Deck 3: An Introduction to Organic Compounds
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/102
العب
ملء الشاشة (f)
Deck 3: An Introduction to Organic Compounds
1
Which of the following is a tertiary amine?
A)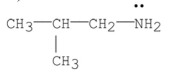
B)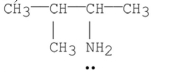
C)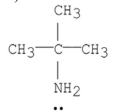
D)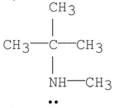
E)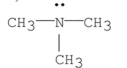
A)

B)

C)

D)

E)


2
Which of the following compounds diisopropyl ether?
A)
B)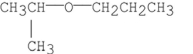
C)
D)
E)
A)
B)
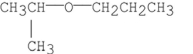
C)
D)
E)
3
Which of the following is sec-butyl alcohol?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)
4
What is the common name of the following compound? 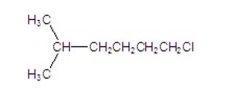
A) heptyl chloride
B) sec-heptyl chloride
C) isoheptyl chloride
D) tert-heptyl chloride
E) n-heptyl chloride
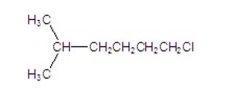
A) heptyl chloride
B) sec-heptyl chloride
C) isoheptyl chloride
D) tert-heptyl chloride
E) n-heptyl chloride

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
5
How many hydrogens are in limonene? 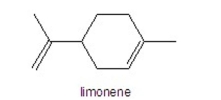
A) 8
B) 10
C) 12
D) 16
E) 18

A) 8
B) 10
C) 12
D) 16
E) 18

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
6
There are 8 alkyl bromides with a molecular formula of C5H11Br. How many of these are tertiary alkyl bromides?
A) 0
B) 1
C) 2
D) 3
E) 8
A) 0
B) 1
C) 2
D) 3
E) 8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
7
How many constitutional isomers have a molecular formula of C6H14?
A) 4
B) 5
C) 6
D) 7
E) 8
A) 4
B) 5
C) 6
D) 7
E) 8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which of the following compounds has the highest boiling point?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
9
What is the name of the following compound?
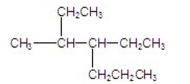
A) 2-ethyl-3-propylpentane
B) 2,3-diethylhexane
C) 4-ethyl-5-methylheptane
D) 4-methyl-3-ethylheptane
E) 4-ethyl-3-methylheptane

A) 2-ethyl-3-propylpentane
B) 2,3-diethylhexane
C) 4-ethyl-5-methylheptane
D) 4-methyl-3-ethylheptane
E) 4-ethyl-3-methylheptane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
10
What is the name of the following compound?
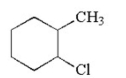
A) 1-chloro-2-methylcyclohexane
B) 1-methyl-2-chlorocyclohexane
C) 1 -chloro-5-methylcyclohexane
D) 1-methyl-5-chlorocyclohexane
E) 1, k2-chloromethylcyclohexane

A) 1-chloro-2-methylcyclohexane
B) 1-methyl-2-chlorocyclohexane
C) 1 -chloro-5-methylcyclohexane
D) 1-methyl-5-chlorocyclohexane
E) 1, k2-chloromethylcyclohexane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
11
What is the name of the following compound?
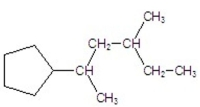
A) 2-cyclopentyl-4-methylhexane
B) 5 -cyclopentyl-3-methylhexane
C) 1 -cyclopentyl-1,3-dimethylpentane
D) 2 -cyclopentyl-4-methylheptane
E) 5 -cyclopentyl-3-methylheptane

