Deck 27: Pericyclic Reactions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/47
العب
ملء الشاشة (f)
Deck 27: Pericyclic Reactions
1
Which of the following statements about a p-bonding molecular orbital is true?
A)A p-bonding molecular orbital is formed when two p orbitals of similar phase overlap.
B)A p-bonding molecular orbital is lower in energy than a s-bonding molecular orbital.
C)A p-bonding molecular orbital is formed when two p orbitals of opposite phase overlap.
D)Both the statements "a p-bonding molecular orbital is formed when two p orbitals of similar phase overlap" and a p-bonding molecular orbital is lower in energy than a s-bonding molecular orbital" are true.
A)A p-bonding molecular orbital is formed when two p orbitals of similar phase overlap.
B)A p-bonding molecular orbital is lower in energy than a s-bonding molecular orbital.
C)A p-bonding molecular orbital is formed when two p orbitals of opposite phase overlap.
D)Both the statements "a p-bonding molecular orbital is formed when two p orbitals of similar phase overlap" and a p-bonding molecular orbital is lower in energy than a s-bonding molecular orbital" are true.
Both the statements "a p-bonding molecular orbital is formed when two p orbitals of similar phase overlap" and a p-bonding molecular orbital is lower in energy than a s-bonding molecular orbital" are true.
2
How many bonding molecular orbitals are present in 1,3,5,7,9-decapentaene?
A)4
B)5
C)10
D)12
A)4
B)5
C)10
D)12
5
3
Which of the following statements about pericyclic reactions is true?
A)In pericyclic reactions, bonds are broken and formed in multiple steps.
B)In pericyclic reactions, all bonds are broken and formed in a single step.
C)One intermediate has been identified in pericyclic reactions.
D)The transition state in a pericyclic reaction is acyclic.
A)In pericyclic reactions, bonds are broken and formed in multiple steps.
B)In pericyclic reactions, all bonds are broken and formed in a single step.
C)One intermediate has been identified in pericyclic reactions.
D)The transition state in a pericyclic reaction is acyclic.
In pericyclic reactions, all bonds are broken and formed in a single step.
4
Which of the following is not a type of pericyclic reactions?
A)cycloadditions
B)nucleophilic reactions
C)electrocyclic reactions
D)sigmatropic rearrangements
A)cycloadditions
B)nucleophilic reactions
C)electrocyclic reactions
D)sigmatropic rearrangements

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
5
How many p molecular orbitals are present in 1,3,5,7,9-decapentaene?
A)4
B)5
C)10
D)12
A)4
B)5
C)10
D)12

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which of the following statements about sigmatropic reactions is true?
A)The reactants contain one more p bond than the product.
B)The product contains one more p bond than the reactant.
C)A p bond is broken in the reactant.
D)A s bond is broken in the reactant.
A)The reactants contain one more p bond than the product.
B)The product contains one more p bond than the reactant.
C)A p bond is broken in the reactant.
D)A s bond is broken in the reactant.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
7
How many bonding molecular orbitals are present in 1,3,5-hexatriene?
A)3
B)4
C)5
D)6
A)3
B)4
C)5
D)6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which of the following is a type of pericyclic reaction?
A)electrocyclic reactions
B)acyclic reactions
C)cycloelimination reactions
D)electrophilic reactions
A)electrocyclic reactions
B)acyclic reactions
C)cycloelimination reactions
D)electrophilic reactions

