Deck 10: The Liquid and Solid States
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/107
العب
ملء الشاشة (f)
Deck 10: The Liquid and Solid States
1
Which of the following statements is correct?
A)A liquid which has a high boiling point has a higher vapor pressure at a given temperature than a liquid with a low boiling point.
B)Liquids boil at higher temperatures in the mountains due to the higher altitude.
C)Evaporation is an exothermic process, since heat is given off when a substance vaporizes.
D)Since acetone (nail polish remover) evaporates more readily than water, one would assume that acetone has weaker intermolecular forces.
E)The phase change from solid to gas is exothermic.
A)A liquid which has a high boiling point has a higher vapor pressure at a given temperature than a liquid with a low boiling point.
B)Liquids boil at higher temperatures in the mountains due to the higher altitude.
C)Evaporation is an exothermic process, since heat is given off when a substance vaporizes.
D)Since acetone (nail polish remover) evaporates more readily than water, one would assume that acetone has weaker intermolecular forces.
E)The phase change from solid to gas is exothermic.
Since acetone (nail polish remover) evaporates more readily than water, one would assume that acetone has weaker intermolecular forces.
2
The pictured change of state occurs at constant temperature even though heat is being removed.Where does this change of state occur on the cooling curve? 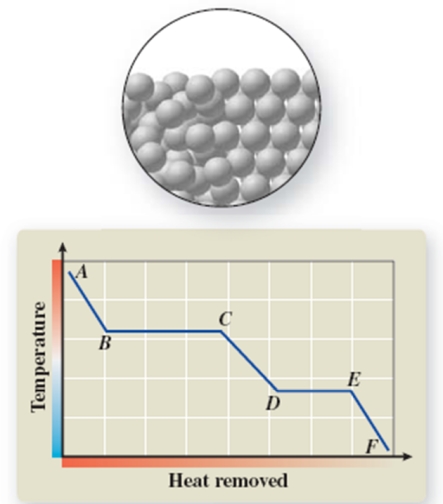
A)AB
B)BC
C)CD
D)DE
E)EF

A)AB
B)BC
C)CD
D)DE
E)EF
DE
3
Three phases of water are shown in the figure.List the terms used to identify the phase changes indicated by the arrows. 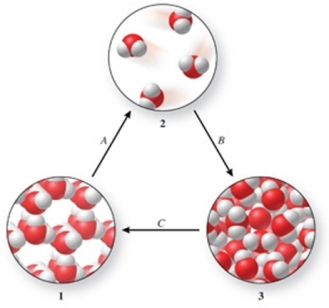
A)A = sublimation, B = vaporization, C = freezing
B)A = vaporization, B = condensation, C = melting
C)A = vaporization, B = deposition, C = melting
D)A = sublimation, B = condensation, C = freezing
E)A = condensation, B = sublimation, C = freezing

A)A = sublimation, B = vaporization, C = freezing
B)A = vaporization, B = condensation, C = melting
C)A = vaporization, B = deposition, C = melting
D)A = sublimation, B = condensation, C = freezing
E)A = condensation, B = sublimation, C = freezing
A = sublimation, B = condensation, C = freezing
4
The pictured change of state occurs at constant temperature even though heat is being removed.Where does this change of state occur on the cooling curve? 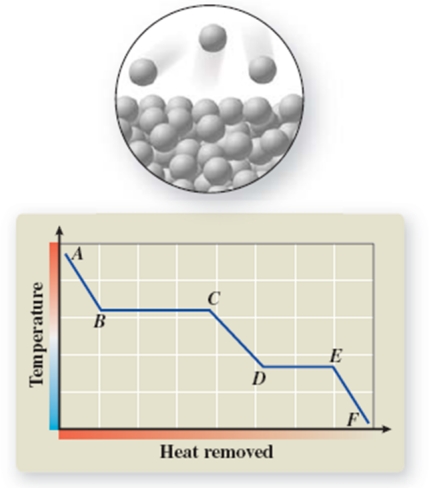
A)AB
B)BC
C)CD
D)DE
E)EF