A) 2-cyclopentyl-4-methylhexane
B) 5 -cyclopentyl-3-methylhexane
C) 1 -cyclopentyl-1,3-dimethylpentane
D) 2 -cyclopentyl-4-methylheptane
E) 5 -cyclopentyl-3-methylheptane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which of the following compounds cannot form hydrogen bonds between its molecules?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
13
What is the IUPAC name of the following compound?
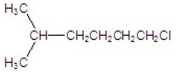
A) 2-methylheptane
B) 1-chloro-5-methylhexane
C) 6-chloro-2-methylhexane
D) 1,1-dimethyl-5-chloropentane
E) 5-methyl-1-chlorohexane
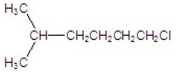
A) 2-methylheptane
B) 1-chloro-5-methylhexane
C) 6-chloro-2-methylhexane
D) 1,1-dimethyl-5-chloropentane
E) 5-methyl-1-chlorohexane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of the following has the largest bond angle?
A) the C-O-C bond in an ether
B) the C-N-C bond in a secondary amine
C) the C-C-C bond in an alkane
D) the C-O-C bond in an alcohol
E) They are all equal.
A) the C-O-C bond in an ether
B) the C-N-C bond in a secondary amine
C) the C-C-C bond in an alkane
D) the C-O-C bond in an alcohol
E) They are all equal.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
15
What is the common name of the following compound? 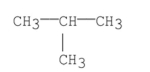
A) isobutane
B) isopropylmethane
C) tert-butane
D) n-butane
E) sec-butane

A) isobutane
B) isopropylmethane
C) tert-butane
D) n-butane
E) sec-butane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
16
How many tertiary carbons are in the following compound? 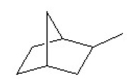
A) 2
B) 3
C) 4
D) 5
E) 6

A) 2
B) 3
C) 4
D) 5
E) 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
17
What is the name of the following compound?
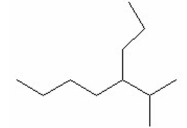
A) 2-methyl-3-ethylheptane
B) 3-ethyl-2-methylheptane
C) 5 -isopropyloctane
D) 4-isopropyloctane
E) 2-methyl-3-propylheptane

A) 2-methyl-3-ethylheptane
B) 3-ethyl-2-methylheptane
C) 5 -isopropyloctane
D) 4-isopropyloctane
E) 2-methyl-3-propylheptane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
18
What is the molecular formula of the following compound?
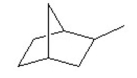
A)
B)
C)
D)
E)

A)
B)
C)
D)
E)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
19
What is the common name of the following compound?
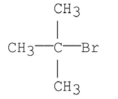
A) Isobutyl bromide
B)
C) Neobutyl bromide
D)
E) Isopropyl methyl bromide

A) Isobutyl bromide
B)
C) Neobutyl bromide
D)
E) Isopropyl methyl bromide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
20
If an acyclic alkane contains n carbons, how many hydrogens does it contain?
A) n
B) n+2
C) n-2
D) 2n
E) 2n + 2
A) n
B) n+2
C) n-2
D) 2n
E) 2n + 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which of the following is a staggered conformer for rotation about the Cl-C2 bond in the following compound? 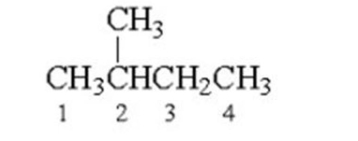
I.
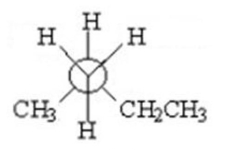
II.
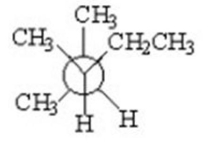
III.
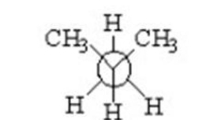
IV.
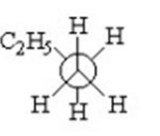
V.
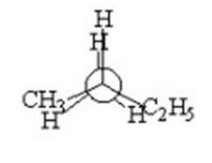
A) I
B) II
C) III
D) IV
E) V
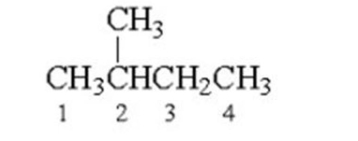
I.

II.

