Deck 4: Chemical Quantities and Chemical Reactions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/135
العب
ملء الشاشة (f)
Deck 4: Chemical Quantities and Chemical Reactions
1
What is a biochemical pathway?
A) the path that nutrients take through the body when they are metabolized
B) the complex blood transport system
C) a chemical reaction in the body that releases energy
D) a chemical reaction that releases carbon dioxide
E) a particular sequence of chemical reactions in the body
A) the path that nutrients take through the body when they are metabolized
B) the complex blood transport system
C) a chemical reaction in the body that releases energy
D) a chemical reaction that releases carbon dioxide
E) a particular sequence of chemical reactions in the body
a particular sequence of chemical reactions in the body
2
Which statement best describes why we use moles when measuring quantities of atoms and molecules?
A) Moles are used because they are units of mass.
B) Moles are used because one mole is the same numerical value as the atomic mass of an element.
C) Moles are used because atoms and molecules are too small to measure singly.
D) Moles are used because chemists like moles.
E) Moles are not involved in measuring quantities of atoms and molecules.
A) Moles are used because they are units of mass.
B) Moles are used because one mole is the same numerical value as the atomic mass of an element.
C) Moles are used because atoms and molecules are too small to measure singly.
D) Moles are used because chemists like moles.
E) Moles are not involved in measuring quantities of atoms and molecules.
Moles are used because atoms and molecules are too small to measure singly.
3
A molecule of oxygen, O2, has a molar mass of 32.00 g/mole. What is the mass of 4.52 *1023 O2 molecules?
A) 24 grams
B) 4.52 *1023 amu
C) 4.52 * 1023 grams
D) 0.75 grams
E) 0.23 grams
A) 24 grams
B) 4.52 *1023 amu
C) 4.52 * 1023 grams
D) 0.75 grams
E) 0.23 grams
24 grams
4
What is the mass of one mole of the hydroxide ion (-OH)?
A) 8 g
B) 15 g
C) 17 g
D) 7 g
E) 5 g
A) 8 g
B) 15 g
C) 17 g
D) 7 g
E) 5 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
5
How many carbon atoms are in 1 mole of carbon?
A) 1
B) 12
C) 12.011
D) 6
E) 6.02 * 1023
A) 1
B) 12
C) 12.011
D) 6
E) 6.02 * 1023

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
6
How many grams of iron are in 1.28 moles of iron?
A) 0.0230 g
B) 20.3 g
C) 33.3 g
D) 43.6 g
E) 71.4 g
A) 0.0230 g
B) 20.3 g
C) 33.3 g
D) 43.6 g
E) 71.4 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
7
Where in the body does ethanol metabolism occur?
A) the stomach
B) the liver
C) the kidneys
D) the small intestine
E) the mouth
A) the stomach
B) the liver
C) the kidneys
D) the small intestine
E) the mouth

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
8
How many moles of carbon are in 27.1 g of carbon?
A) 2.26 moles
B) 325 moles
C) 0.443 moles
D) 4.52 moles
E) 163 moles
A) 2.26 moles
B) 325 moles
C) 0.443 moles
D) 4.52 moles
E) 163 moles

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which of the following statements describes how molecular mass and molar mass differ?
A) Molecular mass is measured in amu, whereas molar mass is measured in grams/mole.
B) Molar mass is measured in amu, whereas molecular mass is measured in grams/mole.
C) A molar mass is the mass of one unit of an ionic lattice, whereas molecular mass is the mass of one molecule.
D) A molar mass is the mass of one molecule, whereas molecular mass is the mass of one unit of an ionic lattice.
E) There is no difference between these terms.
A) Molecular mass is measured in amu, whereas molar mass is measured in grams/mole.
B) Molar mass is measured in amu, whereas molecular mass is measured in grams/mole.
C) A molar mass is the mass of one unit of an ionic lattice, whereas molecular mass is the mass of one molecule.
D) A molar mass is the mass of one molecule, whereas molecular mass is the mass of one unit of an ionic lattice.
E) There is no difference between these terms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
10
Does one mole of the antibiotic penicillin G (C16H18N2O4S) weigh more or less than one mole of the antibiotic streptomycin (C21H39N7O12)?
A) Actually, they should weigh the same because a mole always has the same mass.
B) Actually, they should weigh the same because a mole always has the same number of particles in it.
C) Streptomycin should weigh more because its individual molecules are larger.
D) Penicillin should weigh more because its individual molecules are larger.
E) It is not possible to determine which one would weigh more because mole size varies.
A) Actually, they should weigh the same because a mole always has the same mass.
B) Actually, they should weigh the same because a mole always has the same number of particles in it.
C) Streptomycin should weigh more because its individual molecules are larger.
D) Penicillin should weigh more because its individual molecules are larger.
E) It is not possible to determine which one would weigh more because mole size varies.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
11
How many copper atoms are in 0.5 moles of copper?
A) 0.5
B) 14
C) 3
D) 6.02 * 1012
E) 3.01 * 1023
A) 0.5
B) 14
C) 3
D) 6.02 * 1012
E) 3.01 * 1023

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
12
How many moles is 9.1*1024 ions of Na+?
A) 5.5 *1048 moles
B) 0.17 moles
C) 1 mole
D) 15 moles
E) 0.67 moles
A) 5.5 *1048 moles
B) 0.17 moles
C) 1 mole
D) 15 moles
E) 0.67 moles

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
13
How many moles of the antibiotic tetracycline (C22H24N2O8) are in a 50-mg dose?
A) 8880 moles
B) 22.2 moles
C) 8.88 moles
D) 0.113 moles
E) 1.13 *10-4 moles
A) 8880 moles
B) 22.2 moles
C) 8.88 moles
D) 0.113 moles
E) 1.13 *10-4 moles