A)AB
B)BC
C)CD
D)DE
E)EF

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which of the following statements is correct?
A)The phase change from solid to liquid is exothermic.
B)Liquids boil at lower than normal temperatures in Death Valley, since it is below sea level.
C)A substance with a low boiling point would be expected to have a relatively low vapor pressure at a low temperature.
D)The phase change from liquid to gas is exothermic.
E)When a substance condenses, heat energy is given off.
A)The phase change from solid to liquid is exothermic.
B)Liquids boil at lower than normal temperatures in Death Valley, since it is below sea level.
C)A substance with a low boiling point would be expected to have a relatively low vapor pressure at a low temperature.
D)The phase change from liquid to gas is exothermic.
E)When a substance condenses, heat energy is given off.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
6
What phase transition is occurring between points D and E on the heating curve? 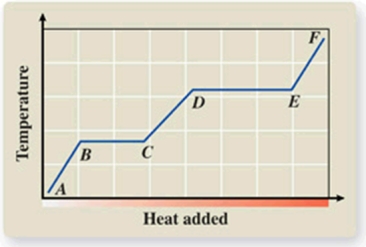
A)melting
B)freezing
C)evaporation
D)sublimation
E)deposition

A)melting
B)freezing
C)evaporation
D)sublimation
E)deposition

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following statements is correct?
A)When the temperature of a sample of liquid is increased, the molecules move faster, but there is no change in the vapor pressure of the sample.
B)The melting point of metallic sodium is 97.8oC, so at a temperature of 25oC, it is a liquid.
C)If you cook an egg on a mountain, it will require a short cooking time, due to the high elevation.
D)When liquids evaporate, it requires an input of energy from the surroundings, so the surroundings become warm.
E)Nitrogen gas has a boiling point of -196oC, so if it is cooled to -200oC, it condenses.
A)When the temperature of a sample of liquid is increased, the molecules move faster, but there is no change in the vapor pressure of the sample.
B)The melting point of metallic sodium is 97.8oC, so at a temperature of 25oC, it is a liquid.
C)If you cook an egg on a mountain, it will require a short cooking time, due to the high elevation.
D)When liquids evaporate, it requires an input of energy from the surroundings, so the surroundings become warm.
E)Nitrogen gas has a boiling point of -196oC, so if it is cooled to -200oC, it condenses.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
8
What phase transition is occurring between points B and C on the cooling curve? 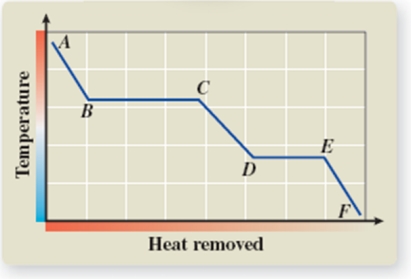
A)melting
B)condensation
C)evaporation
D)sublimation
E)deposition

A)melting
B)condensation
C)evaporation
D)sublimation
E)deposition

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
9
What phase transition is occurring between points D and E on the cooling curve? 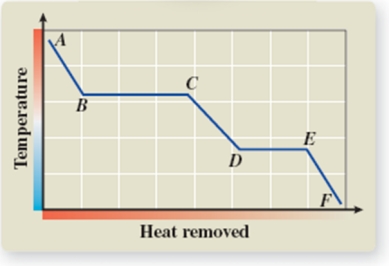
A)melting
B)freezing
C)evaporation
D)sublimation
E)deposition

A)melting
B)freezing
C)evaporation
D)sublimation
E)deposition

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
10
The pictured change of state occurs at constant temperature even though heat is being added.Where does this change of state occur on the cooling curve? 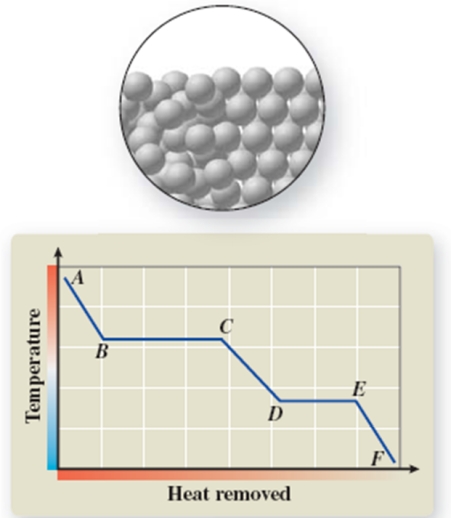
A)AB
B)BC
C)CD
D)DE
E)EF

