Deck 17: Temperature, Thermal Expansion, and the Ideal Gas Law
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/83
العب
ملء الشاشة (f)
Deck 17: Temperature, Thermal Expansion, and the Ideal Gas Law
1
At constant volume, the absolute pressure of a gas is inversely proportional to the absolute temperature.
False
2
When a bimetallic strip is cooled, the strip will bend toward the side
A)with the larger coefficient of linear expansion.
B)with the smaller coefficient of linear expansion.
C)with the higher temperature.
D)with the lower temperature.
E)depends of the method of heating
A)with the larger coefficient of linear expansion.
B)with the smaller coefficient of linear expansion.
C)with the higher temperature.
D)with the lower temperature.
E)depends of the method of heating
with the larger coefficient of linear expansion.
3
Two systems are in thermal equilibrium. The two systems are at the same
A)pressure.
B)volume.
C)height.
D)pressure and temperature.
E)temperature.
A)pressure.
B)volume.
C)height.
D)pressure and temperature.
E)temperature.
temperature.
4
Equal volumes of gas at the same pressure and temperature contain equal numbers of molecules.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
5
Distinguish between the three phases of matter from the atomic or microscopic point of view.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
6
Describe the anomalous thermal expansion property of water and its significance.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
7
State Charles's law.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
8
State Gay-Lassac's law.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
9
State Avogadro's hypothesis.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
10
State Boyle's law.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
11
When a bimetallic strip is heated, the strip will bend toward the side
A)with the larger coefficient of linear expansion.
B)with the smaller coefficient of linear expansion.
C)with the higher temperature.
D)with the lower temperature.
E)depends of the method of heating
A)with the larger coefficient of linear expansion.
B)with the smaller coefficient of linear expansion.
C)with the higher temperature.
D)with the lower temperature.
E)depends of the method of heating

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
12
A machinist needs to remove a tight fitting pin of material A from a hole in a block made of material B. The machinist heats both the pin and and the block to the same high temperature and removes the pin easily. What statement relates the coefficient of thermal expansion of material A to that of material B?
A)Material B has a greater coefficient of expansion than does material A.
B)The situation is not possible, heating block B will shrink the hole in the material as the material expands with increasing temperature.
C)Material B has the same coefficient of expansion as does material A.
D)Material B has a negative coefficient of expansion and A has a positive coefficient of expansion.
E)Material A has a greater coefficient of expansion than does material B.
A)Material B has a greater coefficient of expansion than does material A.
B)The situation is not possible, heating block B will shrink the hole in the material as the material expands with increasing temperature.
C)Material B has the same coefficient of expansion as does material A.
D)Material B has a negative coefficient of expansion and A has a positive coefficient of expansion.
E)Material A has a greater coefficient of expansion than does material B.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
13
State the Zeroth Law of Thermodynamics.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
14
The volume of a given amount of gas is inversely proportional to the absolute temperature when the pressure is kept constant.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
15
If a thermometer measures the temperature of two objects as being equal, you can conclude that the objects will be in thermal equilibrium if they are brought into thermal contact.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
16
The triple point of water occurs only at a unique temperature and pressure.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
17
Describe the two step process used to calculate thermal stress in a beam with ends which are rigidly fixed.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
18
If a thermometer measures the temperature of two objects as being equal, you can conclude that if the objects are placed in thermal contact, no heat will flow between them.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
19
The volume of a gas is inversely proportional to the absolute pressure applied to it when the temperature is kept constant.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
20
Explain the operation of a constant-volume gas thermometer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
21
What is wrong with the following statements? A fixed quantity of ideal gas at constant volume is warmed from a temperature of 20°C to 40°C. The pressure in the gas doubles during this process because the temperature doubles.
A)The appropriate temperature does not double during this process, so the pressure does not double either.
B)It is impossible to keep a fixed quantity of gas at constant volume during the temperature change specified in the problem.
C)The pressure changes to one half its initial value during this process.
D)The conclusion regarding the pressure cannot be reached because the mass of the ideal gas particles is unknown.
E)The pressure change will depend on whether the ideal gas particles are monatomic or polyatomic.
A)The appropriate temperature does not double during this process, so the pressure does not double either.
B)It is impossible to keep a fixed quantity of gas at constant volume during the temperature change specified in the problem.
C)The pressure changes to one half its initial value during this process.
D)The conclusion regarding the pressure cannot be reached because the mass of the ideal gas particles is unknown.
E)The pressure change will depend on whether the ideal gas particles are monatomic or polyatomic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
22
FIGURE 17-1 
Consider a flat steel plate with a hole through its center as shown in Fig.17-1. When the plate's temperature is decreased, the hole will
A)contract only if it takes up more than half the plate's surface area.
B)expand if it takes up less than half the plate's surface area.
C)contract.
D)expand.
E)remain the same size.