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
14
The ionic compound CaCO3 has a formula mass of
A) 50 g/mole.
B) 50 amu.
C) 100 g/mole.
D) 100 amu.
E) 68.1 amu.
A) 50 g/mole.
B) 50 amu.
C) 100 g/mole.
D) 100 amu.
E) 68.1 amu.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
15
An infant dose of acetaminophen (C8H9NO2) is 0.080
A) 5.3 * 10-4 molecules
B) 3.2 * 1020 molecules
C) 7.2 * 1023 molecules
D) 1.2 molecules
E) 2 * 10-24 molecules
A) 5.3 * 10-4 molecules
B) 3.2 * 1020 molecules
C) 7.2 * 1023 molecules
D) 1.2 molecules
E) 2 * 10-24 molecules

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which of the following statements describes how formula mass and molecular mass differ?
A) A formula mass is measured in amu, whereas a molecular mass is measured in grams/mole.
B) A formula mass is measured in grams/mole, whereas a molecular mass is measured in amu.
C) A formula mass is the mass of one unit of an ionic lattice, whereas molecular mass is the mass of one molecule.
D) A formula mass is the mass of one molecule, whereas molecular mass is the mass of one unit of an ionic lattice.
E) There is no difference between these terms.
A) A formula mass is measured in amu, whereas a molecular mass is measured in grams/mole.
B) A formula mass is measured in grams/mole, whereas a molecular mass is measured in amu.
C) A formula mass is the mass of one unit of an ionic lattice, whereas molecular mass is the mass of one molecule.
D) A formula mass is the mass of one molecule, whereas molecular mass is the mass of one unit of an ionic lattice.
E) There is no difference between these terms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
17
Calculate the mass of one mole of dopamine, a neurotransmitter with the molecular formula C8H11NO2.
A) 22.0 g
B) 43.0 g
C) 82.0 g
D) 153 g
E) 164 g
A) 22.0 g
B) 43.0 g
C) 82.0 g
D) 153 g
E) 164 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which of the following values is Avogadro's number?
A) 6
B) 6.02
C) 6.02 * 1023
D) 6.02 * 1026
E) 6.02 * 10123
A) 6
B) 6.02
C) 6.02 * 1023
D) 6.02 * 1026
E) 6.02 * 10123

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which of the following equations best describes how to calculate the molar mass of Mg(OH)2?
A) 24.3 g/mole + 16.0 g/mole + 1.01 g/mole
B) 24.3 g/mole + 16.0 g/mole + (2 * 1.01 g/mole)
C) 24.3 g/mole + (2 *16.0 g/mole) + 1.01 g/mole
D) 24.3 g/mole + 2 * (16.0 g/mole + 1.01 g/mole)
E) 2 *(24.3 g/mole + 16.0 g/mole + 1.01 g/mole)
A) 24.3 g/mole + 16.0 g/mole + 1.01 g/mole
B) 24.3 g/mole + 16.0 g/mole + (2 * 1.01 g/mole)
C) 24.3 g/mole + (2 *16.0 g/mole) + 1.01 g/mole
D) 24.3 g/mole + 2 * (16.0 g/mole + 1.01 g/mole)
E) 2 *(24.3 g/mole + 16.0 g/mole + 1.01 g/mole)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
20
Calculate the molecular mass of aspirin, C9H8O4.
A) 29 g/mole
B) 126 g/mole
C) 180. g/mole
D) 126 amu
E) 180. amu
A) 29 g/mole
B) 126 g/mole
C) 180. g/mole
D) 126 amu
E) 180. amu

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which of these figures illustrate chemical reactions? 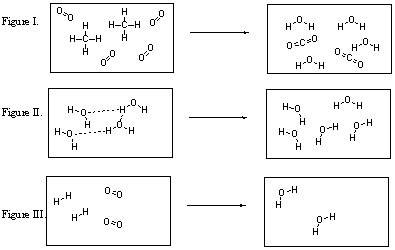
A) I, II, and III are all chemical reactions.
B) Only I and II are chemical reactions.
C) Only I and III are chemical reactions.
D) Only II and III are chemical reactions.
E) None of these figures are chemical reactions.

A) I, II, and III are all chemical reactions.
B) Only I and II are chemical reactions.
C) Only I and III are chemical reactions.
D) Only II and III are chemical reactions.
E) None of these figures are chemical reactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
22
Below are several statements. Which of these statements apply to chemical reactions?
I. A chemical reaction always involves two reactants.
II. Physical changes are a type of chemical reaction.
III. Energy changes are involved in chemical reactions.
IV. Atoms are gained and lost during a chemical reaction.
V. Bonds are broken and new bonds formed during a chemical reaction.
A) All of these statements apply to chemical reactions.
B) Only I applies to chemical reactions.
C) I, II, and III all apply to chemical reactions.
D) IV and V both apply to chemical reactions.
E) III and V both apply to chemical reactions.
I. A chemical reaction always involves two reactants.
II. Physical changes are a type of chemical reaction.
III. Energy changes are involved in chemical reactions.
IV. Atoms are gained and lost during a chemical reaction.
V. Bonds are broken and new bonds formed during a chemical reaction.
A) All of these statements apply to chemical reactions.
B) Only I applies to chemical reactions.
C) I, II, and III all apply to chemical reactions.
D) IV and V both apply to chemical reactions.
E) III and V both apply to chemical reactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
23
If Jane Doe has a blood carbon dioxide concentration of 0.022 mol/L, how many carbon dioxide molecules are in each liter of her blood?
A) 3.7 *10-26
B) 0.022
C) 1.32 *1022
D) 6.02 * 1023
E) 2.7 * 1025
A) 3.7 *10-26
B) 0.022
C) 1.32 *1022
D) 6.02 * 1023
E) 2.7 * 1025

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
24
Jane Doe has a cholesterol (C27H46O) count of 178 mg/dL. You would like to calculate the number of cholesterol molecules that Jane Doe has in each deciliter of blood. Which of the following calculations is used to solve this problem?
A)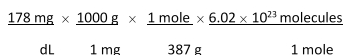
B)
C)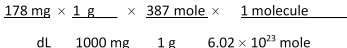
D)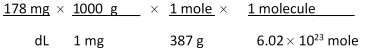
E)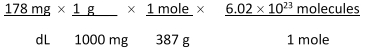
A)
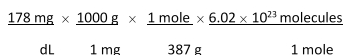
B)