A)AB
B)BC
C)CD
D)DE
E)EF

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
11
From the vapor pressure curve of propane, it can be seen that the normal boiling point of propane is about _________. 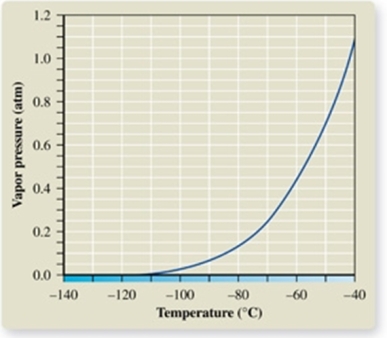
A)-137oC
B)-120oC
C)-60oC
D)-42oC
E)0oC

A)-137oC
B)-120oC
C)-60oC
D)-42oC
E)0oC

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
12
What phase change is occurring in the figure, and is it endothermic or exothermic? 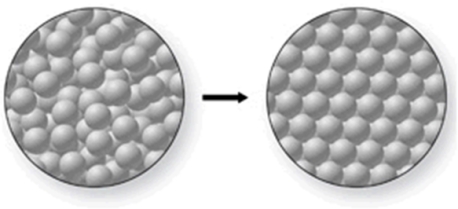
A)melting; exothermic
B)freezing; endothermic
C)freezing; exothermic
D)melting; endothermic
E)condensation; endothermic

A)melting; exothermic
B)freezing; endothermic
C)freezing; exothermic
D)melting; endothermic
E)condensation; endothermic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
13
Three phases of water are shown in the figure.List the terms used to identify the phase changes indicated by the arrows. 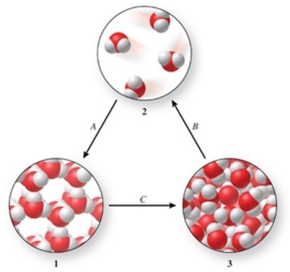
A)A = sublimation, B = vaporization, C = freezing
B)A = condensation, B = sublimation, C = melting
C)A = freezing, B = vaporization, C = melting
D)A = deposition, B = vaporization, C = melting
E)A = deposition, B = sublimation, C = freezing

A)A = sublimation, B = vaporization, C = freezing
B)A = condensation, B = sublimation, C = melting
C)A = freezing, B = vaporization, C = melting
D)A = deposition, B = vaporization, C = melting
E)A = deposition, B = sublimation, C = freezing

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
14
What phase transition is occurring between points B and C on the heating curve? 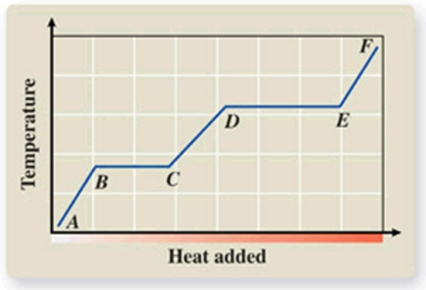
A)melting
B)condensation
C)evaporation
D)sublimation
E)deposition

A)melting
B)condensation
C)evaporation
D)sublimation
E)deposition

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
15
The atmospheric pressure on Mount Everest is about 0.30 atm.From the vapor pressure curve of propane, it can be seen the boiling point of propane at this elevation is about _________. 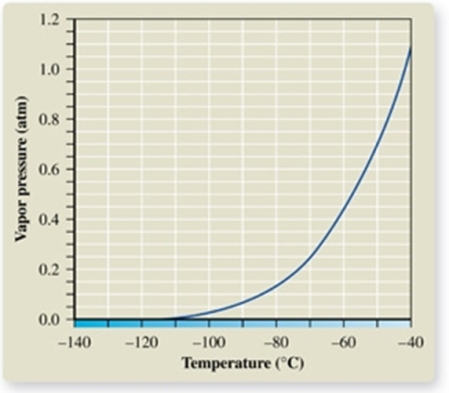
A)-137oC
B)-120oC
C)-67oC
D)-42oC
E)0oC