Consider a flat steel plate with a hole through its center as shown in Fig.17-1. When the plate's temperature is decreased, the hole will
A)contract only if it takes up more than half the plate's surface area.
B)expand if it takes up less than half the plate's surface area.
C)contract.
D)expand.
E)remain the same size.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
23
Express 14°F in °C.
A)-18°C
B)-10°C
C)46°C
D)14°C
E)57°C
A)-18°C
B)-10°C
C)46°C
D)14°C
E)57°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
24
An industrial fabrication process stamps out thin metal pieces, each in the shape of a square with a circular hole in the middle, from a large thin sheet. These pieces are mounted in a large machine. As these pieces heat up during regular machine operation, the diameter of the circular hole will
A)decrease.
B)stay the same.
C)increase.
D)behave unpredictably causing the machine to malfunction.
A)decrease.
B)stay the same.
C)increase.
D)behave unpredictably causing the machine to malfunction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
25
FIGURE 17-1 
Consider a flat steel plate with a hole through its center as shown in Fig. 17-1. When the plate's temperature is increased, the hole will
A)expand only if it takes up more than half the plate's surface area.
B)contract if it takes up less than half the plate's surface area.
C)always contract.
D)always expand.
E)remain the same size.

Consider a flat steel plate with a hole through its center as shown in Fig. 17-1. When the plate's temperature is increased, the hole will
A)expand only if it takes up more than half the plate's surface area.
B)contract if it takes up less than half the plate's surface area.
C)always contract.
D)always expand.
E)remain the same size.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
26
Two liters of a perfect gas are at 0°C and 1 atm. The gas is nitrogen, N2.
(a) Determine the number of moles.
(b) Determine the number of molecules.
(c) Determine the mass of the gas.
(a) Determine the number of moles.
(b) Determine the number of molecules.
(c) Determine the mass of the gas.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
27
A metal cylindrical sleeve has an inner radius A and an outer radius B. The metal has a positive thermal expansion coefficient. When the sleeve's temperature increases
A)the outer radius decreases and the inner radius increases.
B)the inner radius decreases and the outer radius increases.
C)both the inner and outer radii decrease.
D)both the inner and outer radii increase.
E)The sleeve's length decreases.
A)the outer radius decreases and the inner radius increases.
B)the inner radius decreases and the outer radius increases.
C)both the inner and outer radii decrease.
D)both the inner and outer radii increase.
E)The sleeve's length decreases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
28
Gallium boils at 2205°C. What is the corresponding temperature in the Fahrenheit scale?
A)2205°F
B)3805°F
C)4001°F
D)5223°F
E)5548°F
A)2205°F
B)3805°F
C)4001°F
D)5223°F
E)5548°F