C)
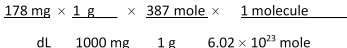
D)
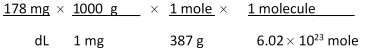
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
25
Jane Doe has her cholesterol measured and the resulting lab report gives a value of 0.548 *10-3 mol/dL. You know that the normal range of cholesterol (C27H46O) is 0.100 - 0.240 g/dL. Is Jane Doe's cholesterol within the normal range?
A) No, it is above 0.240 g/dL.
B) No, it is below 0.100 g/dL.
C) Yes, it is between 0.100 g/dL and 0.240 g/dL.
D) Yes, it is above 0.240 g/dL.
E) Yes, it is below 0.100 g/dL.
A) No, it is above 0.240 g/dL.
B) No, it is below 0.100 g/dL.
C) Yes, it is between 0.100 g/dL and 0.240 g/dL.
D) Yes, it is above 0.240 g/dL.
E) Yes, it is below 0.100 g/dL.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which of the figures violates the conservation of mass? 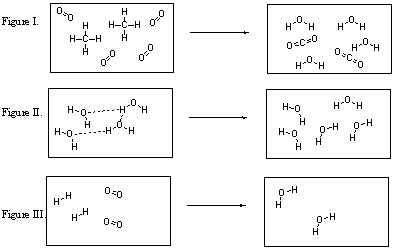
A) All of these figures violate the conservation of mass.
B) Figure I violates the conservation of mass.
C) Figure II violates the conservation of mass.
D) Figure III violates the conservation of mass.
E) Figures II and III both violate the conservation of mass.

A) All of these figures violate the conservation of mass.
B) Figure I violates the conservation of mass.
C) Figure II violates the conservation of mass.
D) Figure III violates the conservation of mass.
E) Figures II and III both violate the conservation of mass.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which of the following transformations is a chemical reaction?
A) water boiling
B) burning wood
C) condensation on the outside of a water glass
D) dry ice changing from a solid to a gas
E) All of the above
A) water boiling
B) burning wood
C) condensation on the outside of a water glass
D) dry ice changing from a solid to a gas
E) All of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
28
A normal blood oxygen (O2) level is 8.0 mmol/L. What is the normal mass of oxygen per liter of blood?
A) 2.50*10-4 g
B) 0.256 g
C) 4.00 g
D) 256 g
E) 2.56 * 105 g
A) 2.50*10-4 g
B) 0.256 g
C) 4.00 g
D) 256 g
E) 2.56 * 105 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
29
An important part of metabolism is the breakdown of proteins into smaller parts called amino acids. During the course of this reaction, some bonds are broken and others are formed. Select the choice that correctly lists the bonds that are broken and those that are formed. 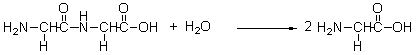
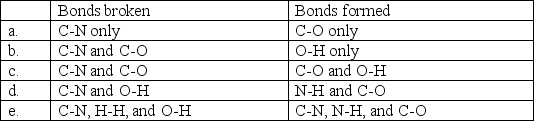
A)a
B)b
C)c
D)d
E)e
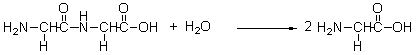

A)a
B)b
C)c
D)d
E)e

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which of the following must occur for there to be a chemical reaction?
A) Heat is released.
B) There is a change of state.
C) A gas is produced.
D) Bonds break and/or new ones are formed.
E) All of the above
A) Heat is released.
B) There is a change of state.
C) A gas is produced.
D) Bonds break and/or new ones are formed.
E) All of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which of the following transformations is illustrated in Figure II? 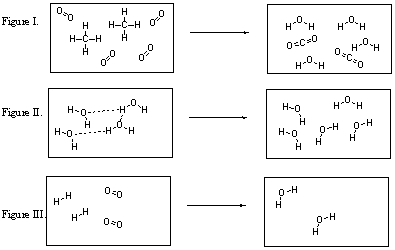
A) Figure II illustrates the breaking of hydrogen bonds.
B) Figure II illustrates the breaking of covalent bonds.
C) Figure II illustrates a chemical reaction.
D) Figure II illustrates a nuclear reaction.

A) Figure II illustrates the breaking of hydrogen bonds.
B) Figure II illustrates the breaking of covalent bonds.
C) Figure II illustrates a chemical reaction.
D) Figure II illustrates a nuclear reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
32
In which figure are new O-H bonds being formed? 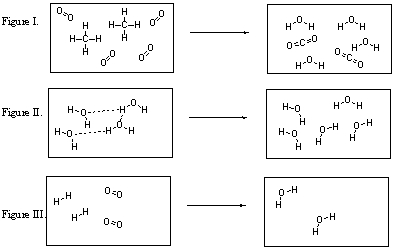
A) Figure I
B) Figure II
C) Figure III
D) Figures I and II
E) Figures I and III

A) Figure I
B) Figure II
C) Figure III
D) Figures I and II
E) Figures I and III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
33
If Jane Doe has a blood glucose (C6H12O6) concentration of 0.102 g/dL, how many glucose molecules does she have in each deciliter of blood?
A) 3.41 * 1026
B) 3.41 * 1023
C) 3.41 *1020
D) 6.41 * 1022
E) 5.67 *10-4
A) 3.41 * 1026
B) 3.41 * 1023
C) 3.41 *1020
D) 6.41 * 1022
E) 5.67 *10-4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
34
Jane Doe has a cholesterol (C27H46O) count of 178 mg/dL. You would like to calculate the number of cholesterol molecules that Jane Doe has in each deciliter of blood. Which of the following unit conversions are required to calculate the number of cholesterol molecules per deciliter? I. 10 dL II. 1 mole III. 1 g____ IV. 6.02 * 1023 molecules
1 L 387 g 1000 mg 1 mole
A) All of the conversions are needed.
B) I and IV
C) III and IV
D) I, II, and III
E) II, III, and IV
1 L 387 g 1000 mg 1 mole
A) All of the conversions are needed.
B) I and IV
C) III and IV
D) I, II, and III
E) II, III, and IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
35
Jane Doe has a cholesterol (C27H46O) count of 178 mg/dL. How many cholesterol molecules does Jane Doe have in each deciliter of blood?
A) 7.64 * 10-22
B) 1.14 * 10-22
C) 2.77 *1020
D) 4.45 * 1025
E) 2.77 * 1026
A) 7.64 * 10-22
B) 1.14 * 10-22
C) 2.77 *1020
D) 4.45 * 1025
E) 2.77 * 1026