A)-137oC
B)-120oC
C)-67oC
D)-42oC
E)0oC

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
16
The pictured change of state occurs at constant temperature even though heat is being added.Where does this change of state occur on the cooling curve? 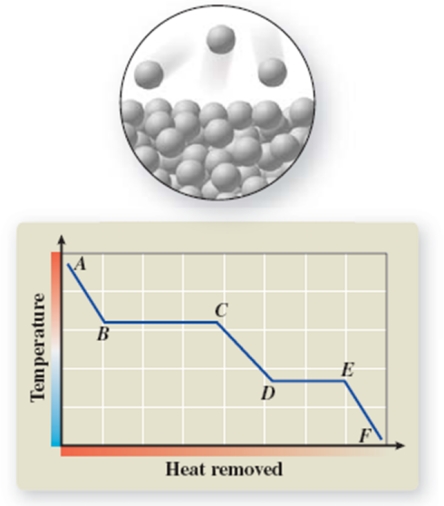
A)AB
B)BC
C)CD
D)DE
E)EF

A)AB
B)BC
C)CD
D)DE
E)EF

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which of the molecular-level images in the figure shows the liquid and vapor phases in equilibrium in a closed container? 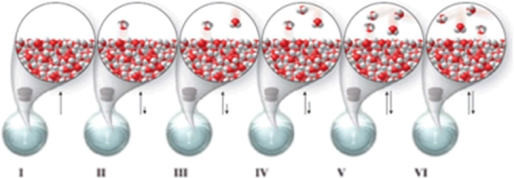
A)I, II, and III
B)none of the images
C)I
D)V
E)V and VI

A)I, II, and III
B)none of the images
C)I
D)V
E)V and VI

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
18
What phase change is occurring in the figure, and is it endothermic or exothermic? 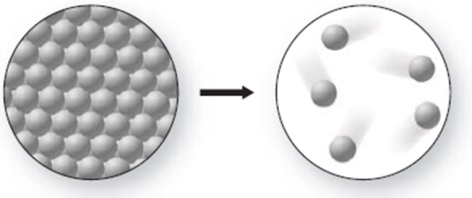
A)melting; exothermic
B)freezing; endothermic
C)freezing; exothermic
D)melting; endothermic
E)sublimation; endothermic

A)melting; exothermic
B)freezing; endothermic
C)freezing; exothermic
D)melting; endothermic
E)sublimation; endothermic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
19
From the vapor pressure curve of acetone, it can be seen that the normal boiling point of acetone is about _________. 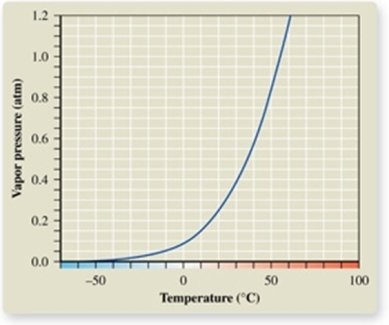
A)-50oC
B)0oC
C)50oC
D)57oC
E)100oC

A)-50oC
B)0oC
C)50oC
D)57oC
E)100oC

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which of the following statements is correct?
A)At the melting point of a substance, the solid, liquid, and gas phases are all in equilibrium.
B)The freezing point and the melting point of a substance are the same.
C)There is no fundamental difference in the melting process for covalent substances and ionic substances.
D)When iodine vapor cools at atmospheric pressure, it condenses to the liquid state.
E)In order for a substance to go from the liquid phase to the gas phase, it must give off energy to the surroundings.
A)At the melting point of a substance, the solid, liquid, and gas phases are all in equilibrium.
B)The freezing point and the melting point of a substance are the same.
C)There is no fundamental difference in the melting process for covalent substances and ionic substances.
D)When iodine vapor cools at atmospheric pressure, it condenses to the liquid state.
E)In order for a substance to go from the liquid phase to the gas phase, it must give off energy to the surroundings.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which of the following is not an attractive force that acts between the individual molecules of CH3OH?
A)London dispersion forces
B)dipole-dipole forces
C)hydrogen-bonding forces
D)covalent bonds
E)none of these is correct
A)London dispersion forces
B)dipole-dipole forces
C)hydrogen-bonding forces
D)covalent bonds
E)none of these is correct

