Deck 5: Alkenes Thermodynamics and Kinetics
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/56
العب
ملء الشاشة (f)
Deck 5: Alkenes Thermodynamics and Kinetics
1
Which of the curved arrows are correct for this reaction? 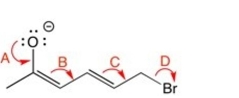
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D
A
B
C
D
B
C
D
2
Name the following compound:
A) vinyl iodide
B) allyl iodide
C) 1-iodo-2-propene
D) iodomethylethene
E) 2 -iodo-1-propene
A) vinyl iodide
B) allyl iodide
C) 1-iodo-2-propene
D) iodomethylethene
E) 2 -iodo-1-propene
allyl iodide
3
Which of the curved arrows are correct for this reaction? 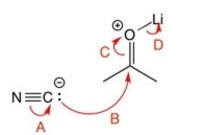
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D
B
C
C
4
Which set of curved arrows correctly shows the flow of electrons in this reaction?
A)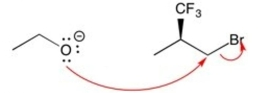
B)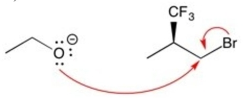
C)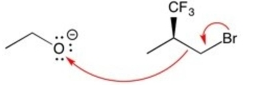
D)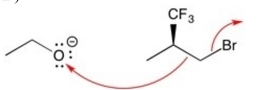
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which of the following statements about ethene C2H4, is not correct?
A) The H-C-H bond angles are approximately 109.5°.
B) All of the hydrogen atoms are in the same plane.
C) It has five sigma bonds.
D) The carbon atoms are sp2 hybridized.
E) The bond angles are approximately 120°.
A) The H-C-H bond angles are approximately 109.5°.
B) All of the hydrogen atoms are in the same plane.
C) It has five sigma bonds.
D) The carbon atoms are sp2 hybridized.
E) The bond angles are approximately 120°.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
6
Name the following compound:
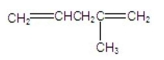
A) 2-methyl-2,4-pentadiene
B) 4-methyl-1,4-pentadiene
C) 2-methylene-4-pentene
D) 4-methylene-2-pentene
E) 2-methyl-1,4-pentadiene

A) 2-methyl-2,4-pentadiene
B) 4-methyl-1,4-pentadiene
C) 2-methylene-4-pentene
D) 4-methylene-2-pentene
E) 2-methyl-1,4-pentadiene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the curved arrows are correct for this reaction? 
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
8
How many transition states are in the following reaction coordinate diagram?
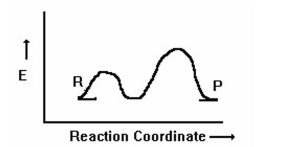
A) 3
B) 4
C) 5
D) 2
E) 1

A) 3
B) 4
C) 5
D) 2
E) 1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
9
Name the following compound:
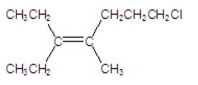
A) 7 -chloro-3-ethyl-4-methyl-3-heptene
B) 1-chloro-5-ethyl-4-methyl-3-heptene
C) 1-chloro-3-pentenyl-2-pentene
D) cis-7-chloro-3-ethyl-4-methyl-3-heptene
E) trans- 7 -chloro-3-ethyl-4-methyl-3-heptene

A) 7 -chloro-3-ethyl-4-methyl-3-heptene
B) 1-chloro-5-ethyl-4-methyl-3-heptene
C) 1-chloro-3-pentenyl-2-pentene
D) cis-7-chloro-3-ethyl-4-methyl-3-heptene
E) trans- 7 -chloro-3-ethyl-4-methyl-3-heptene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which of the following is vinyl chloride?
A)
B)
C)
D)
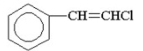
E)
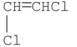
A)
B)
C)
D)

E)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
11
What is the name of the following compound?
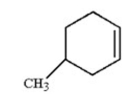
A) 5-methylcyclohexene
B) 4-methylcyclohexene
C) 1-methyl-3-cyclohexene
D) 1-methyl-4-cyclohexene
E) methylcyclohexene