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which of the following statements about porbitals is true?
A)P Orbitals are formed by the linear combination of two sp3 orbitals.
B)P Orbitals are formed by the linear combination of two sp2 orbitals.
C)P Orbitals are formed by the linear combination of two sp orbitals.
D)P Orbitals are formed by the linear combination of two p orbitals.
A)P Orbitals are formed by the linear combination of two sp3 orbitals.
B)P Orbitals are formed by the linear combination of two sp2 orbitals.
C)P Orbitals are formed by the linear combination of two sp orbitals.
D)P Orbitals are formed by the linear combination of two p orbitals.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which of the following statements about cycloaddition reactions is true?
A)Cycloaddition reactions occur only intermolecularly.
B)Cycloaddition reactions occur only intramolecularly.
C)Cycloaddition reactions form a cyclic product with two new p bonds.
D)Cycloaddition reactions can be intramolecular or intermolecular.
A)Cycloaddition reactions occur only intermolecularly.
B)Cycloaddition reactions occur only intramolecularly.
C)Cycloaddition reactions form a cyclic product with two new p bonds.
D)Cycloaddition reactions can be intramolecular or intermolecular.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which of the following statements about molecular orbitals is true?
A)The number of molecular orbitals formed is different from the number of atomic orbitals used.
B)The number of molecular orbitals formed is equal to the number of atomic orbitals used.
C)The number of molecular orbitals formed is equal to twice the number of atomic orbitals used.
D)The number of molecular orbitals formed is equal to half the number of atomic orbitals used.
A)The number of molecular orbitals formed is different from the number of atomic orbitals used.
B)The number of molecular orbitals formed is equal to the number of atomic orbitals used.
C)The number of molecular orbitals formed is equal to twice the number of atomic orbitals used.
D)The number of molecular orbitals formed is equal to half the number of atomic orbitals used.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which of the following statements about pericyclic reactions is true?
A)Pericyclic reactions occur by way of ionic intermediates.
B)Pericyclic reactions occur by way of radical intermediates.
C)Pericyclic reactions involve multiple steps.
D)Reactive intermediates are not formed in pericyclic reactions.
A)Pericyclic reactions occur by way of ionic intermediates.
B)Pericyclic reactions occur by way of radical intermediates.
C)Pericyclic reactions involve multiple steps.
D)Reactive intermediates are not formed in pericyclic reactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which of the following statements about electrocyclic ring closure is not true?
A)It is an intramolecular reaction.
B)It requires heat or light.
C)The cyclic product contains one more s bond and one fewer p bond than the reactants.
D)The cyclic product contains one more p bond and one fewer s bond than the reactants.
A)It is an intramolecular reaction.
B)It requires heat or light.
C)The cyclic product contains one more s bond and one fewer p bond than the reactants.
D)The cyclic product contains one more p bond and one fewer s bond than the reactants.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of the following statements about a p* antibonding molecular orbital is true?
A)A p* antibonding molecular orbital is formed when two p orbitals of similar phase overlap.
B)A p* antibonding molecular orbital is formed when two p orbitals of opposite phase overlap.
C)A p* antibonding molecular orbital is a higher-energy molecular orbital than a p bonding molecular orbital.
D)Both the statements "a p* antibonding molecular orbital is formed when two p orbitals of opposite phase overlap" and "a p* antibonding molecular orbital is a higher-energy molecular orbital than a p bonding molecular orbital" are true.
A)A p* antibonding molecular orbital is formed when two p orbitals of similar phase overlap.
B)A p* antibonding molecular orbital is formed when two p orbitals of opposite phase overlap.
C)A p* antibonding molecular orbital is a higher-energy molecular orbital than a p bonding molecular orbital.
D)Both the statements "a p* antibonding molecular orbital is formed when two p orbitals of opposite phase overlap" and "a p* antibonding molecular orbital is a higher-energy molecular orbital than a p bonding molecular orbital" are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which of the following statements about pericyclic reactions is not true?
A)Pericyclic reactions are concerted reactions.
B)Pericyclic reactions proceed through a cyclic transition state.
C)Pericyclic reactions are not stereospecific.
D)Pericyclic reactions require light or heat.
A)Pericyclic reactions are concerted reactions.
B)Pericyclic reactions proceed through a cyclic transition state.
C)Pericyclic reactions are not stereospecific.
D)Pericyclic reactions require light or heat.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
16
How many p molecular orbitals are present in 1,3,5-hexatriene?
A)3
B)4
C)5
D)6
A)3
B)4
C)5
D)6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
17
Predict the major organic product(s) of the following electrocyclic reaction. 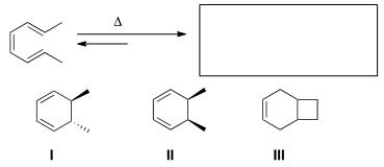
A)Only I
B)Only II
C)Only III
D)Only I and II