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
22
Calculate the amount of heat energy required to evaporate 55.0 g of water at 100.0 C.(molar heat of vaporization of liquid water = 4.07 x 104 J/mol)
A)1.24 x 102 kJ
B)2.24 x 103 kJ
C)4.03 x 104 kJ
D)1.24 x 108 kJ
E)2.43 x 10-2 kJ
A)1.24 x 102 kJ
B)2.24 x 103 kJ
C)4.03 x 104 kJ
D)1.24 x 108 kJ
E)2.43 x 10-2 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
23
Calculate the amount of heat energy, in units of kilojoules, required to convert 50.0 g of liquid ethanol, CH3CH2OH, at 25.5 C to gaseous ethanol at 92.0 C.(Cethanol liquid = 2.44 J/g C; Cethanol gas = 1.42 J/g C; molar heat of vaporization of liquid ethanol = 3.86 x 104 J/mol.The boiling point of ethanol is 78.4 C and its molar mass is 46.07 g/mol.)
A)49.3 kJ
B)1.93 x 103 kJ
C)0.862 kJ
D)1.35 x 102 kJ
E)2.67 kJ
A)49.3 kJ
B)1.93 x 103 kJ
C)0.862 kJ
D)1.35 x 102 kJ
E)2.67 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
24
Which of the following substances is most likely to be a gas at room temperature and atmospheric pressure?
A)Cs
B)SO2
C)I2
D)CCl4
E)Ni
A)Cs
B)SO2
C)I2
D)CCl4
E)Ni

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
25
Calculate the amount of heat energy required to convert 35.0 g of ice at -12.5 C to water at 24.0 C.(Cwater = 4.18 J/g C; Cice = 2.03 J/g C; molar heat of fusion of ice = 6.01 x 103 J/mol)
A)8.88 x 102 J
B)1.17 x 104 J
C)1.61 x 1010 J
D)3.51 x 103 J
E)1.61 x 104 J
A)8.88 x 102 J
B)1.17 x 104 J
C)1.61 x 1010 J
D)3.51 x 103 J
E)1.61 x 104 J

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
26
The diagram represents the physical state of a substance.Where does this physical state occur on the heating curve? 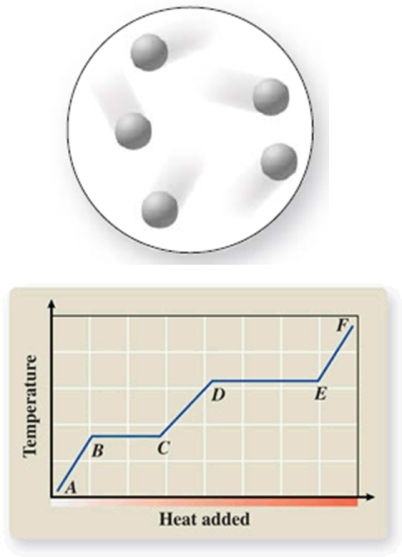
A)AB
B)BC
C)CD
D)DE
E)EF

A)AB
B)BC
C)CD
D)DE
E)EF

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which of the following substances is most likely to be a gas at room temperature and atmospheric pressure?
A)I2
B)Pb
C)Ar
D)CCl4
E)C8H18
A)I2
B)Pb
C)Ar
D)CCl4
E)C8H18