A) 5-methylcyclohexene
B) 4-methylcyclohexene
C) 1-methyl-3-cyclohexene
D) 1-methyl-4-cyclohexene
E) methylcyclohexene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which of the curved arrows are correct for this reaction? 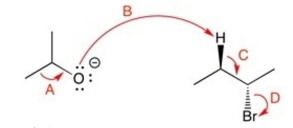
A) A
B) B
C) C
D) D

A) A
B) B
C) C
D) D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
13
Name the following compound:
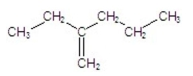
A) 2-ethyl-1-pentene
B) 2-propyl-1-butene
C) 3-methylenehexane
D) 3-methyl-3-hexene
E) ethyl propyl ethene

A) 2-ethyl-1-pentene
B) 2-propyl-1-butene
C) 3-methylenehexane
D) 3-methyl-3-hexene
E) ethyl propyl ethene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
14
How many carbons are in the planar double-bond system of 3-methylcyclopentene?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which of the following statements about propene , is correct?
A) All nine atoms lie in the same plane.
B) The compound has a cis and trans isomer.
C) It is a Lewis acid.
D) It has eight sigma bonds.
E) All the carbon atoms are hybridized.
A) All nine atoms lie in the same plane.
B) The compound has a cis and trans isomer.
C) It is a Lewis acid.
D) It has eight sigma bonds.
E) All the carbon atoms are hybridized.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
16
What is the following compound's common name?
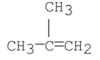
A) t-butylene
B) sec-butylene
C) isobutylene
D) butylene
E) methylpropylene

A) t-butylene
B) sec-butylene
C) isobutylene
D) butylene
E) methylpropylene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
17
What is the systematic name of the following compound?
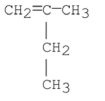
A) 2-methyl-1-butene
B) isopentene
C) 2-methybutene
D) 2-ethylpropene
E) 3-methyl-3-butene

A) 2-methyl-1-butene
B) isopentene
C) 2-methybutene
D) 2-ethylpropene
E) 3-methyl-3-butene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
18
Name the following compound:
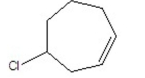
A) 4-chlorocyclohexene
B) 1-chloro-3-cyclohexene
C) 1-chloro-3-cycloheptene
D) 4-chlorocycloheptane
E) 4-chlorocycloheptene

A) 4-chlorocyclohexene
B) 1-chloro-3-cyclohexene
C) 1-chloro-3-cycloheptene
D) 4-chlorocycloheptane
E) 4-chlorocycloheptene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which of the following is not a nucleophile?
A)H+
B) Br-
C) NH3
D)
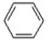
E) CH3OCH3
A)H+
B) Br-
C) NH3
D)

E) CH3OCH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which of the following is not an electrophile?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which of the following describes the reaction shown below?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
22
Draw the structure of vinyl bromide.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
23
An increase in which of the following will occur if the reaction temperature is increased?
I. energy of activation
II) collision frequency
III) fraction of collisions with sufficient energy
A) I and II
B) I and III
C) II and III
D) I, II, and III
E) I
I. energy of activation
II) collision frequency
III) fraction of collisions with sufficient energy
A) I and II
B) I and III
C) II and III
D) I, II, and III
E) I

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
24
Name the following compound: 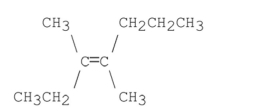


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
25
Draw the structure of 1,3-cyclopentadiene.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
26
The Gibbs free energy shows that the free energy ° ________.
A) increases as both Keq and T increase
B) increases most when Keq increases and T decreases
C) increases most when Keq decreases and T increases
D) increases as both Keq and T decrease
E) increases as reactant concentrations increase
A) increases as both Keq and T increase
B) increases most when Keq increases and T decreases
C) increases most when Keq decreases and T increases
D) increases as both Keq and T decrease
E) increases as reactant concentrations increase

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
27
Name the following compound: 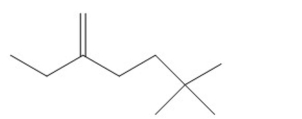