A)Only I
B)Only II
C)Only III
D)Only I and II

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which of the following statements about electrocyclic reactions is true?
A)An electrocyclic reaction is generally irreversible.
B)Generally, an acyclic triene is favored over a six-membered ring at equilibrium.
C)Generally, a four-membered ring is favored over an acyclic diene at equilibrium.
D)An electrocyclic reaction is generally reversible.
A)An electrocyclic reaction is generally irreversible.
B)Generally, an acyclic triene is favored over a six-membered ring at equilibrium.
C)Generally, a four-membered ring is favored over an acyclic diene at equilibrium.
D)An electrocyclic reaction is generally reversible.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
19
How many nodes are present in Y5 of 1,3,5,7,9-decapentaene?
A)2
B)3
C)4
D)5
A)2
B)3
C)4
D)5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which of the following statements about an electrocyclic ring-opening reaction is not true?
A)An electrocyclic ring-opening reaction is an intramolecular reaction.
B)An electrocyclic ring-opening reaction requires a source of energy (heat or light).
C)The product of an electrocyclic ring-opening reaction contains one fewer p bond than the reactant.
D)The product of an electrocyclic ring-opening reaction contains one more p bond than the reactant.
A)An electrocyclic ring-opening reaction is an intramolecular reaction.
B)An electrocyclic ring-opening reaction requires a source of energy (heat or light).
C)The product of an electrocyclic ring-opening reaction contains one fewer p bond than the reactant.
D)The product of an electrocyclic ring-opening reaction contains one more p bond than the reactant.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which of the following statements about sigmatropic rearrangements and orbital symmetry is not true?
A)Reactions involving six atoms or fewer must take place by suprafacial pathways.
B)In a suprafacial rearrangement, the new sbond forms on the opposite side of the psystem as the broken s bond.
C)In an antarafacial rearrangement, the new sbond forms on the opposite side of the psystem as the broken s bond.
D)In a suprafacial rearrangement, the new sbond forms on the same side of the p system as the broken s bond.
A)Reactions involving six atoms or fewer must take place by suprafacial pathways.
B)In a suprafacial rearrangement, the new sbond forms on the opposite side of the psystem as the broken s bond.
C)In an antarafacial rearrangement, the new sbond forms on the opposite side of the psystem as the broken s bond.
D)In a suprafacial rearrangement, the new sbond forms on the same side of the p system as the broken s bond.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which of the following statements about cycloaddition reactions is true?
A)Cycloaddition reactions can be initiated by heat only.
B)Cycloaddition reactions can be initiated by light only.
C)Cycloaddition reactions can be initiated by heat or light.
D)Cycloaddition reactions are identified by the number of p electrons in the two products.
A)Cycloaddition reactions can be initiated by heat only.
B)Cycloaddition reactions can be initiated by light only.
C)Cycloaddition reactions can be initiated by heat or light.
D)Cycloaddition reactions are identified by the number of p electrons in the two products.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
23
Why is the Diels-Alder reaction called a thermal [4+2] cycloaddition?
A)Because the reaction is initiated by heat; the diene has four p electrons and the dienophile has two p electrons.
B)Because the reaction is initiated by light; the diene has four p electrons and the dienophile has two p electrons.
C)Because the reaction is initiated by heat; the dienophile has four p electrons and the diene has two pelectrons.
D)Because the reaction is initiated by light; the dienophile has four p electrons and the diene has two pelectrons.
A)Because the reaction is initiated by heat; the diene has four p electrons and the dienophile has two p electrons.
B)Because the reaction is initiated by light; the diene has four p electrons and the dienophile has two p electrons.
C)Because the reaction is initiated by heat; the dienophile has four p electrons and the diene has two pelectrons.
D)Because the reaction is initiated by light; the dienophile has four p electrons and the diene has two pelectrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
24
Which of the following statements about sigmatropic reactions is not true?
A)A sigmatropic reaction is an intramolecular pericyclic reaction.
B)In a sigmatropic reaction, s bond is broken in one of the reactants.
C)The p bonds rearrange in a sigmatropic reaction.
D)The number of p bonds in the reactants and product differs in a sigmatropic reaction.
A)A sigmatropic reaction is an intramolecular pericyclic reaction.
B)In a sigmatropic reaction, s bond is broken in one of the reactants.
C)The p bonds rearrange in a sigmatropic reaction.
D)The number of p bonds in the reactants and product differs in a sigmatropic reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which of the following statements about photochemical electrocyclic reactions is not true?
A)In photochemical reactions, we consider the orbitals of the HOMO of the excited state to determine the course of the reaction.
B)Photochemical electrocyclic reactions occur in a conrotatory fashion for a conjugated polyene with an odd number of pbonds.
C)Photochemical electrocyclic reactions occur in a disrotatory fashion for a conjugated polyene with an even number of pbonds.
D)In photochemical reactions, we consider the orbitals of the LUMO of the excited state to determine the course of the reaction.
A)In photochemical reactions, we consider the orbitals of the HOMO of the excited state to determine the course of the reaction.
B)Photochemical electrocyclic reactions occur in a conrotatory fashion for a conjugated polyene with an odd number of pbonds.
C)Photochemical electrocyclic reactions occur in a disrotatory fashion for a conjugated polyene with an even number of pbonds.
D)In photochemical reactions, we consider the orbitals of the LUMO of the excited state to determine the course of the reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
26
Predict the major organic product(s) of the following electrocyclic reaction. 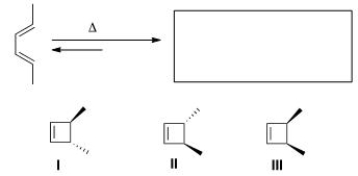
A)Only I
B)Only II
C)Only III
D)Only I and II