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which of the following statements regarding intermolecular forces is incorrect?
A)An intermolecular force is an attractive force that operates between molecules.
B)Bonding forces are much stronger than intermolecular forces.
C)London dispersion forces occur in all atoms and molecules.
D)London dispersion forces are the result of permanent dipoles in atoms or molecules.
E)Molecules which have hydrogen bonded to F, O, or N can undergo hydrogen-bonding.
A)An intermolecular force is an attractive force that operates between molecules.
B)Bonding forces are much stronger than intermolecular forces.
C)London dispersion forces occur in all atoms and molecules.
D)London dispersion forces are the result of permanent dipoles in atoms or molecules.
E)Molecules which have hydrogen bonded to F, O, or N can undergo hydrogen-bonding.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
29
Calculate the amount of heat energy, in units of kilojoules, required to melt 75.0 g of ice at 0.0 C.(molar heat of fusion of ice = 6.01 x 103 J/mol)
A)4.50 x 102 kJ
B)25.0 kJ
C)13.0 kJ
D)2.25 x 103 kJ
E)4.50 x 10-1 kJ
A)4.50 x 102 kJ
B)25.0 kJ
C)13.0 kJ
D)2.25 x 103 kJ
E)4.50 x 10-1 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
30
Calculate the amount of heat energy, in units of kilojoules, required to evaporate 35.0 g of ethanol, CH3CH2OH, at 78.4 C, ethanol's boiling point.(The molar heat of vaporization of liquid ethanol is 3.86 x 104 J/mol.The molar mass of ethanol is 46.07 g/mol.)
A)29.3 kJ
B)1.35 x 103 kJ
C)1.10 x 103 kJ
D)8.38 x 102 kJ
E)9.21 x 10-4 kJ
A)29.3 kJ
B)1.35 x 103 kJ
C)1.10 x 103 kJ
D)8.38 x 102 kJ
E)9.21 x 10-4 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which of the following substances is most likely to be a gas at room temperature and atmospheric pressure?
A)Ni
B)Hg
C)CO2
D)C7H16
E)CBr4
A)Ni
B)Hg
C)CO2
D)C7H16
E)CBr4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
32
The physical properties of a substance influenced by the strength of intermolecular forces include all of the following except:
A)density.
B)boiling point.
C)surface tension.
D)melting point.
E)mass.
A)density.
B)boiling point.
C)surface tension.
D)melting point.
E)mass.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
33
Calculate the amount of heat energy required to evaporate 27.5 g of water at 100.0 C.(molar heat of vaporization of liquid water = 4.07 x 104 J/mol)
A)62.1 kJ
B)1.12 x 103 kJ
C)2.02 x 104 kJ
D)6.20 x 107 kJ
E)1.22 x 10-2 kJ
A)62.1 kJ
B)1.12 x 103 kJ
C)2.02 x 104 kJ
D)6.20 x 107 kJ
E)1.22 x 10-2 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
34
Calculate the amount of heat energy required to melt 25.0 g of ice at 0.0 C.(molar heat of fusion of ice = 6.01 x 103 J/mol)
A)1.50 x 102 kJ
B)8.34 kJ
C)4.33 kJ
D)7.50 x 102 kJ
E)1.50 x 10-1 kJ
A)1.50 x 102 kJ
B)8.34 kJ
C)4.33 kJ
D)7.50 x 102 kJ
E)1.50 x 10-1 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
35
Calculate the amount of heat energy required to convert 27.5 g of water at 62.5 C to steam at 124.0 C.(Cwater = 4.18 J/g C; Cice = 2.03 J/goC; Csteam = 2.02 J/g C; molar heat of fusion of ice = 6.01 x 103 J/mol; molar heat of vaporization of liquid water = 4.07 x 104 J/mol)
A)6.15 x 104 kJ
B)1.23 x 102 kJ
C)4.31 x 102 kJ
D)31.8 kJ
E)1.33 kJ
A)6.15 x 104 kJ
B)1.23 x 102 kJ
C)4.31 x 102 kJ
D)31.8 kJ
E)1.33 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
36
Calculate the amount of heat energy required to convert 55.0 g of water at 62.5oC to steam at 124.0 C.(Cwater = 4.18 J/g C; Csteam = 2.02 J/g C; molar heat of vaporization of liquid water = 4.07 x 104 J/mol)
A)2.25 x 103 kJ
B)1.23 x 102 kJ
C)0.862 kJ
D)1.35 x 102 kJ
E)2.67 kJ
A)2.25 x 103 kJ
B)1.23 x 102 kJ
C)0.862 kJ
D)1.35 x 102 kJ
E)2.67 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
37
The diagram represents the physical state of a substance.Where does this physical state occur on the cooling curve? 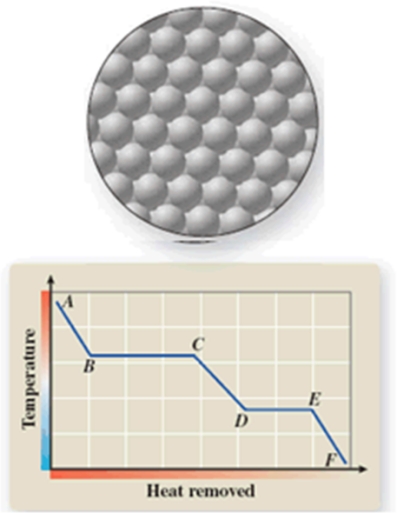
A)AB
B)BC
C)CD
D)DE
E)EF

