largest diameter? 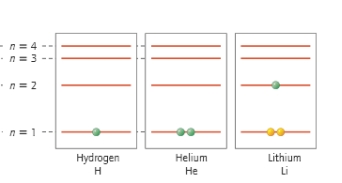
A) H
B) He
C) H and He
D) Li
E) All of the above have the same diameter.

A) H
B) He
C) H and He
D) Li
E) All of the above have the same diameter.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
50
Which atom has two core electrons and one valence electron? 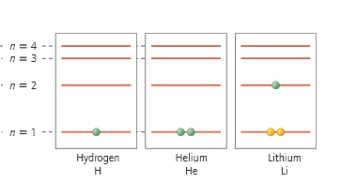
A) H
B) He
C) H and He
D) Li
E) All of the above

A) H
B) He
C) H and He
D) Li
E) All of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
51
How many electrons are in the valence shell? 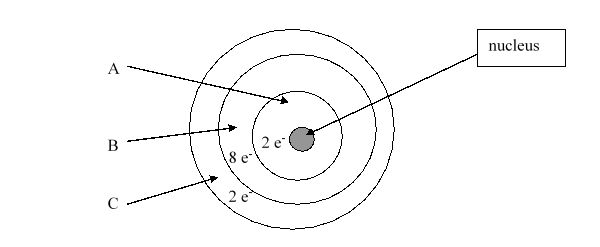
A) 12
B) 10
C) 8
D) 4
E) 2

A) 12
B) 10
C) 8
D) 4
E) 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which electron shell is lowest in energy? 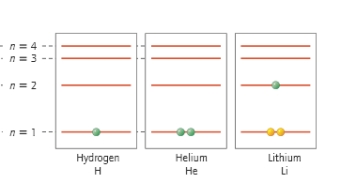
A) n =1
B) n =2
C) n =3
D) n =4
E) All electrons are equal in energy.

A) n =1
B) n =2
C) n =3
D) n =4
E) All electrons are equal in energy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
53
Electron shells closest to the nucleus are occupied before electron shells farther from the nucleus because the electrons shells closest to the nucleus are
A) pulled toward the nucleus by gravity.
B) protected by the outer shell.
C) attracted to the nucleus for reasons unknown.
D) more stable than electrons farther from the nucleus.
E) Actually, electrons farther from the nucleus are filled first.
A) pulled toward the nucleus by gravity.
B) protected by the outer shell.
C) attracted to the nucleus for reasons unknown.
D) more stable than electrons farther from the nucleus.
E) Actually, electrons farther from the nucleus are filled first.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
54
According to the periodic table, how many electron shells do the elements in the third row have?
A) 1
B) 2
C) 3
D) 4
E) It depends on the specific element.
A) 1
B) 2
C) 3
D) 4
E) It depends on the specific element.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
55
The element chlorine has three electron shells. How many electrons are in each shell? 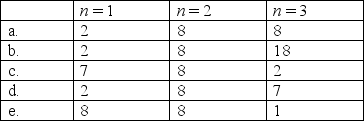
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
56
According to the periodic table, how many valence electrons do the elements in group 7A have?
A) 5
B) 6
C) 7
D) 8
E) It depends on the specific element.
A) 5
B) 6
C) 7
D) 8
E) It depends on the specific element.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
57
Which of the following is NOT a building block element?
A) C
B) H
C) O
D) N
E) These are all building block elements.
A) C
B) H
C) O
D) N
E) These are all building block elements.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
58
Adding _____ to drinking water is a common practice in many cities, meant to strengthen tooth enamel and decrease dental cavities.
A) iodine
B) flourine
C) zinc
D) iron
E) oxygen
A) iodine
B) flourine
C) zinc
D) iron
E) oxygen

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
59
According to the periodic table, how many valence electrons do the elements in the third row have?
A) 3
B) 4
C) 5
D) 8
E) It depends on the specific element.
A) 3
B) 4
C) 5
D) 8
E) It depends on the specific element.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
60
Which shell is the valence shell? 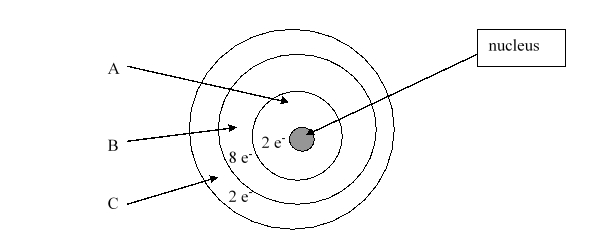
A) shell A
B) shell B
C) shell C
D) shells A and B
E) shells B and C

A) shell A
B) shell B
C) shell C
D) shells A and B
E) shells B and C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
61
The size of atoms generally increases as the number of
A) electron shells increases.
B) electrons in the valence shell increases.
C) neutrons in atoms increases.
D) protons increases.
E) All atoms are the same size.
A) electron shells increases.
B) electrons in the valence shell increases.
C) neutrons in atoms increases.
D) protons increases.
E) All atoms are the same size.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
62
Which of the following is NOT the same for different isotopes of the same element?
A) atomic number
B) number of protons
C) number of electrons
D) charge
E) mass number
A) atomic number
B) number of protons
C) number of electrons
D) charge
E) mass number

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
63
Which of the following groups of elements forms anions?
A) noble gases
B) transition metals
C) alkaline metals
D) alkali earth metals
E) halogens
A) noble gases
B) transition metals
C) alkaline metals
D) alkali earth metals
E) halogens

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
64
The energy diagram below is for an ion of magnesium. To make this ion, the atom that it came from had to_________. 
A) become a different atom.
B) gain electrons.
C) become a nonmetal.
D) lose electrons.
E) become a metal.