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
29
On a cold winter day, you turn up the thermostat in your house. Assume that your house is well sealed and that no air enters or leaves the house. When the air temperature in your house rises a short while later, what statement is correct regarding the air pressure?
A)The air pressure is higher at the higher temperature.
B)The air pressure is lower at the higher temperature.
C)The air pressure does not change at the higher temperature.
D)The air pressure increases at first but then decreases again as the temperature approaches its higher value.
E)The air pressure decreases at first but then increases again as the temperature approaches its lower value.
A)The air pressure is higher at the higher temperature.
B)The air pressure is lower at the higher temperature.
C)The air pressure does not change at the higher temperature.
D)The air pressure increases at first but then decreases again as the temperature approaches its higher value.
E)The air pressure decreases at first but then increases again as the temperature approaches its lower value.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
30
Two metal spheres are made of the same material and have the same diameter, but one is solid and the other is hollow. If their temperature is increased by the same amount,
A)the solid sphere becomes bigger than the hollow one.
B)the hollow sphere becomes bigger than the solid one.
C)the two spheres remain of equal size.
D)the solid sphere becomes heavier and the hollow one lighter.
E)the solid sphere becomes lighter and the hollow one heavier.
A)the solid sphere becomes bigger than the hollow one.
B)the hollow sphere becomes bigger than the solid one.
C)the two spheres remain of equal size.
D)the solid sphere becomes heavier and the hollow one lighter.
E)the solid sphere becomes lighter and the hollow one heavier.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
31
The number of molecules in one mole of a substance
A)depends on the molecular weight of the substance.
B)depends on the atomic weight of the substance.
C)depends on the density of the substance.
D)depends on the temperature of the substance.
E)is the same for all substances.
A)depends on the molecular weight of the substance.
B)depends on the atomic weight of the substance.
C)depends on the density of the substance.
D)depends on the temperature of the substance.
E)is the same for all substances.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
32
Express 50°C in °F.
A)50°F
B)45.6°F
C)59.8°F
D)122°F
E)148°F
A)50°F
B)45.6°F
C)59.8°F
D)122°F
E)148°F

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
33
Nitrogen boils at -196°C. What is the corresponding temperature in the Fahrenheit scale?
A)-315°F
B)-196°F
C)-346°F
D)-290°F
E)-321°F
A)-315°F
B)-196°F
C)-346°F
D)-290°F
E)-321°F