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
36
What are the products of the reaction illustrated in Figure I? 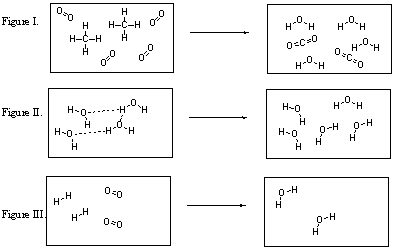
A) CH4 and O2
B) CO2 and H2O
C) CH4 only
D) O2 only
E) CO2 only

A) CH4 and O2
B) CO2 and H2O
C) CH4 only
D) O2 only
E) CO2 only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
37
How is a nuclear reaction different from a chemical reaction?
A) They are identical.
B) Only in a chemical reaction can atoms change into different atoms.
C) Chemical reactions involve only the nucleus of the atom.
D) Only in a nuclear reaction can atoms change into different atoms.
E) Nuclear reactions release energy and chemical reactions absorb energy.
A) They are identical.
B) Only in a chemical reaction can atoms change into different atoms.
C) Chemical reactions involve only the nucleus of the atom.
D) Only in a nuclear reaction can atoms change into different atoms.
E) Nuclear reactions release energy and chemical reactions absorb energy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
38
A chemical reaction involves many changes, but not everything changes during a reaction. Which of the following is NOT a change that occurs during a chemical reaction?
A) Atoms change in number or identity.
B) Bonds break.
C) Heat is released and/or absorbed.
D) New bonds form.
E) New molecules are formed.
A) Atoms change in number or identity.
B) Bonds break.
C) Heat is released and/or absorbed.
D) New bonds form.
E) New molecules are formed.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
39
Hydrogen fuel cells are a promising alternative to gasoline engines in part because they release water instead of carbon dioxide. In a hydrogen fuel cell, hydrogen gas (H2) and oxygen gas (O2) react to make water as shown below. Which of the following statements is a true statement about how this reaction occurs? 2 H2(g) + O2(g) → 2 H2O(g)
A) Two molecules of H2 and a molecule of O2 must collide at one time.
B) A molecule of H2 and a molecule of O2 collide.
C) An O-H bond must break.
D) An H-H bond must form.
E) The reactants must undergo a change of state for this reaction to occur.
A) Two molecules of H2 and a molecule of O2 must collide at one time.
B) A molecule of H2 and a molecule of O2 collide.
C) An O-H bond must break.
D) An H-H bond must form.
E) The reactants must undergo a change of state for this reaction to occur.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which of the following equations is used to convert 91.0 milligrams of urea (CH4N2O) to moles of urea?
A)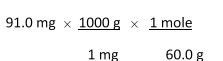
B)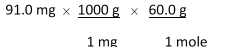
C)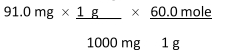
D)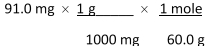
E)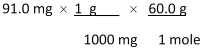
A)
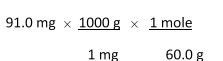
B)
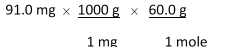
C)
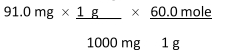
D)
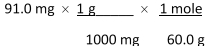
E)
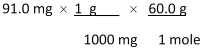

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
41
Below are several chemical equations, each labeled with the names of parts of a chemical equation. Which has the parts labeled correctly. 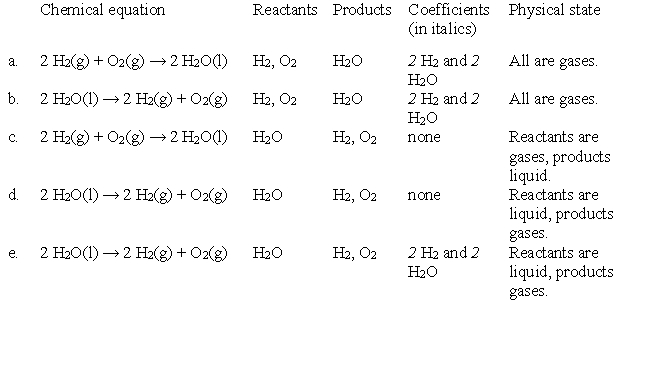
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
42
What does it mean for mass to be conserved?
A) Mass is neither created nor destroyed in the course of a chemical reaction.
B) Mass is a valuable resource and should not be wasted.
C) Molecules do not degrade under normal circumstances.
D) Atoms sometimes disappear over the course of a chemical reaction.
E) Atoms sometimes appear, disappear or are changed over the course of a chemical reaction.
A) Mass is neither created nor destroyed in the course of a chemical reaction.
B) Mass is a valuable resource and should not be wasted.
C) Molecules do not degrade under normal circumstances.
D) Atoms sometimes disappear over the course of a chemical reaction.
E) Atoms sometimes appear, disappear or are changed over the course of a chemical reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
43
Coefficients are important components of chemical equations. What is the significance of coefficients in a chemical equation? I. Coefficients indicate the ratio of masses of molecules.
II) Coefficients indicate the ratio of numbers of atoms and molecules.
III) Coefficients indicate the ratio of numbers of moles of molecules and atoms.
A) I only
B) II only
C) III only
D) I and II
E) II and III
II) Coefficients indicate the ratio of numbers of atoms and molecules.
III) Coefficients indicate the ratio of numbers of moles of molecules and atoms.
A) I only
B) II only
C) III only
D) I and II
E) II and III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
44
What is the meaning of the arrow in the following chemical equation? 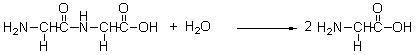
A) Heat is released from the reaction.
B) A product is substituted for a reactant.
C) The reactants collide.
D) The reactants are transformed into the products.
E) Heat is applied to the reactants.
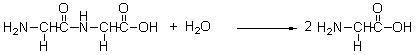
A) Heat is released from the reaction.
B) A product is substituted for a reactant.
C) The reactants collide.
D) The reactants are transformed into the products.
E) Heat is applied to the reactants.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
45
Which of the following characteristics of a chemical reaction is never included in a chemical equation?
A) the quantity of reactants
B) the relative proportions of reactants and products
C) the amount of energy absorbed or released by the reaction
D) the identity of the products and reactants
E) the physical state of the reactants and products
A) the quantity of reactants
B) the relative proportions of reactants and products
C) the amount of energy absorbed or released by the reaction
D) the identity of the products and reactants
E) the physical state of the reactants and products