A)Only I
B)Only II
C)Only III
D)Only I and II

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
27
What type of cycloaddition reaction is shown in the following equation?
![<strong>What type of cycloaddition reaction is shown in the following equation? </strong> A)[2+2] B)[4+2] C)[4+4] D)[0+2]](https://d2lvgg3v3hfg70.cloudfront.net/TB5872/11eb4ad7_6c08_c492_99b2_db5326882b71_TB5872_00.jpg)
A)[2+2]
B)[4+2]
C)[4+4]
D)[0+2]
![<strong>What type of cycloaddition reaction is shown in the following equation? </strong> A)[2+2] B)[4+2] C)[4+4] D)[0+2]](https://d2lvgg3v3hfg70.cloudfront.net/TB5872/11eb4ad7_6c08_c492_99b2_db5326882b71_TB5872_00.jpg)
A)[2+2]
B)[4+2]
C)[4+4]
D)[0+2]

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
28
What type of sigmatropic rearrangement is illustrated below?
![<strong>What type of sigmatropic rearrangement is illustrated below? </strong> A)[1,3] B)[1,4] C)[3,3] D)[1,5]](https://d2lvgg3v3hfg70.cloudfront.net/TB5872/11eb4ad7_6c08_eba3_99b2_9b1efecd7def_TB5872_00.jpg)
A)[1,3]
B)[1,4]
C)[3,3]
D)[1,5]
![<strong>What type of sigmatropic rearrangement is illustrated below? </strong> A)[1,3] B)[1,4] C)[3,3] D)[1,5]](https://d2lvgg3v3hfg70.cloudfront.net/TB5872/11eb4ad7_6c08_eba3_99b2_9b1efecd7def_TB5872_00.jpg)
A)[1,3]
B)[1,4]
C)[3,3]
D)[1,5]