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
34
Internal human body temperature is often stated to be normal at 98.6°F. What is this temperature on the Celsius scale?
A)120°C
B)145°C
C)37.0°C
D)72.6°C
E)22.8°C
A)120°C
B)145°C
C)37.0°C
D)72.6°C
E)22.8°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
35
When both the pressure and the absolute temperature of an ideal gas are increased each by a factor of two, the volume occupied by the gas will
A)increase by a factor of four.
B)increase by a factor of two.
C)not change.
D)decrease by a factor of two.
E)decrease by a factor of four.
A)increase by a factor of four.
B)increase by a factor of two.
C)not change.
D)decrease by a factor of two.
E)decrease by a factor of four.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
36
The surface water temperature on a large, deep lake is 3°C. A sensitive temperature probe is lowered several meters into the lake. What temperature will the probe record?
A)a temperature warmer than 3°C
B)a temperature less than 3°C
C)a temperature equal to 3°C
D)There is not enough information to determine.
A)a temperature warmer than 3°C
B)a temperature less than 3°C
C)a temperature equal to 3°C
D)There is not enough information to determine.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
37
For a fixed amount of gas, if the absolute temperature of the gas is doubled, what happens to the pressure of the gas?
A)The answer cannot be determined without volume information.
B)The pressure of the gas becomes double the original pressure.
C)The pressure of the gas becomes triple the original pressure.
D)The pressure of the gas becomes one half the original pressure.
E)The pressure of the gas becomes four times the original pressure.
A)The answer cannot be determined without volume information.
B)The pressure of the gas becomes double the original pressure.
C)The pressure of the gas becomes triple the original pressure.
D)The pressure of the gas becomes one half the original pressure.
E)The pressure of the gas becomes four times the original pressure.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
38
If the pressure acting on an ideal gas at constant temperature is tripled, its volume is
A)reduced to one-third.
B)increased by a factor of three.
C)increased by a factor of two.
D)reduced to one-half.
E)unchanged.
A)reduced to one-third.
B)increased by a factor of three.
C)increased by a factor of two.
D)reduced to one-half.
E)unchanged.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
39
On a hot summer day, you turn the thermostat in your house way down. Assume that your house is well sealed and that no air enters or leaves the house. When the air temperature in your house falls a short while later, what statement is correct regarding the air pressure?
A)The air pressure is higher at the lower temperature.
B)The air pressure is lower at the lower temperature.
C)The air pressure does not change at the lower temperature.
D)The air pressure increases at first but then decreases again as the temperature approaches its higher value.
E)The air pressure decreases at first but then increases again as the temperature approaches its lower value.
A)The air pressure is higher at the lower temperature.
B)The air pressure is lower at the lower temperature.
C)The air pressure does not change at the lower temperature.
D)The air pressure increases at first but then decreases again as the temperature approaches its higher value.
E)The air pressure decreases at first but then increases again as the temperature approaches its lower value.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
40
A mole of diatomic oxygen molecules and a mole of diatomic nitrogen molecules at STP have
A)the same average molecular speeds.
B)the same number of molecules.
C)the same diffusion rates.
D)all of the above
E)none of the above
A)the same average molecular speeds.
B)the same number of molecules.
C)the same diffusion rates.
D)all of the above
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
41
The coefficient of volume expansion of olive oil is 0.68 × 10-3K-1. A 1-liter glass beaker is filled to the brim with olive oil at room temperature. The beaker is placed on a range and the temperature of the oil and beaker increases by 25 C°. As a result, 0.0167 liters of olive oil spill over the top of the beaker. What is the coefficient of linear expansion of glass?
A)1 × 10-6K-1
B)4 × 10-6K-1
C)1 × 10-5K-1
D)2 × 10-5K-1
E)3 ×10-5K-1
A)1 × 10-6K-1
B)4 × 10-6K-1
C)1 × 10-5K-1
D)2 × 10-5K-1
E)3 ×10-5K-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
42
A rod has a length 2.0000 m at 20.0°C. The length of the rod increases to 2.0005 m when the temperature increases to 40.0°C. What is the coefficient of thermal expansion of the material from which the rod is made?
A)2.00 × 10-5/K
B)2.50 × 10-5/K
C)1.25 × 10-5/K
D)5.00 × 10-3/K
E)5.00 × 10-5/K
A)2.00 × 10-5/K
B)2.50 × 10-5/K
C)1.25 × 10-5/K
D)5.00 × 10-3/K
E)5.00 × 10-5/K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
43
The coefficient of linear expansion of steel is 12 × 10-6K-1. What is the change in length of a 25-m steel bridge span when it undergoes a temperature change of 40 K?
A)1.2 cm
B)1.4 cm
C)1.6 cm
D)1.8 cm
E)2.0 cm
A)1.2 cm
B)1.4 cm
C)1.6 cm
D)1.8 cm
E)2.0 cm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
44
The density of water at 0°C is 999.84 kg/m3 and at 4°C it is 999.96 kg/m3. A 1.0-liter container, full to the brim with water at 4°C is placed in the refrigerator. By the time that the temperature of the water reaches 0°C, how much of the water has spilled from the container, assuming that the contraction of the container is negligible?
A)1.1 × 10-7m3
B)1.2 × 10-7m3
C)1.3 × 10-7m3
D)1.4 × 10-7m3
E)1.5 × 10-7m3
A)1.1 × 10-7m3
B)1.2 × 10-7m3
C)1.3 × 10-7m3
D)1.4 × 10-7m3
E)1.5 × 10-7m3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
45
The coefficient of linear expansion of lead is 29 × 10-6K-1. What change in temperature will cause a 10-m long lead bar to change in length by 3.0 mm?
A)5.0 K
B)10 K
C)15 K
D)20 K
E)25 K
A)5.0 K
B)10 K
C)15 K
D)20 K
E)25 K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
46
Suppose that a steel bridge, 1000 m long, were built without any expansion joints. Suppose that only one end of the bridge was held fixed. What would the difference in the length of the bridge be between winter and summer, taking a typical winter temperature as 0°C, and a typical summer temperature as 40°C? The coefficient of thermal expansion of steel is 10.5 × 10-6K-1.
A)0.42 m
B)0.11 mm
C)0.11 m
D)0.42 mm
E)0.37 cm
A)0.42 m
B)0.11 mm
C)0.11 m
D)0.42 mm
E)0.37 cm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
47
1 L of water at 20°C will occupy what volume at 80°C? Water has a volume expansion coefficient of 210 × 10-6/C°.
A)1.6 L
B)1.326 L
C)1.013 L
D)0.987 L
E)0.9987 L
A)1.6 L
B)1.326 L
C)1.013 L
D)0.987 L
E)0.9987 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
48
FIGURE 17-2 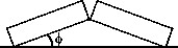
A bolt hole in a brass plate has a diameter of 1.200 cm at 20°C. What is the diameter of the hole when the plate is heated to 220°C? (The coefficient of linear thermal expansion for brass is 19 × 10-6 /C°.)
A)1.205 cm
B)1.125 cm
C)1.195 cm
D)1.200 cm
E)1.210 cm