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
46
The following chemical equation, the decomposition of hydrogen peroxide to water, is not balanced. What must be done to the equation to make it balanced? 2 H2O2 (l) → 2 H2O (l)
A) Change the coefficients from 2 to 1.
B) Add an atom of oxygen to the products.
C) Add a molecule of O2 to the products.
D) Change the state of matter in the products.
E) Change the subscripts of H2O to H2O2.
A) Change the coefficients from 2 to 1.
B) Add an atom of oxygen to the products.
C) Add a molecule of O2 to the products.
D) Change the state of matter in the products.
E) Change the subscripts of H2O to H2O2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
47
The balanced equation for nitrogen fixing is given below. What do the numbers in front of the molecules (the coefficients) mean? N2 + 3 H2 → 2 NH3
A) Three moles of H2 react with one mole of N2 to give two moles of NH3.
B) Three grams of H2 react with one gram of N2 to give two grams of NH3.
C) Three atoms of H react with one atom of N to give two molecules of NH3.
D) Two grams of N react with six grams of H to give eight grams of NH3.
E) All of the above
A) Three moles of H2 react with one mole of N2 to give two moles of NH3.
B) Three grams of H2 react with one gram of N2 to give two grams of NH3.
C) Three atoms of H react with one atom of N to give two molecules of NH3.
D) Two grams of N react with six grams of H to give eight grams of NH3.
E) All of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
48
What is the primary relationship between a chemical reaction and a chemical equation?
A) There is no relationship between a chemical reaction and a chemical equation.
B) A chemical equation is the written representation of a chemical reaction.
C) A chemical equation follows the law of conservation of mass but a chemical reaction does not.
D) A chemical equation shows the proportion of reactants and products but a chemical reaction does not necessarily reflect those proportions.
E) Chemical equations and chemical reactions are exactly the same thing.
A) There is no relationship between a chemical reaction and a chemical equation.
B) A chemical equation is the written representation of a chemical reaction.
C) A chemical equation follows the law of conservation of mass but a chemical reaction does not.
D) A chemical equation shows the proportion of reactants and products but a chemical reaction does not necessarily reflect those proportions.
E) Chemical equations and chemical reactions are exactly the same thing.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
49
Hydrogen peroxide loses its potency when it is stored for extended periods of time because it spontaneously breaks down. The following figure illustrates this breakdown, but it is missing a product molecule. Which of the following symbols represents the missing product in this reaction? 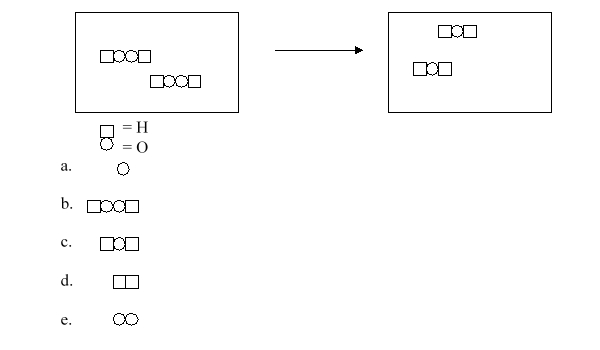
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
50
The following reaction is the formation of a peptide from two amino acids. This sort of reaction occurs in the body as part of the synthesis of proteins. This reaction is missing a component. Which of the following is missing? 2 NH2CH2COOH → NH2CH2CONHCH2COOH
A) Nothing is missing.
B) H2O is missing from the reactant side of the equation.
C) H2O is missing from the product side of the equation.
D) O2 is missing from the reactant side of the equation.
E) O2 is missing from the product side of the equation.
A) Nothing is missing.
B) H2O is missing from the reactant side of the equation.
C) H2O is missing from the product side of the equation.
D) O2 is missing from the reactant side of the equation.
E) O2 is missing from the product side of the equation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
51
During metabolism, table sugar (sucrose) is broken down into monosaccharides. Which of the following equations could you use to determine the moles of monosaccharides produced when 3 moles of sucrose react with 3 moles of water? 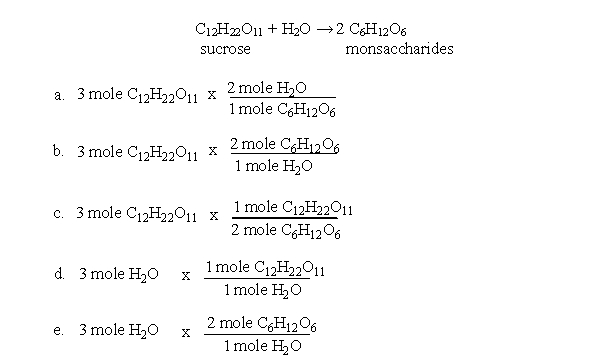
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
52
What does it mean for a chemical equation to be balanced?
A) A balanced chemical equation describes a chemical reaction found in nature.
B) A balanced chemical equation describes a chemical reaction that produces only some product, so there is an equal amount of reactant and product.
C) A balanced chemical equation contains an equal number of each type of atom on both sides of the equation.
D) A balanced chemical equation can run either forward or backward.
E) A balanced chemical equation represents a chemical reaction that is part of metabolism.
A) A balanced chemical equation describes a chemical reaction found in nature.
B) A balanced chemical equation describes a chemical reaction that produces only some product, so there is an equal amount of reactant and product.
C) A balanced chemical equation contains an equal number of each type of atom on both sides of the equation.
D) A balanced chemical equation can run either forward or backward.
E) A balanced chemical equation represents a chemical reaction that is part of metabolism.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
53
The industrial nitrogen fixing reaction used in the preparation of fertilizers and other nitrogen containing compounds is called the Haber process. The chemical equation for this process is shown below. According to this reaction, how many moles of ammonia would you produce if you reacted one mole of N2 with three moles of H2? N2 + 3 H2 → 2 NH3
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
54
In the body, glucose is broken down in the presence of oxygen into carbon dioxide and water. The balanced chemical equation for this reaction is shown below. According to this equation, how many molecules of carbon dioxide are produced when three molecules of glucose are metabolized in the presence of 18 moles of oxygen? C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
A) 0
B) 6
C) 12
D) 18
E) 24
A) 0
B) 6
C) 12
D) 18
E) 24