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which step has the greatest activation energy in the reverse direction? 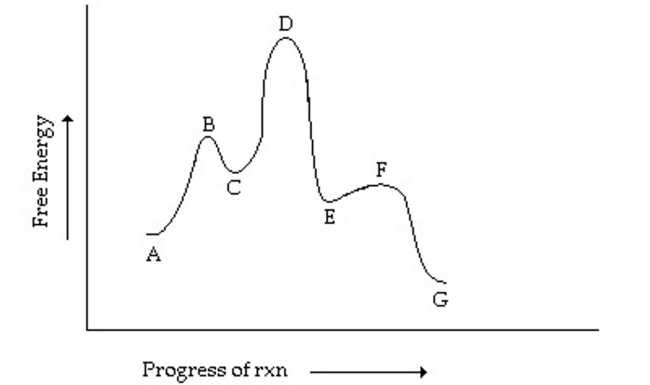
A) A going to C
B) C going to E
C) E going to G
D) E going to C
E) C going to A

A) A going to C
B) C going to E
C) E going to G
D) E going to C
E) C going to A

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which of the following describes the reaction whose reaction coordinate diagram is shown below? 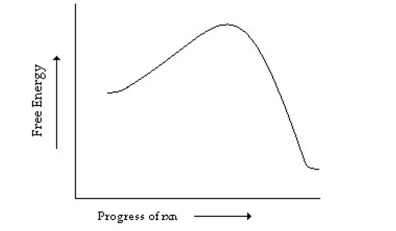
A) endergonic with no transition state
B) exergonic with no transition state
C) endergonic with a transition state
D) exergonic with a transition state
E) endergonic with an intermediate

A) endergonic with no transition state
B) exergonic with no transition state
C) endergonic with a transition state
D) exergonic with a transition state
E) endergonic with an intermediate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
30
Name the following compound: 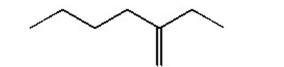


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which of the following describes intermediates transition states?
A) Transition states occur at minima on reaction coordinate diagrams.
B) Both transition states and intermediates occur at maxima on reaction coordinate diagrams.
C) An intermediate is always produced after the rate-determining step of a reaction mechanism.
D) Transition states have partially formed bonds whereas intermediates have fully formed bonds.
E) none of the above
A) Transition states occur at minima on reaction coordinate diagrams.
B) Both transition states and intermediates occur at maxima on reaction coordinate diagrams.
C) An intermediate is always produced after the rate-determining step of a reaction mechanism.
D) Transition states have partially formed bonds whereas intermediates have fully formed bonds.
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
32
An increase in which of the following results in a decrease in the rate of a reaction?
A) temperature
B) concentration
C) collision frequency
D) energy of activation
E) fraction of collisions with proper orientation
A) temperature
B) concentration
C) collision frequency
D) energy of activation
E) fraction of collisions with proper orientation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following contributes to make ° more negative?
A) use of a catalyst
B) a more positive
C) a more positive
D) a larger rate constant
E) none of the above
A) use of a catalyst
B) a more positive
C) a more positive
D) a larger rate constant
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
34
Name the following compound: 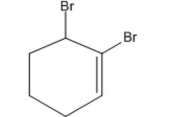


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
35
What is the value of for the reaction shown below?
Bond energies are:
A) +32.5
B) -57.5
C) -32.5
D) +57.5
E) -8.5
Bond energies are:
A) +32.5
B) -57.5
C) -32.5
D) +57.5
E) -8.5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
36
What is the free energy of activation for reaction in the following reaction coordinate diagram? 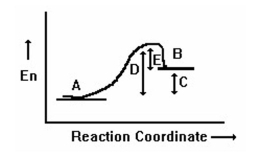
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
37
Name the following compound. Include E or Z if necessary. 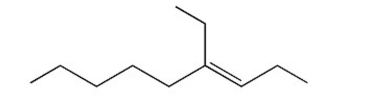


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
38
Draw and name the six alkenes that have molecular formula C5H10.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
39
Name the following compound: 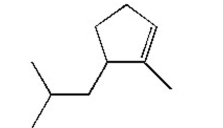


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
40
What is the hybridization and bond angle for a carbon in ethene?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
41
If the temperature of a reaction is doubled, how will the Gibbs free energy of the reaction change?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
42
Name the following compound. Include E or Z if necessary. 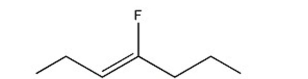