A bolt hole in a brass plate has a diameter of 1.200 cm at 20°C. What is the diameter of the hole when the plate is heated to 220°C? (The coefficient of linear thermal expansion for brass is 19 × 10-6 /C°.)
A)1.205 cm
B)1.125 cm
C)1.195 cm
D)1.200 cm
E)1.210 cm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
49
FIGURE 17-2 
Two identical concrete slabs lie flat and in contact with each other as shown in Fig. 17-2. If the temperature increases by 40°C, the lower edges opposite the contact edges remained fixed in position, and the lower edges of the contact side remain in contact, at what angle φ will the slabs be tilted? The coefficient of thermal expansion of the concrete is 10 × 10-6/K.
A)18.7°
B)0.0283°
C)14.5°
D)1.62°
E)answer depends on length of slabs

Two identical concrete slabs lie flat and in contact with each other as shown in Fig. 17-2. If the temperature increases by 40°C, the lower edges opposite the contact edges remained fixed in position, and the lower edges of the contact side remain in contact, at what angle φ will the slabs be tilted? The coefficient of thermal expansion of the concrete is 10 × 10-6/K.
A)18.7°
B)0.0283°
C)14.5°
D)1.62°
E)answer depends on length of slabs

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
50
A bimetallic strip is made out of two strips of metal bonded together. Each strip is 20.0 cm long and 1.00 mm thick. One strip has a coefficient of thermal expansion 4.00 × 10-6/K and the other has a coefficient of thermal expansion 3.00 × 10-6/K. At 20.0°C the strip is straight. What is the inner radius of curvature of the composite strip at 200°C?
A)15.2 cm
B)2.22 m
C)1.27 m
D)34.8 cm
E)5.56 m
A)15.2 cm
B)2.22 m
C)1.27 m
D)34.8 cm
E)5.56 m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
51
A bimetallic strip is made by bonding together a 1.0-mm thick and 15.0-cm long steel strip to a similar strip made of aluminum. The strip is straight when the temperature is 20.0°C. What will the radius of curvature of the strip be when the temperature is 40.0°C? The coefficient of thermal expansion of steel is 10.5 × 10-6K-1 and that of aluminum is 23.0 × 10-6K-1.
A)4.0 m
B)3.6 m
C)4.8 m
D)5.2 m
E)4.4 m
A)4.0 m
B)3.6 m
C)4.8 m
D)5.2 m
E)4.4 m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
52
You want to insert an aluminum rod, which at 20°C has a radius of 1.00020 cm into a copper tube which has a radius of 1.00010 cm at the same temperature. You decide to put both of them in the refrigerator. At what temperature will the rod just fit if both are cooled to the same temperature? The coefficient of thermal expansion for aluminum is 2.4 × 10-5K-1, and that of copper is 1.7 × 10-5K-1.
A)7.8°C
B)6.3°C
C)9.2°C
D)14.7°C
E)5.7°C
A)7.8°C
B)6.3°C
C)9.2°C
D)14.7°C
E)5.7°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
53
The temperature changes from 35°F during the night to 75°F during the day. What is the temperature change on the Celsius scale?
A)72 C°
B)40 C°
C)32 C°
D)22 C°
A)72 C°
B)40 C°
C)32 C°
D)22 C°