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
55
Which of the following reactions is balanced?
A) CH3CH2CH3 + O2 → CO2 + H2O
B) CH3CH2CH3 + O2 → 3 CO2 + 4 H2O
C) CH3CH2CH3 + 10 O2 → 3 CO2 + 4 H2O
D) CH3CH2CH3 + 5 O2 → 3 CO2 + 4 H2O
E) None of these are balanced.
A) CH3CH2CH3 + O2 → CO2 + H2O
B) CH3CH2CH3 + O2 → 3 CO2 + 4 H2O
C) CH3CH2CH3 + 10 O2 → 3 CO2 + 4 H2O
D) CH3CH2CH3 + 5 O2 → 3 CO2 + 4 H2O
E) None of these are balanced.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
56
The balanced equation for the combustion of propane is given below. What do the numbers in front of the molecules (the coefficients) mean? CH3CH2CH3 + O2 → 3 CO2 + 4 H2O
A) Three molecules of CO2 are produced when one molecule of CH3CH2CH3 reacts with one molecule of O2.
B) Six molecules of CO2 are produced when two molecules of CH3CH2CH3 react with two molecules of O2.
C) Nine moles of CO2 are produced when three moles of CH3CH2CH3 react with three moles of O2.
D) Four moles of H2O are produced when one mole of CH3CH2CH3 reacts with one mole of O2.
E) All of the above
A) Three molecules of CO2 are produced when one molecule of CH3CH2CH3 reacts with one molecule of O2.
B) Six molecules of CO2 are produced when two molecules of CH3CH2CH3 react with two molecules of O2.
C) Nine moles of CO2 are produced when three moles of CH3CH2CH3 react with three moles of O2.
D) Four moles of H2O are produced when one mole of CH3CH2CH3 reacts with one mole of O2.
E) All of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
57
Under what circumstances is mass conserved?
A) Mass is conserved only in the laboratory.
B) Mass is conserved only theoretically.
C) Mass is conserved everywhere except the human body.
D) Mass is conserved in nature, but not in the laboratory.
E) Mass is conserved during all chemical reactions.
A) Mass is conserved only in the laboratory.
B) Mass is conserved only theoretically.
C) Mass is conserved everywhere except the human body.
D) Mass is conserved in nature, but not in the laboratory.
E) Mass is conserved during all chemical reactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
58
Your friend combines vinegar (which contains acetic acid, CH3COOH) and baking soda (NaHCO3) and ends up with a runny foam. Your friend is pretty sure that the reaction produces water and carbon dioxide, which he can see just by looking at the foam, but he is unsure of what else the reaction produces. Given the partial reaction below, what do you tell your friend? NaHCO3 + CH3COOH → H2O + CO2 + ?
A) This reaction also produces CH3COONa.
B) This reaction also produces CH4.
C) This reaction also produces O2.
D) This reaction only produces H2O and CO2.
E) This reaction probably produces something else, but it is not possible to determine the identity of the other product(s).
A) This reaction also produces CH3COONa.
B) This reaction also produces CH4.
C) This reaction also produces O2.
D) This reaction only produces H2O and CO2.
E) This reaction probably produces something else, but it is not possible to determine the identity of the other product(s).

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
59
The following chemical equation is not balanced. What must be done to the equation to make it balanced? 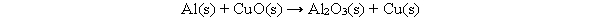
A) Change the coefficients to: 2 for Al, 3 for CuO and 3 for Cu.
B) Change the subscripts of CuO to CuO3.
C) Add a molecule of AlO.
D) Change the state of matter in the reactants.
E) Change the subscripts of Al2O3 to be AlO.
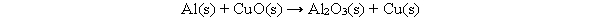
A) Change the coefficients to: 2 for Al, 3 for CuO and 3 for Cu.
B) Change the subscripts of CuO to CuO3.
C) Add a molecule of AlO.
D) Change the state of matter in the reactants.
E) Change the subscripts of Al2O3 to be AlO.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
60
During metabolism, table sugar (sucrose) is broken down into monosaccharides. According to the following chemical equation, how many moles of monosaccharides will be produced when 6 moles of sucrose react with 6 moles of water? C12H22O11 + H2O → 2 C6H12O6
Sucrose monosaccharides
A) 0
B) 2
C) 4
D) 6
E) 12
Sucrose monosaccharides
A) 0
B) 2
C) 4
D) 6
E) 12