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
29
What type of sigmatropic rearrangement is illustrated below?
![<strong>What type of sigmatropic rearrangement is illustrated below? </strong> A)[1,3] B)[1,4] C)[3,3] D)[1,5]](https://d2lvgg3v3hfg70.cloudfront.net/TB5872/11eb4ad7_6c09_12b5_99b2_cb71330f109e_TB5872_00.jpg)
A)[1,3]
B)[1,4]
C)[3,3]
D)[1,5]
![<strong>What type of sigmatropic rearrangement is illustrated below? </strong> A)[1,3] B)[1,4] C)[3,3] D)[1,5]](https://d2lvgg3v3hfg70.cloudfront.net/TB5872/11eb4ad7_6c09_12b5_99b2_cb71330f109e_TB5872_00.jpg)
A)[1,3]
B)[1,4]
C)[3,3]
D)[1,5]

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
30
Predict the major organic product(s) of the following electrocyclic reaction. 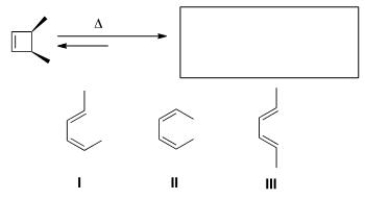
A)Only I
B)Only II
C)Only III
D)Only I and II

A)Only I
B)Only II
C)Only III
D)Only I and II

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which of the following statements about [2+2] cycloaddition reactions between two alkenes is true?
A)The reaction is initiated by heat.
B)The reaction is initiated by light.
C)The product is a cyclopentane derivative.
D)Each reactant contains two s electrons that participate in the formation of new bonds.
A)The reaction is initiated by heat.
B)The reaction is initiated by light.
C)The product is a cyclopentane derivative.
D)Each reactant contains two s electrons that participate in the formation of new bonds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
32
What type of cycloaddition reaction is shown below?
![<strong>What type of cycloaddition reaction is shown below? </strong> A)[2+2] B)[4+2] C)[4+4] D)[0+2]](https://d2lvgg3v3hfg70.cloudfront.net/TB5872/11eb4ad7_6c08_9d81_99b2_f30387679422_TB5872_00.jpg)
A)[2+2]
B)[4+2]
C)[4+4]
D)[0+2]
![<strong>What type of cycloaddition reaction is shown below? </strong> A)[2+2] B)[4+2] C)[4+4] D)[0+2]](https://d2lvgg3v3hfg70.cloudfront.net/TB5872/11eb4ad7_6c08_9d81_99b2_f30387679422_TB5872_00.jpg)
A)[2+2]
B)[4+2]
C)[4+4]
D)[0+2]

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
33
Predict the major organic product(s) of the following electrocyclic reaction. 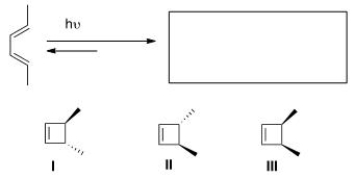
A)Only I
B)Only II
C)Only III
D)Only I and II

A)Only I
B)Only II
C)Only III
D)Only I and II

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
34
What type of sigmatropic rearrangement is illustrated below?
![<strong>What type of sigmatropic rearrangement is illustrated below? </strong> A)[1,3] B)[1,4] C)[3,3] D)[1,5]](https://d2lvgg3v3hfg70.cloudfront.net/TB5872/11eb4ad7_6c09_12b4_99b2_7117e9b440b7_TB5872_00.jpg)
A)[1,3]
B)[1,4]
C)[3,3]
D)[1,5]
![<strong>What type of sigmatropic rearrangement is illustrated below? </strong> A)[1,3] B)[1,4] C)[3,3] D)[1,5]](https://d2lvgg3v3hfg70.cloudfront.net/TB5872/11eb4ad7_6c09_12b4_99b2_7117e9b440b7_TB5872_00.jpg)
A)[1,3]
B)[1,4]
C)[3,3]
D)[1,5]