III.

IV.

V.

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
22
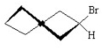
Which of the following is the most stable conformer of bromocyclohexane? I.
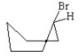
II.
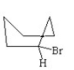
III.
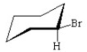
IV.
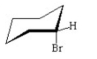
V.
A) I
B) II
C) III
D) IV
E) V

II.

III.

IV.

V.

A) I
B) II
C) III
D) IV
E) V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which of the following compounds has the greatest van der Waal's interactions between its molecules?
A)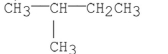
B)
C)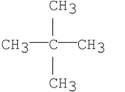
D)
E)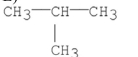
A)
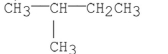
B)
C)

D)
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
24
Assuming roughly equivalent molecular weights, which of the following compounds has the highest boiling point?
A) a tertiary amine
B) a quaternary ammonium salt
C) an alcohol
D) an ether
E) an alkyl chloride
A) a tertiary amine
B) a quaternary ammonium salt
C) an alcohol
D) an ether
E) an alkyl chloride

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which of the following describes the most stable conformer of methylcyclohexane?
A) Both groups are equatorial.
B) Both groups are axial.
C) The tert-butyl group is equatorial and the methyl group is axial.
D) The tert-butyl group is axial and the methyl group is equatorial.
E) none of the above
A) Both groups are equatorial.
B) Both groups are axial.
C) The tert-butyl group is equatorial and the methyl group is axial.
D) The tert-butyl group is axial and the methyl group is equatorial.
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which conformer has the greatest energy in the potential energy diagram for chair-chair interconversion of cyclohexane?
A) twist-boat
B) planar
C) boat
D) half-chair
E) chair
A) twist-boat
B) planar
C) boat
D) half-chair
E) chair

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which of the following compounds has the highest boiling point?
A)
B)
C)
D)
E)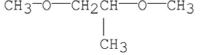
A)
B)
C)
D)
E)
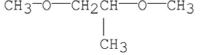

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which of the following compounds cannot form hydrogen bonds between its molecules?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
29
Identify the relative positions of the methyl groups in the most stable conformer of butane viewed along th C2-C3 bond?
A) anti
B) eclipsed
C) gauche
D) totally eclipsed
E) adjacent
A) anti
B) eclipsed
C) gauche
D) totally eclipsed
E) adjacent

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which of the following compounds is the most soluble H2CO?
A) CH3OCH3
B) CH3CH2OH
C) CH3CH2Cl
D) CH3CH2CH3
E) CH3CHO
A) CH3OCH3
B) CH3CH2OH
C) CH3CH2Cl
D) CH3CH2CH3
E) CH3CHO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
31
The eclipsed and staggered forms of ethane differ in ________.
A) molecular formula
B) configuration
C) conformation
D) constitution
E) structure
A) molecular formula
B) configuration
C) conformation
D) constitution
E) structure