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
61
A common, over-the-counter antacid is Al(OH)3. This antacid reacts with gastric juice (HCl) in the stomach, producing AlCl3 and H2O. Which of the following equations can be used to correctly determine how much gastric juice (HCl) reacts with an antacid tablet containing 0.25 grams Al(OH)3?
A)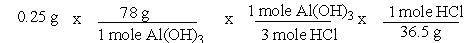
B)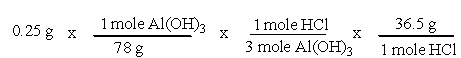
C)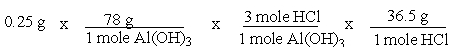
D)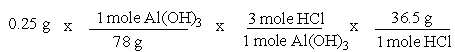
E)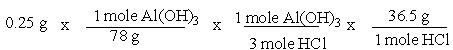
A)
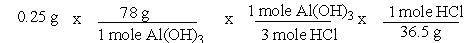
B)
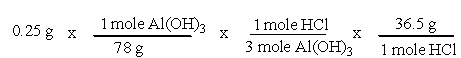
C)
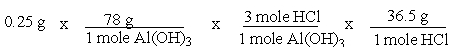
D)
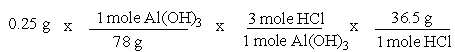
E)
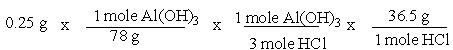

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
62
How many atoms of magnesium are present in the reactants of this chemical equation? Mg(OH)2 + 2 HCl → MgCl2 + 2 H2O
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
63
Combustion reactions are ___________ because products of the reaction are _________ in potential energy than the reactants.
A) endothermic; higher
B) exothermic; higher
C) endothermic; lower
D) exothermic; lower
E) endothermic; the same
A) endothermic; higher
B) exothermic; higher
C) endothermic; lower
D) exothermic; lower
E) endothermic; the same

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
64
The steps for performing stoichiometry calculations are diagramed below: 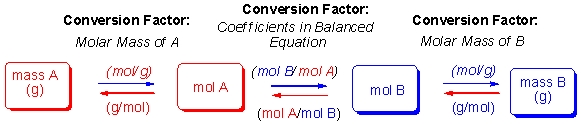 According to these steps, which of the following equations would allow you to calculate the yield of monosaccharides when 28 g of sucrose is broken down as shown in the following equation?
According to these steps, which of the following equations would allow you to calculate the yield of monosaccharides when 28 g of sucrose is broken down as shown in the following equation? 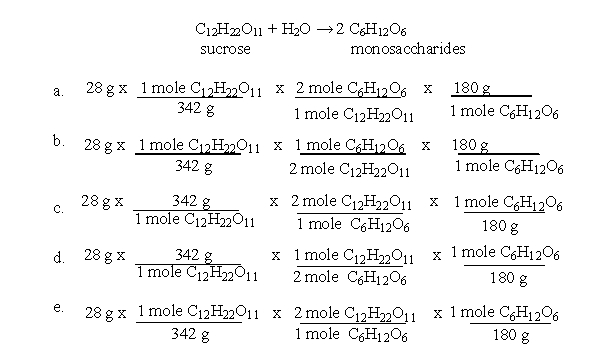
A)a
B)b
C)c
D)d
E)e
 According to these steps, which of the following equations would allow you to calculate the yield of monosaccharides when 28 g of sucrose is broken down as shown in the following equation?
According to these steps, which of the following equations would allow you to calculate the yield of monosaccharides when 28 g of sucrose is broken down as shown in the following equation? 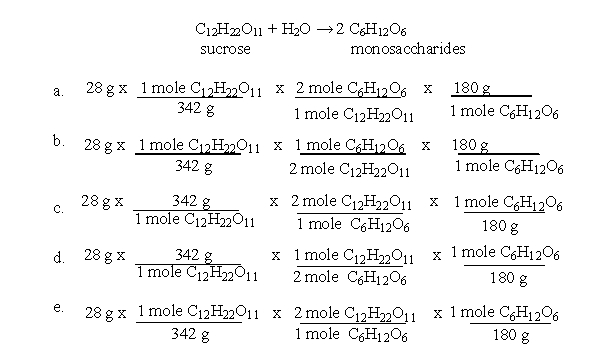
A)a
B)b
C)c
D)d
E)e