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
35
Which of the following statements about cycloaddition reactions is not true?
A)Cycloaddition reactions form a cyclic product with two new s bonds.
B)The course of the reaction is determined by the symmetry of the molecular orbitals of the products.
C)Cycloaddition reactions are concerted.
D)Cycloaddition reactions are stereospecific.
A)Cycloaddition reactions form a cyclic product with two new s bonds.
B)The course of the reaction is determined by the symmetry of the molecular orbitals of the products.
C)Cycloaddition reactions are concerted.
D)Cycloaddition reactions are stereospecific.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which of the following statements about thermal electrocyclic reactions is not true?
A)The number of s bonds in the conjugated polyene determines whether rotation is conrotatory or disrotatory.
B)Thermal electrocyclic reactions occur in a disrotatory fashion for a conjugated polyene with an odd number of p bonds.
C)Thermal electrocyclic reactions occur in a conrotatory fashion for a conjugated polyene with an even number of p bonds.
D)In thermal reactions, we consider the orbitals of the HOMO of the ground state electronic configuration to determine the course of the reaction.
A)The number of s bonds in the conjugated polyene determines whether rotation is conrotatory or disrotatory.
B)Thermal electrocyclic reactions occur in a disrotatory fashion for a conjugated polyene with an odd number of p bonds.
C)Thermal electrocyclic reactions occur in a conrotatory fashion for a conjugated polyene with an even number of p bonds.
D)In thermal reactions, we consider the orbitals of the HOMO of the ground state electronic configuration to determine the course of the reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which of the following statements about orbital symmetry and cycloaddition reactions is true?
A)Thermal cycloadditions involving an even number of pbonds proceed by a suprafacial pathway.
B)Thermal cycloadditions involving an odd number of pbonds proceed by an antarafacial pathway.
C)Photochemical cycloadditions involving an even number of p bonds proceed by an antarafacial pathway.
D)Photochemical cycloadditions involving an even number of p bonds proceed by a suprafacial pathway.
A)Thermal cycloadditions involving an even number of pbonds proceed by a suprafacial pathway.
B)Thermal cycloadditions involving an odd number of pbonds proceed by an antarafacial pathway.
C)Photochemical cycloadditions involving an even number of p bonds proceed by an antarafacial pathway.
D)Photochemical cycloadditions involving an even number of p bonds proceed by a suprafacial pathway.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which of the following statements about orbital symmetry and cycloaddition reactions is true?
A)Thermal cycloadditions involving an even number of pbonds proceed by a suprafacial pathway.
B)Thermal cycloadditions involving an odd number of pbonds proceed by a suprafacial pathway.
C)Photochemical cycloadditions involving an odd number of p bonds proceed by a suprafacial pathway.
D)Photochemical sycloadditions involving an even number of p bonds proceed by an antarafacial pathway.
A)Thermal cycloadditions involving an even number of pbonds proceed by a suprafacial pathway.
B)Thermal cycloadditions involving an odd number of pbonds proceed by a suprafacial pathway.
C)Photochemical cycloadditions involving an odd number of p bonds proceed by a suprafacial pathway.
D)Photochemical sycloadditions involving an even number of p bonds proceed by an antarafacial pathway.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
39
Which of the following is not a correct designation for a sigmatropic rearrangement?
A)[1,3]
B)[1,5]
C)[3,3]
D)[3,1]
A)[1,3]
B)[1,5]
C)[3,3]
D)[3,1]

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
40
What are the two modes of bond formation in cycloaddition reactions?
A)Suprafacial and antarafacial bond formations
B)Superfacial and antifacial bond formations
C)Suprafacial and synfacial bond formations
D)Synfacial and antifacial bond formations
A)Suprafacial and antarafacial bond formations
B)Superfacial and antifacial bond formations
C)Suprafacial and synfacial bond formations
D)Synfacial and antifacial bond formations