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
32
List n-hexane, 2, 3-dimethylbutane, and 2-methylpentane in order of increasing boiling point.
A) 2,3 -dimethylbutane <2 -methylpentane
B) 2 -methylpentane
C) 2 -methylpentane <2,3 -dimethylbutane
D) n -hexane <2 -methylpentane <2,3 -dimethylbutane
E) n -hexane <2,3 -dimethylbutane <2 -methylpentane
A) 2,3 -dimethylbutane <2 -methylpentane
B) 2 -methylpentane
C) 2 -methylpentane <2,3 -dimethylbutane
D) n -hexane <2 -methylpentane <2,3 -dimethylbutane
E) n -hexane <2,3 -dimethylbutane <2 -methylpentane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following statements about the conformers that result from rotation about the C2-C3 bond of butane is correct?
A) The highest energy conformer is one in which methyl groups are eclipsed by hydrogens.
B) The gauche conformer is eclipsed.
C) Steric strain is absent in an eclipsed conformer.
D) An eclipsed conformer is more stable than a staggered conformer.
E) none of the above
A) The highest energy conformer is one in which methyl groups are eclipsed by hydrogens.
B) The gauche conformer is eclipsed.
C) Steric strain is absent in an eclipsed conformer.
D) An eclipsed conformer is more stable than a staggered conformer.
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which of the following compounds has the lowest boiling point?
A) CH3Cl
B) CH4
C) CH2Cl2
D) CHCl3
E) CCl4
A) CH3Cl
B) CH4
C) CH2Cl2
D) CHCl3
E) CCl4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
35
Which of the following correctly ranks the cycloalkanes in order of increasing ring strain?
A) cyclopropane < cyclobutane < cyclohexane < cycloheptane
B) cyclohexane < cyclopentane < cyclobutane < cyclopropane
C) cyclopentane < cyclobutane < cyclopentane < cyclopropane
D) cyclopentane < cyclopropane < cyclobutane < cyclohexane
E) cyclopropane < cyclopentane < cyclobutane < cyclohexane
A) cyclopropane < cyclobutane < cyclohexane < cycloheptane
B) cyclohexane < cyclopentane < cyclobutane < cyclopropane
C) cyclopentane < cyclobutane < cyclopentane < cyclopropane
D) cyclopentane < cyclopropane < cyclobutane < cyclohexane
E) cyclopropane < cyclopentane < cyclobutane < cyclohexane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
36
What is the strongest intermolecular force between molecules of ethyl alcohol?
A) induced dipole-induced dipole
B) dipole-dipole, specifically hydrogen bonding
C) dipole-dipole, but not hydrogen bonding
D) ion-dipole
E) ion-ion
A) induced dipole-induced dipole
B) dipole-dipole, specifically hydrogen bonding
C) dipole-dipole, but not hydrogen bonding
D) ion-dipole
E) ion-ion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which of the following correctly lists the conformers of cyclohexane in order of increasing energy?
A) chair < boat < twist-boat < half-chair
B) half-chair < boat < twist-boat < chair
C) chair < twist-boat < half-chair < boat
D) chair < twist-boat < boat < half-chair
E) half-chair < twist-boat < boat < chair
A) chair < boat < twist-boat < half-chair
B) half-chair < boat < twist-boat < chair
C) chair < twist-boat < half-chair < boat
D) chair < twist-boat < boat < half-chair
E) half-chair < twist-boat < boat < chair

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which conformers of butane, viewed along the Cl-C2 bond, has the lowest energy?
A) the gauche conformer
B) the eclipsed and gauche conformers
C) the anti conformer
D) the eclipsed conformer
E) the anti and gauche conformers
A) the gauche conformer
B) the eclipsed and gauche conformers
C) the anti conformer
D) the eclipsed conformer
E) the anti and gauche conformers

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
39
Which of the following compounds has the lowest boiling point?
A)
B)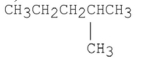
C)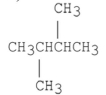
D)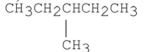
E) CH3CH2CH2CH2CH2CH2CH3
A)
B)
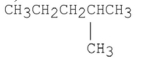
C)

D)
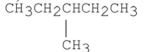
E) CH3CH2CH2CH2CH2CH2CH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which of the following has the greatest solubility ?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
41
How many secondary hydrogens does the following compound have? 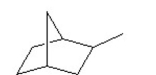
A) 4
B) 6
C) 8
D) 10
E) 12

A) 4
B) 6
C) 8
D) 10
E) 12

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
42
Which of the following is the least the least stable conformer of 1-tert-butyl-3- methylcyclohexane.
A) tert-butyl is axial and the methyl is equatorial.
B) tert-butyl is axial and the methyl is axial.
C) tert-butyl is equatorial and the methyl is axial.
D) tert-butyl is equatorial and the methyl is equatorial.
E) All are equally stable.
A) tert-butyl is axial and the methyl is equatorial.
B) tert-butyl is axial and the methyl is axial.
C) tert-butyl is equatorial and the methyl is axial.
D) tert-butyl is equatorial and the methyl is equatorial.
E) All are equally stable.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
43
Draw 6-ethyl-2,6,7-trimethyl-5-propylnonane.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
44
Name the alkane shown below. 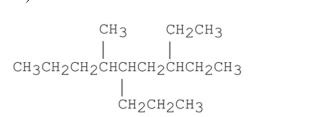