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
43
Why do reactions occur at a faster rate as T increases?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
44
Under what conditions is 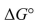 ° equal
° equal 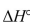 ° for a reaction?
° for a reaction?
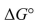 ° equal
° equal 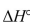 ° for a reaction?
° for a reaction?
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
45
Draw the curved arrows to show how CH3CH=CHCH3 reacts with HBr to form a carbocation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
46
Which of the following are nucleophiles and which are electrophiles? 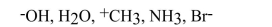
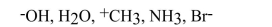

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
47
Draw all constitutional isomers with molecular formula C4H8.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
48
Draw the structure of two alkenes with molecular formula C4H8 that do not have cis-trans isomers.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
49
Which is the rate-determining step in the conversion of A to G in the reaction coordinate
diagram shown below?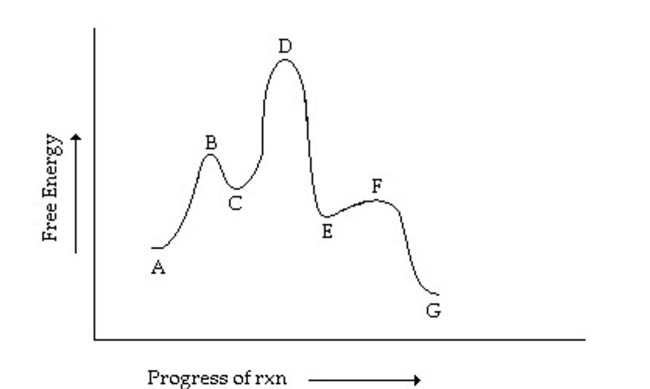
diagram shown below?


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
50
Consider the conversion of C to D via a one-step reaction. The activation energy of this reaction is 3 kcal/mol. The energy difference between D and the transition state of the reaction is 7 kcal/mol. What is the 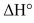 or the reaction?
or the reaction?
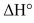 or the reaction?
or the reaction?
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
51
Which letters designate intermediates in the reaction coordinate diagram shown below? 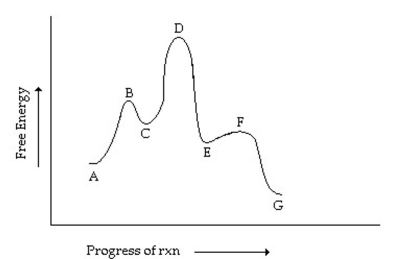


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
52
What is the free energy of activation of a one-step reaction? How is it related to the rate constant of the reaction?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
53
Draw the structure of (Z)-1-chloro-2-methyl-2-butene.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
54
Consider the one-step conversion of F to G. Given that the reaction is endergonic by 5 kcal/mol and that the energy difference between G and the transition state for the reaction is 15 kcal/mol, sketch a reaction coordinate diagram for this reaction. Make sure to show how the given energy differences are consistent with your sketch.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
55
Based on the following reaction coordinate diagram, which compound, A or C, is formed faster from B? Which is more stable, A or C? 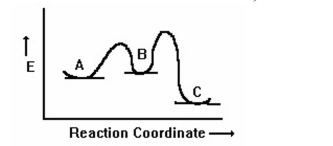


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck
56
Which step has the greatest rate constant in the forward direction in the reaction coordinate
diagram shown below?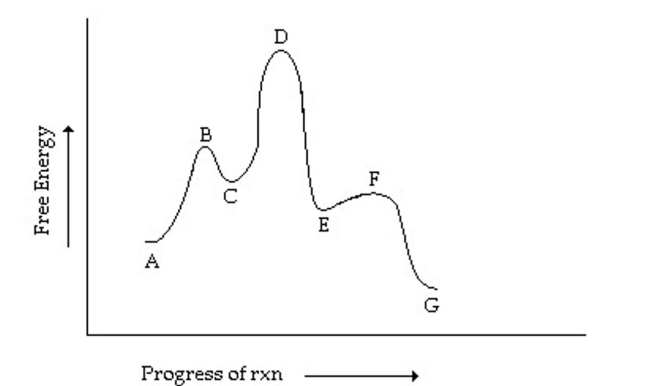
diagram shown below?


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 56 في هذه المجموعة.
فتح الحزمة
k this deck