A) become a different atom.
B) gain electrons.
C) become a nonmetal.
D) lose electrons.
E) become a metal.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
65
How many electrons are in the valence shell of the ion represented by the energy diagram shown below? 
A) 8
B) 10
C) 12
D) 22
E) 0

A) 8
B) 10
C) 12
D) 22
E) 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
66
Which of the following elements forms a cation?
A) carbon
B) phosphorous
C) krypton
D) nickel
E) iodine
A) carbon
B) phosphorous
C) krypton
D) nickel
E) iodine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
67
Which of the following elements has a mass number of 35 and an atomic number of 17?
A) chlorine
B) bromine
C) argon
D) tellurium
E) sulfur-35
A) chlorine
B) bromine
C) argon
D) tellurium
E) sulfur-35

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
68
Isotopes are
A) elements with the same number of electrons but different numbers of protons.
B) elements with the same number of protons but different numbers of electrons.
C) elements with the same number of electrons but different numbers of neutrons.
D) elements with the same number of protons but different numbers of neutrons.
E) elements with the same number of neutrons but different numbers of protons.
A) elements with the same number of electrons but different numbers of protons.
B) elements with the same number of protons but different numbers of electrons.
C) elements with the same number of electrons but different numbers of neutrons.
D) elements with the same number of protons but different numbers of neutrons.
E) elements with the same number of neutrons but different numbers of protons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
69
The energy diagram below is for an ion of magnesium. The charge on this ion is _______ making the ion a _______. 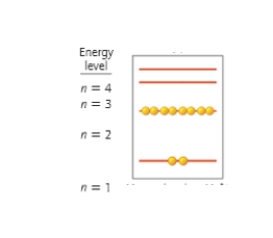

A) (-10; anion)
B) (-2; anion)
C) (-2; cation)
D) + 2; anion
E) + 2; cation


A) (-10; anion)
B) (-2; anion)
C) (-2; cation)
D) + 2; anion
E) + 2; cation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
70
Which of the following is NOT true for the atoms 12C, 13C, and 14C?
A) They all have six electrons.
B) They all have the same mass number.
C) They all have the same atomic number.
D) They are isotopes.
E) They all have six protons.
A) They all have six electrons.
B) They all have the same mass number.
C) They all have the same atomic number.
D) They are isotopes.
E) They all have six protons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
71
Nonmetals typically ______ and metals typically ______.
A) donate electrons; accept electrons
B) accept electrons; donate electrons
C) donate protons; accept protons
D) accept protons; donate protons
E) donate neutrons; accept neutrons
A) donate electrons; accept electrons
B) accept electrons; donate electrons
C) donate protons; accept protons
D) accept protons; donate protons
E) donate neutrons; accept neutrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
72
Elements in group 17 (7A) of the periodic table are likely to form ions with the charge
A) (-3.)
B) (-2.)
C) (-1.)
D) +1.
E) +2.
A) (-3.)
B) (-2.)
C) (-1.)
D) +1.
E) +2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
73
Radioactive isotopes are
A) very stable isotopes.
B) highly chemically reactive.
C) unstable isotopes.
D) charged species.
E) unusually nonreactive.
A) very stable isotopes.
B) highly chemically reactive.
C) unstable isotopes.
D) charged species.
E) unusually nonreactive.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
74
Which of the following is NOT a type of radiation that comes from the decay of radioisotopes?
A) microwaves
B) x-rays
C) gamma rays
D) alpha particle
E) beta particle
A) microwaves
B) x-rays
C) gamma rays
D) alpha particle
E) beta particle

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
75
Elements in group 6A (16) have a -2 charge when they are in compounds. Which of the following statements best explains the reason for this observation?
A) These elements accept two electrons to increase in energy.
B) These elements donate two electrons during the bonding process.
C) These elements accept two electrons so that their valence shells are full.
D) These elements donate two electrons to minimize their mass.
E) There is no way to rationalize this; it is just an observation.
A) These elements accept two electrons to increase in energy.
B) These elements donate two electrons during the bonding process.
C) These elements accept two electrons so that their valence shells are full.
D) These elements donate two electrons to minimize their mass.
E) There is no way to rationalize this; it is just an observation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
76
The conventional way of writing the symbol for an ion of calcium is
A) calcium-18.
B) Ar.
C) Ca - 18.
D) Ca+2.
E) Ca+2.
A) calcium-18.
B) Ar.
C) Ca - 18.
D) Ca+2.
E) Ca+2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
77
Which element would you expect to be the largest?
A) fluorine
B) chlorine
C) argon
D) calcium
E) hydrogen
A) fluorine
B) chlorine
C) argon
D) calcium
E) hydrogen

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
78
Oxygen is a _______ and therefore _______ when it forms an ion.
A) metal; loses electrons
B) metal; gains electrons
C) nonmetal; loses electrons
D) nonmetal; gains electrons
E) metalloid; loses electrons
A) metal; loses electrons
B) metal; gains electrons
C) nonmetal; loses electrons
D) nonmetal; gains electrons
E) metalloid; loses electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
79
Select the choice in which atomic number, mass number, number of neutrons, and number of protons listed is correct for phosphorous-32. 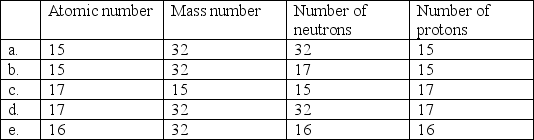
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
80
Elements in group 1A of the periodic table are likely to form ions with the charge
A) (-3.)
B) (-2.)
C) (-1.)
D) +1.
E) +2.
A) (-3.)
B) (-2.)
C) (-1.)
D) +1.
E) +2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck