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
45
Which of the following has a conformer with two equatorial alkyl substituents?
A) 1,1 -dimethylcyclohexane
B) cis-1,2-dimethylcyclohexane
C) cis-1,3-diethylcyclohexane
D) cis-1,4-diethylcyclohexane
E) trans-1,3-diethylcyclohexane
A) 1,1 -dimethylcyclohexane
B) cis-1,2-dimethylcyclohexane
C) cis-1,3-diethylcyclohexane
D) cis-1,4-diethylcyclohexane
E) trans-1,3-diethylcyclohexane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
46
How many hydrogens are attached to the indicated carbon? 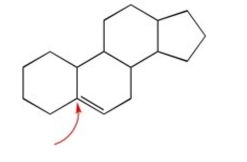
A) zero
B) one
C) two
D) three

A) zero
B) one
C) two
D) three

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
47
Name the alkane shown below. 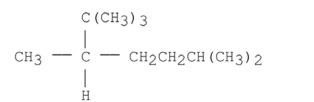
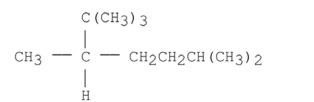

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
48
Does this Newman projection depict the conformer of the molecule with the lowest-energy? 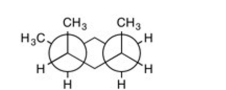
A) Yes
B) No
C) There is not enough information to tell.

A) Yes
B) No
C) There is not enough information to tell.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
49
Draw 4-isopropyl-2-methylheptane.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
50
Name the alkane shown below. 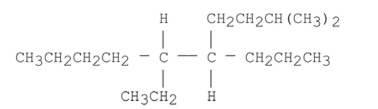


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
51
Name the alkane shown below. 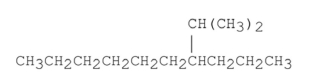
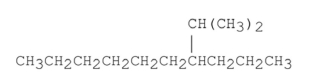

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
52
2-Ethylpropane is not a correct name. Draw the compound and name it correctly.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
53
Name the alkane shown below. 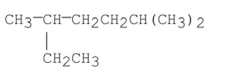
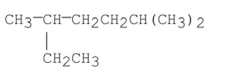

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
54
How many hydrogens are attached to the indicated carbon? 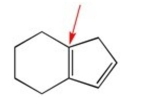
A) zero
B) one
C) two
D) three

A) zero
B) one
C) two
D) three

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
55
Name the compound shown below.
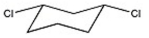
A) trans-1,2-dichlorocyclohexane
B) cis-1,2-dichlorocyclohexane
C) trans-1,3-dichlorocyclohexane
D) cis-1,3-dichlorocyclohexane
E) trans-1,4 -dichlorocyclohexane

A) trans-1,2-dichlorocyclohexane
B) cis-1,2-dichlorocyclohexane
C) trans-1,3-dichlorocyclohexane
D) cis-1,3-dichlorocyclohexane
E) trans-1,4 -dichlorocyclohexane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
56
Name the alkane shown below. 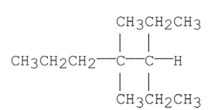


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
57
Draw 3-ethyl-3-methylhexane.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
58
Draw 4-tert-butyloctane.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
59
How many hydrogens are attached to the indicated carbon? 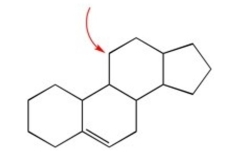
A) zero
B) one
C) two
D) three