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
54
Water at room temperature of 20.0°C is poured into an aluminum cylinder which has graduation markings etched on the inside. The reading in the graduations is 300.0 cc. The cylinder with the water in it is then immersed in a constant temperature bath at a temperature of 100°C. What is the reading for the level of water on the graduations of the cylinder after the water and the cylinder reach thermal equilibrium with the bath? The volume coefficient of expansion of water is 2.07 × 10-4K-1, and the linear coefficient of expansion of aluminum is 23.0 × 10-6K-1.
A)305.0 cc
B)303.5 cc
C)304.0 cc
D)304.5 cc
E)303.3 cc
A)305.0 cc
B)303.5 cc
C)304.0 cc
D)304.5 cc
E)303.3 cc

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
55
A thermometer is made such that the freezing point of water is defined to be 60.0°H and the boiling point of water at sea level is defined to be 520°H. What is the Celsius temperature at 240°H?
A)-13.8°C
B)34.6°C
C)31.0°C
D)-7.8°C
E)39.1°C
A)-13.8°C
B)34.6°C
C)31.0°C
D)-7.8°C
E)39.1°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
56
A student finds a blank liquid bulb thermometer and calibrates the scale by placing it in ice water and placing a reference mark at the level of the fluid. The student places the thermometer in boiling water and finds that the fluid level is 12.0 cm above the reference mark. What is the temperature when the fluid level is 2.4 cm above the reference mark?
A)2.4°C
B)24°C
C)25°C
D)28°C
E)20°C
A)2.4°C
B)24°C
C)25°C
D)28°C
E)20°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
57
The Concorde's exterior is made of aluminum, which has a coefficient of linear expansion of 24 × 10-6K-1. At 15°C, the Concorde measures 62.1 m in length. When the plane is in flight, friction with the air increases the temperature of the exterior skin to 200°C. What is the change in the length of the outer skin of the Concorde?
A)20 cm
B)24 cm
C)28 cm
D)32 cm
E)36 cm
A)20 cm
B)24 cm
C)28 cm
D)32 cm
E)36 cm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
58
The volume measuring system for a gasoline pump at the service station is calibrated at 20.0°C. If the temperature of the gasoline drops to 10.0°C, what percentage extra amount of mass of gasoline do you receive when making a purchase? The coefficient of volume expansion for gasoline is 9.5 × 10-4/K.
A)1.33%
B)1.90%
C)1.05%
D)2.24%
E)0.95%
A)1.33%
B)1.90%
C)1.05%
D)2.24%
E)0.95%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
59
The coefficient of linear expansion of aluminum is 24 × 10-6K-1 and the coefficient of volume expansion of olive oil is 0.68 × 10-3K-1. A novice cook, in preparation for deep-frying some potatoes fills a 1.00-liter aluminum pot to the brim and heats the oil and the pot from an initial temperature of 15°C to 190°C. To his consternation some olive oil spills over the top. How much?
A)0.11 liters
B)0.12 liters
C)0.13 liters
D)0.14 liters
E)0.15 liters
A)0.11 liters
B)0.12 liters
C)0.13 liters
D)0.14 liters
E)0.15 liters