A)AB
B)BC
C)CD
D)DE
E)EF

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
38
The diagram represents the physical state of a substance.Where does this physical state occur on the heating curve? 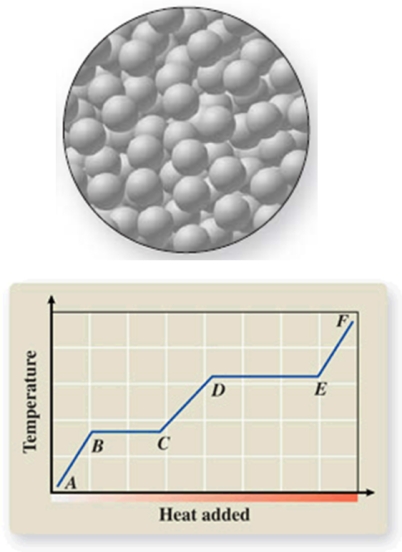
A)AB
B)BC
C)CD
D)DE
E)EF

A)AB
B)BC
C)CD
D)DE
E)EF

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
39
The physical properties of a substance influenced by the strength of intermolecular forces include all of the following except:
A)melting point.
B)boiling point.
C)viscosity.
D)vapor pressure.
E)mass.
A)melting point.
B)boiling point.
C)viscosity.
D)vapor pressure.
E)mass.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
40
The diagram represents the physical state of a substance.Where does this physical state occur on the cooling curve? 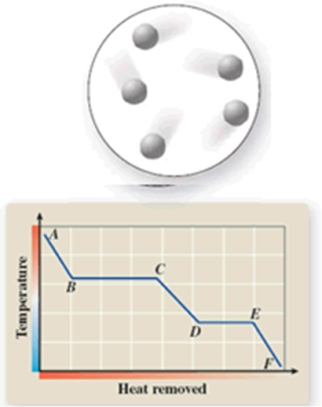
A)AB
B)BC
C)CD
D)DE
E)EF

A)AB
B)BC
C)CD
D)DE
E)EF

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
41
Rank the following substances in order of increasing boiling point: F2, Ne, He, Cl2
A)F2 < Ne < He < Cl2
B)F2 < He < Ne < Cl2
C)F2 < Cl2 < He < Ne
D)He < Ne < F2 < Cl2
E)Cl2 < F2 < Ne < He
A)F2 < Ne < He < Cl2
B)F2 < He < Ne < Cl2
C)F2 < Cl2 < He < Ne
D)He < Ne < F2 < Cl2
E)Cl2 < F2 < Ne < He

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
42
Which of the following has the highest boiling point?
A)H2
B)H2O
C)H2S
D)H2Se
E)H2Te
A)H2
B)H2O
C)H2S
D)H2Se
E)H2Te

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
43
Which of the following has the highest boiling point?
A)CH4
B)CF4
C)CCl4
D)CBr4
E)CI4
A)CH4
B)CF4
C)CCl4
D)CBr4
E)CI4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
44
Which of the following has the highest boiling point?
A)H2
B)HF
C)HCl
D)HBr
E)HI
A)H2
B)HF
C)HCl
D)HBr
E)HI

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
45
Which of the molecules in the figure has hydrogen bonding in the pure liquid state? 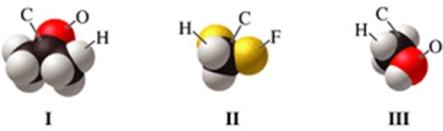
A)I only
B)II only
C)III only
D)II and III
E)I and II

A)I only
B)II only
C)III only
D)II and III
E)I and II

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
46
Which of the following molecules experience dipole-dipole forces?
A)CO2
B)SO2
C)CCl4
D)H2S
E)both SO2 and H2S
A)CO2
B)SO2
C)CCl4
D)H2S
E)both SO2 and H2S

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
47
Which of the molecules in the figure has hydrogen bonding in the pure liquid state? 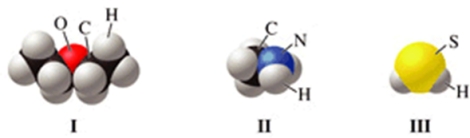
A)I only
B)II only
C)III only
D)II and III
E)I and II