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
41
Which of the following statements about the Claisen rearrangement is true?
A)The Claisen rearrangement occurs readily in a suprafacial pathway under photochemical conditions.
B)The Claisen rearrangement occurs readily in an antarafacial pathway under thermal conditions.
C)The Claisen rearrangement involves three electron pairs; two in p bonds and one in a s bond.
D)The Claisen rearrangement involves the rearrangement of an unsaturated ether to a b,g-unsaturated carbonyl compound.
A)The Claisen rearrangement occurs readily in a suprafacial pathway under photochemical conditions.
B)The Claisen rearrangement occurs readily in an antarafacial pathway under thermal conditions.
C)The Claisen rearrangement involves three electron pairs; two in p bonds and one in a s bond.
D)The Claisen rearrangement involves the rearrangement of an unsaturated ether to a b,g-unsaturated carbonyl compound.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
42
Which of the following statements about a thermal reaction involving an even number of electron pairs is true?
A)A thermal reaction involving an even number of electron pairs is conrotatory or antarafacial.
B)A thermal reaction involving an even number of electron pairs is disrotatory or suprafacial.
C)A thermal reaction involving an even number of electron pairs is conrotatory or suprafacial.
D)A thermal reaction involving an even number of electron pairs is disrotatory or antarafacial.
A)A thermal reaction involving an even number of electron pairs is conrotatory or antarafacial.
B)A thermal reaction involving an even number of electron pairs is disrotatory or suprafacial.
C)A thermal reaction involving an even number of electron pairs is conrotatory or suprafacial.
D)A thermal reaction involving an even number of electron pairs is disrotatory or antarafacial.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
43
Which of the following statements about the Cope rearrangement is not true?
A)The Cope rearrangement involves the rearrangement of a 1,5-diene to an isomeric 1,5-diene.
B)The Cope rearrangement takes place readily in a suprafacial pathway under photochemical conditions.
C)The Cope rearrangement involves three electron pairs; two in p bonds and one in a s bond.
D)The Cope rearrangement takes place readily in a suprafacial pathway, when heated.
A)The Cope rearrangement involves the rearrangement of a 1,5-diene to an isomeric 1,5-diene.
B)The Cope rearrangement takes place readily in a suprafacial pathway under photochemical conditions.
C)The Cope rearrangement involves three electron pairs; two in p bonds and one in a s bond.
D)The Cope rearrangement takes place readily in a suprafacial pathway, when heated.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
44
What is the major organic product of the following Claisen rearrangement?
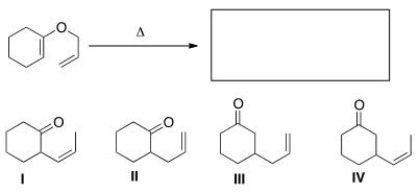
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
45
What is the major organic product of the following Claisen rearrangement?
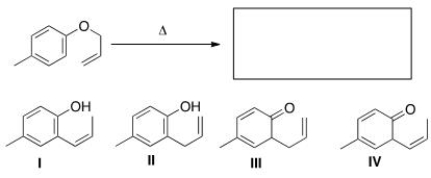
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
46
Consider the following reaction sequence.What is the correct classification of the second reaction in the sequence?
![<strong><sup> </sup>Consider the following reaction sequence.What is the correct classification of the second reaction in the sequence? </strong> A)[2 + 2] Cycloaddition B)[6 + 4] Cycloaddition C)[4 + 4] Cycloaddition D)[4 + 2] Cycloaddition](https://d2lvgg3v3hfg70.cloudfront.net/TB5872/11eb4ad7_6c09_60d9_99b2_fba7559ab0dc_TB5872_00.jpg)
A)[2 + 2] Cycloaddition
B)[6 + 4] Cycloaddition
C)[4 + 4] Cycloaddition
D)[4 + 2] Cycloaddition
![<strong><sup> </sup>Consider the following reaction sequence.What is the correct classification of the second reaction in the sequence? </strong> A)[2 + 2] Cycloaddition B)[6 + 4] Cycloaddition C)[4 + 4] Cycloaddition D)[4 + 2] Cycloaddition](https://d2lvgg3v3hfg70.cloudfront.net/TB5872/11eb4ad7_6c09_60d9_99b2_fba7559ab0dc_TB5872_00.jpg)
A)[2 + 2] Cycloaddition
B)[6 + 4] Cycloaddition
C)[4 + 4] Cycloaddition
D)[4 + 2] Cycloaddition

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
47
What is the major organic product of the following oxy-Cope rearrangement?
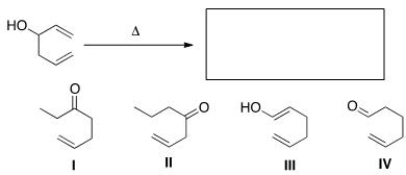
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck