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
60
400 cm3 of mercury at 0°C will expand to what volume at 50°C? Mercury has a volume expansion coefficient of 180 × 10-6/C°.
A)450 cm3
B)422.8 cm3
C)409.7 cm3
D)403.6 cm3
E)401.8 cm3
A)450 cm3
B)422.8 cm3
C)409.7 cm3
D)403.6 cm3
E)401.8 cm3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
61
A car starts out when the air temperature is 288 K. The air pressure in the tires is 460 kPa. After driving a while, the temperature of the air in the tires increases to 298 K. What is the pressure in the tires at that point, assuming the volume remains constant?
A)476 kPa
B)563 kPa
C)488 kPa
D)498 kPa
E)512 kPa
A)476 kPa
B)563 kPa
C)488 kPa
D)498 kPa
E)512 kPa

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
62
Internal human body temperature is often stated to be normal at 98.6°F. What is this temperature on the Kelvin scale?
A)346 K
B)393 K
C)310 K
D)296 K
E)418 K
A)346 K
B)393 K
C)310 K
D)296 K
E)418 K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
63
On a cold day, when the air temperature is 0°C, you empty your lungs and take a deep breath, filling your lungs with 4.2 L of air. If you hold your breath until the temperature of the air in your lungs reaches 37°C, what is the volume of the air in your lungs at that point, assuming the pressure does not change?
A)4.6 L
B)4.4 L
C)5.0 L
D)4.8 L
E)5.2 L
A)4.6 L
B)4.4 L
C)5.0 L
D)4.8 L
E)5.2 L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
64
Convert 14°C to K.
A)46 K
B)100 K
C)187 K
D)287 K
E)474 K
A)46 K
B)100 K
C)187 K
D)287 K
E)474 K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
65
An ideal gas occupies 400 L at an absolute pressure of 500 kPa. Find the absolute pressure if the volume changes to 950 L and the temperature remains constant.
A)500 kPa
B)210 kPa
C)760 kPa
D)950 kPa
E)1150 kPa
A)500 kPa
B)210 kPa
C)760 kPa
D)950 kPa
E)1150 kPa

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
66
An ideal gas in a container of volume 100 cm3 at 20°C has a pressure of 100 N/m2. Determine the number of gas molecules in the container.
A)2.4 × 106
B)4.2 × 106
C)6.0 × 1023
D)2.5 × 1018
E)5.2 × 1018
A)2.4 × 106
B)4.2 × 106
C)6.0 × 1023
D)2.5 × 1018
E)5.2 × 1018

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
67
A sample of helium (He) occupies 44.8 L at STP. What is the mass of the sample?
A)2 g
B)4 g
C)6 g
D)8 g
E)10 g
A)2 g
B)4 g
C)6 g
D)8 g
E)10 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
68
An ideal gas is at a pressure 1.00 × 105 N/m2 and a volume 2.00 m3. If the gas is compressed to a volume 1.00 m3 while the temperature remains constant, what will be the new pressure in the gas?
A)0.500 × 105 N/m2
B)4.00 × 105 N/m2
C)1.00 × 105 N/m2
D)2.00 × 105 N/m2
E)The answer depends on the mass of the gas particles.
A)0.500 × 105 N/m2
B)4.00 × 105 N/m2
C)1.00 × 105 N/m2
D)2.00 × 105 N/m2
E)The answer depends on the mass of the gas particles.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
69
Convert 14°F to K.
A)263 K
B)287 K
C)163 K
D)-10 K
E)474 K
A)263 K
B)287 K
C)163 K
D)-10 K
E)474 K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
70
Carbon dioxide solidifies at 195 K. Express this temperature in degrees Fahrenheit.
A)-78°F
B)-40°F
C)-109°F
D)-163°F
E)-351°F
A)-78°F
B)-40°F
C)-109°F
D)-163°F
E)-351°F