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
65
Pentane (C5H12) reacts with oxygen gas (O2) to form carbon dioxide (CO2) and water (H2O) according to the following reaction. What is the coefficient for carbon dioxide in the balanced equation? C5H12 + _?_ O2 → _?_ CO2 + _?_ H2O
A) 2
B) 4
C) 5
D) 6
E) 8
A) 2
B) 4
C) 5
D) 6
E) 8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
66
The balanced chemical equation for the combustion of propane (C3H8) is given below. How many grams of carbon dioxide (CO2) are released when 35 g of propane are burned in the presence of excess oxygen? C3H8 + 5 O2 → 3 CO2 + 4 H2O
A) 0.80 g
B) 12 g
C) 35 g
D) 105 g
E) 1500 g
A) 0.80 g
B) 12 g
C) 35 g
D) 105 g
E) 1500 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
67
How many atoms of hydrogen are present in the products of this chemical equation? Mg(OH)2 + 2 HCl → MgCl2 + 2 H2O
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
68
Pentane (C5H12) reacts with oxygen gas (O2) to form carbon dioxide (CO2) and water (H2O) according to the following reaction. What is the coefficient for oxygen in the balanced equation? C5H12 + _?_ O2 → _?_ CO2 + _?_ H2O
A) 2
B) 4
C) 5
D) 6
E) 8
A) 2
B) 4
C) 5
D) 6
E) 8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
69
The name for the heat energy released or absorbed in a chemical reaction is
A) energy.
B) endothermic.
C) exothermic.
D) enthalpy.
E) entropy.
A) energy.
B) endothermic.
C) exothermic.
D) enthalpy.
E) entropy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
70
According to the balanced chemical equation below, if, you react 28 grams of butene with excess hydrogen, how many grams of butane would you expect to get? 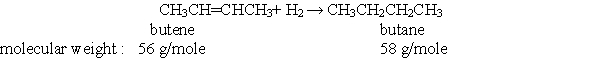
A) 0.0 g
B) 29 g
C) 58 g
D) 112 g
E) 116 g
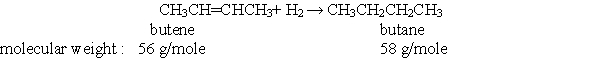
A) 0.0 g
B) 29 g
C) 58 g
D) 112 g
E) 116 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
71
Is this chemical equation balanced? Mg(OH)2 + 2 HCl → MgCl2 + 2 H2O
A) No. The number of magnesiums in the reactants is different from the number in the products.
B) No. The number of hydrogens in the reactants is different from the number in the products.
C) No. The number of oxygens in the reactants is different from the number in the products.
D) No. The number of chlorines in the reactants is different from the number in the products.
E) Yes. The same number of each type of atom is present in the reactants and products.
A) No. The number of magnesiums in the reactants is different from the number in the products.
B) No. The number of hydrogens in the reactants is different from the number in the products.
C) No. The number of oxygens in the reactants is different from the number in the products.
D) No. The number of chlorines in the reactants is different from the number in the products.
E) Yes. The same number of each type of atom is present in the reactants and products.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
72
Pentane (C5H12) reacts with oxygen gas (O2) to form carbon dioxide (CO2) and water (H2O) according to the following reaction. What is the coefficient for water in the balanced equation? 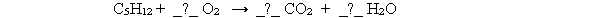
A) 2
B) 4
C) 5
D) 6
E) 8
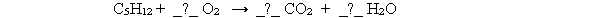
A) 2
B) 4
C) 5
D) 6
E) 8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
73
Acetylene (C2H2) is a small organic molecule used in the industrial preparation of many other molecules and also in welding. Acetylene is made from calcium carbide and water, resulting in acetylene and also calcium hydroxide. Which of the following choices is the correct balanced chemical equation for the preparation of acetylene?
A) CaC2 + H2O → CaO + C2H2
B) CaC2 + H2O → Ca(OH)2 + C2H2
C) CaC2 + 2 H2O → Ca(OH)2 + C2H2
D) CaC2 + 2 H2O → CaO + C2H2 + H2O
E) 2 CaC2 + H2O → Ca(OH)2 + 2 C2H2
A) CaC2 + H2O → CaO + C2H2
B) CaC2 + H2O → Ca(OH)2 + C2H2
C) CaC2 + 2 H2O → Ca(OH)2 + C2H2
D) CaC2 + 2 H2O → CaO + C2H2 + H2O
E) 2 CaC2 + H2O → Ca(OH)2 + 2 C2H2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
74
According to the balanced chemical equation below, if you react 3.0 mole of butene with 3.0 mole of hydrogen, how many moles of butane would you expect to get? 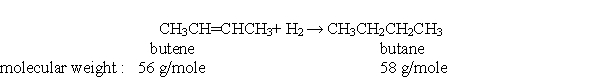
A) 0.0 moles
B) 1.0 mole
C) 2.0 moles
D) 3.0 moles
E) 6.0 moles
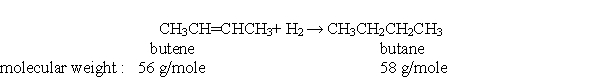
A) 0.0 moles
B) 1.0 mole
C) 2.0 moles
D) 3.0 moles
E) 6.0 moles

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
75
Which of the following biological molecules are the major nutrients that make up the food that we eat?
I. proteins
II. nucleic acids
III. steroids
IV. fats
V. carbohydrates
A) All of these are major nutrients.
B) I, II, IV, and V
C) I and V
D) III, IV, and V
E) I, IV, and V
I. proteins
II. nucleic acids
III. steroids
IV. fats
V. carbohydrates
A) All of these are major nutrients.
B) I, II, IV, and V
C) I and V
D) III, IV, and V
E) I, IV, and V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
76
By moving, breathing, and living, you do work every day. Work requires energy. Where does this energy come from?
A) The energy comes from food; it undergoes chemical reactions in the cells.
B) The energy does not come from anyplace; it is always in our bodies.
C) The energy comes from breaking bonds in food.
D) The energy comes from breaking bonds in food, oxygen, and water.
E) No one really understands where this energy comes from.
A) The energy comes from food; it undergoes chemical reactions in the cells.
B) The energy does not come from anyplace; it is always in our bodies.
C) The energy comes from breaking bonds in food.
D) The energy comes from breaking bonds in food, oxygen, and water.
E) No one really understands where this energy comes from.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
77
How many atoms of oxygen are present in the reactants of this chemical equation? Mg(OH)2 + 2 HCl → MgCl2 + 2 H2O
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
78
The balanced chemical equation for the combustion of butane is given below. How many grams of O2 are needed to completely react with 5.0 g of butane in a butane lighter? 2 C4H10 + 13 O2 → 8 CO2 + 10 H2O
A) 0.42 g
B) 2.8 g
C) 6.0 g
D) 9.1 g
E) 18 g
A) 0.42 g
B) 2.8 g
C) 6.0 g
D) 9.1 g
E) 18 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
79
According to the balanced chemical equation below, if you react 3.0 mole of butene with 3.0 mole of hydrogen, how many grams of butane would you expect to get? 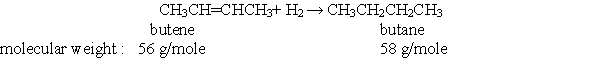
A) 0.0 grams
B) 3.0 gram
C) 58 grams
D) 56 grams
E) 170 grams
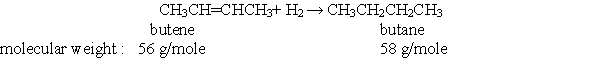
A) 0.0 grams
B) 3.0 gram
C) 58 grams
D) 56 grams
E) 170 grams

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck
80
Which of the following reactions is NOT balanced?
A) 2 Al + 6 HCl → 2 AlCl3 + 3 H2
B) Fe2O3 + 2 Al → 2 Fe + Al2O3
C) C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
D) C2H5OH + O2 → 2 CO2 + 3 H2O
E) CaO + 3 C → CaC2 + CO
A) 2 Al + 6 HCl → 2 AlCl3 + 3 H2
B) Fe2O3 + 2 Al → 2 Fe + Al2O3
C) C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
D) C2H5OH + O2 → 2 CO2 + 3 H2O
E) CaO + 3 C → CaC2 + CO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 135 في هذه المجموعة.
فتح الحزمة
k this deck