A) zero
B) one
C) two
D) three

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
60
Which of the following has a conformer with one equatorial substituent and one axial substituent?
A) trans-1,4-dibromocyclohexane
B) cis-1,4-dibromocyclohexane
C) cis-1,3-dibromocyclohexane
D) trans-1,2-dibromocyclohexane
E) none of them
A) trans-1,4-dibromocyclohexane
B) cis-1,4-dibromocyclohexane
C) cis-1,3-dibromocyclohexane
D) trans-1,2-dibromocyclohexane
E) none of them

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
61
Name the cycloalkane shown below. 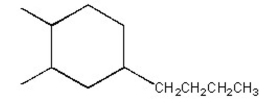


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
62
Draw the structure for isopentyl alcohol.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
63
Classify the following amines as primary, secondary, or tertiary. 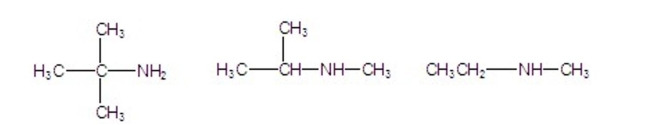


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
64
Explain why the carbon-fluorine bond in 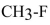 is shorter than the carbon-chlorine bond in
is shorter than the carbon-chlorine bond in 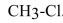 .
.
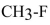 is shorter than the carbon-chlorine bond in
is shorter than the carbon-chlorine bond in 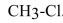 .
.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
65
Name the compound shown below. 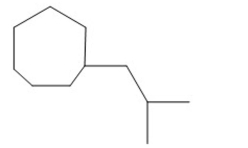


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
66
Which of the compounds shown below has the higher boiling point? Explain your choice. CH3CH2CH2OH or CH3CH2OCH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
67
Name the alkane shown below. 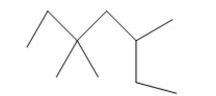


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
68
Name the alkane shown below. 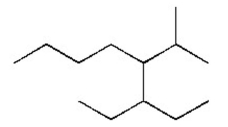


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
69
Would you expect sodium chloride (NaCl) to be soluble in hexane? Explain your answer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
70
Draw all ethers with molecular formula C4H10O.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
71
Name the compound shown below. 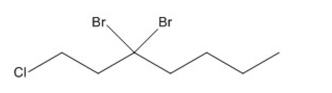


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
72
Draw all possible constitutional isomers for C2H6O and give a common name for each isomer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
73
Which compound is more soluble in water? Explain your choice. CH3OCH3 or CH3CH2OH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
74
Explain why primary and secondary amines form hydrogen bonds but tertiary amines do not.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
75
Name the alkane shown below. 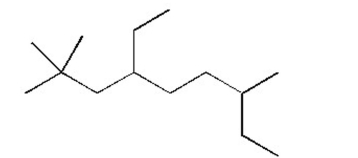


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
76
Name the compound shown below. 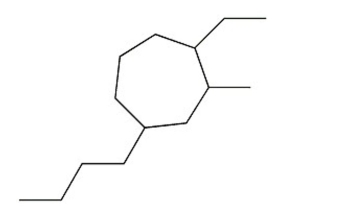


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
77
Explain why the compound shown below has a lower boiling point than CH3CH2CH2CH3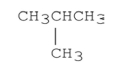


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
78
Name the alkane shown below. 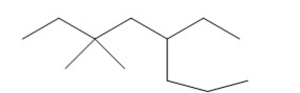


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
79
Draw the structure for sec-butylcyclopentane.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck
80
Explain why trimethylamine, 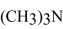 , has a considerably lower boiling point than
, has a considerably lower boiling point than
propylamine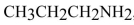 , even though both compounds have the same molecular formula.
, even though both compounds have the same molecular formula.
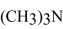 , has a considerably lower boiling point than
, has a considerably lower boiling point thanpropylamine
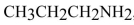 , even though both compounds have the same molecular formula.
, even though both compounds have the same molecular formula.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 102 في هذه المجموعة.
فتح الحزمة
k this deck