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
71
A constant volume closed container of gas is at a pressure 1.00 × 105 N/m2 and a temperature 20°C. What is the pressure if the temperature of the gas is increased to 60.0°C?
A)0.330 × 105 N/m2
B)9.00 × 105 N/m2
C)0.880 × 105 N/m2
D)3.00 × 105 N/m2
E)1.14 × 105 N/m2
A)0.330 × 105 N/m2
B)9.00 × 105 N/m2
C)0.880 × 105 N/m2
D)3.00 × 105 N/m2
E)1.14 × 105 N/m2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
72
What is the temperature of 3.00 moles of an ideal gas at a pressure of 250 kPa held in a volume of 25.0 L?
A)509 K
B)90.5 K
C)752 K
D)272 K
E)251 K
A)509 K
B)90.5 K
C)752 K
D)272 K
E)251 K

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
73
How many moles of H2O molecules are in a 4.00 m3 container at a pressure 8.00 × 105 N/m2 and temperature 600°C?
A)7.72 × 1026
B)641
C)441
D)3.86 × 1026
E)2.65 × 1026
A)7.72 × 1026
B)641
C)441
D)3.86 × 1026
E)2.65 × 1026

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
74
Convert 14 K to °C.
A)46°C
B)287°C
C)25°C
D)-225°C
E)-259°C
A)46°C
B)287°C
C)25°C
D)-225°C
E)-259°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
75
What is the mass density of argon gas at pressure 1.00 × 105 N/m2 and at temperature 300 K? The mean atomic mass of argon is 39.948u.
A)1.40 kg/m3
B)1.00 kg/m3
C)1.20 kg/m3
D)1.60 kg/m3
E)1.80 kg/m3
A)1.40 kg/m3
B)1.00 kg/m3
C)1.20 kg/m3
D)1.60 kg/m3
E)1.80 kg/m3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
76
The interior of a refrigerator has a volume of 0.600 m3. The temperature inside the refrigerator in 282 K, and the pressure is 101 kPa. If the molecular weight of air is 29 g/mol, what is the mass of air inside the refrigerator?
A)498 g
B)560 g
C)135 g
D)267 g
E)750 g
A)498 g
B)560 g
C)135 g
D)267 g
E)750 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
77
A thermometer is made such that the freezing point of water is defined to be 60.0°H and the boiling point of water at sea level is defined to be 520°H. What is absolute zero on the H scale?
A)0.6°H
B)-36°H
C)-980°H
D)-1256°H
E)-1196°H
A)0.6°H
B)-36°H
C)-980°H
D)-1256°H
E)-1196°H

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
78
How many water molecules are there in 36 g of water? Express your answer as a multiple of Avogadro's number NA. (The molecular structure of a water molecule is H2O.)
A)36NA
B)2NA
C)6NA
D)18NA
E)none of the above
A)36NA
B)2NA
C)6NA
D)18NA
E)none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
79
A weather balloon contains 12.0 m3 of hydrogen gas when it is released from a location at which the temperature is 22°C and the pressure is 101 kPa. It rises to a location where the temperature is -30°C and the pressure is 20 kPa. If the balloon is free to expand so that the pressure of the gas inside is equal to the ambient pressure, what is the new volume of the balloon?
A)14.0 m3
B)2.38 m3
C)49.9 m3
D)82.6 m3
E)4.16 m3
A)14.0 m3
B)2.38 m3
C)49.9 m3
D)82.6 m3
E)4.16 m3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck
80
Convert 14 K to °F.
A)287°F
B)-434°F
C)-343°F
D)-259°F
E)474°F
A)287°F
B)-434°F
C)-343°F
D)-259°F
E)474°F

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 83 في هذه المجموعة.
فتح الحزمة
k this deck