A)I only
B)II only
C)III only
D)II and III
E)I and II

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which of the following substances can participate in hydrogen bonding?
A)SiH4
B)CH2Cl2
C)H2O
D)PH3
E)both H2O and PH3
A)SiH4
B)CH2Cl2
C)H2O
D)PH3
E)both H2O and PH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
49
Which of the following has the highest vapor pressure at a given temperature?
A)CH4
B)CF4
C)CCl4
D)CBr4
E)CI4
A)CH4
B)CF4
C)CCl4
D)CBr4
E)CI4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
50
Rank the following substances in order of increasing intermolecular forces: He, HF, F2, Ne
A)He < HF < F2 < Ne
B)He < HF < Ne < F2
C)He < Ne < F2 < HF
D)He < F2 < Ne < HF
E)HF < Ne < F2 < He
A)He < HF < F2 < Ne
B)He < HF < Ne < F2
C)He < Ne < F2 < HF
D)He < F2 < Ne < HF
E)HF < Ne < F2 < He

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
51
Which of the following molecules experience dipole-dipole forces?
A)NO2
B)CO2
C)CBr4
D)SO3
E)all of these choices are correct
A)NO2
B)CO2
C)CBr4
D)SO3
E)all of these choices are correct

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which of the following molecules experience dipole-dipole forces?
A)NO
B)Cl2
C)CH4
D)SiH4
E)all of these
A)NO
B)Cl2
C)CH4
D)SiH4
E)all of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
53
Rank the following substances in order of increasing intermolecular forces: Ne, NH3, H2, O2
A)Ne < NH3 < H2 < O2
B)Ne < NH3 < O2 < H2
C)NH3 < O2 < H2 < Ne
D)O2 < H2 < Ne < NH3
E)H2 < Ne < O2 < NH3
A)Ne < NH3 < H2 < O2
B)Ne < NH3 < O2 < H2
C)NH3 < O2 < H2 < Ne
D)O2 < H2 < Ne < NH3
E)H2 < Ne < O2 < NH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
54
Rank the following substances in order of increasing boiling point: Cl2, Ar, Ne, Br2
A)Cl2 < Ar < Ne < Br2
B)Ar < Ne < Br2 < Cl2
C)Ne < Ar < Br2 < Cl2
D)Ne < Ar < Cl2 < Br2
E)Cl2 < Br2 < Ar < Ne
A)Cl2 < Ar < Ne < Br2
B)Ar < Ne < Br2 < Cl2
C)Ne < Ar < Br2 < Cl2
D)Ne < Ar < Cl2 < Br2
E)Cl2 < Br2 < Ar < Ne

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
55
Which of the following substances can participate in hydrogen bonding?
A)CH4
B)HF
C)CH3COCH3
D)SiH4
E)all of these choices are correct
A)CH4
B)HF
C)CH3COCH3
D)SiH4
E)all of these choices are correct

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
56
Which of the following substances can participate in hydrogen bonding?
A)CH4
B)CHCl3
C)H2S
D)NH3
E)both H2S and NH3
A)CH4
B)CHCl3
C)H2S
D)NH3
E)both H2S and NH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
57
Rank the following substances in order of increasing boiling point: O2, Ne, Ar, H2
A)O2 < Ne < Ar < H2
B)O2 < Ar < Ne < H2
C)H2 < Ne < Ar < O2
D)H2 < Ne < O2 < Ar
E)Ar < O2 < Ne < H2
A)O2 < Ne < Ar < H2
B)O2 < Ar < Ne < H2
C)H2 < Ne < Ar < O2
D)H2 < Ne < O2 < Ar
E)Ar < O2 < Ne < H2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
58
Rank the following substances in order of increasing intermolecular forces: Ar, H2O, N2, O2
A)Ar < H2O < N2 < O2
B)Ar < H2O < O2 < N2
C)H2O < N2 < O2 < Ar
D)N2 < O2 < Ar < H2O
E)Ar < N2 < O2 < H2O
A)Ar < H2O < N2 < O2
B)Ar < H2O < O2 < N2
C)H2O < N2 < O2 < Ar
D)N2 < O2 < Ar < H2O
E)Ar < N2 < O2 < H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
59
Which of the following substances is most likely to be a gas at room temperature?
A)CBr4
B)F2
C)Mn
D)C6H14
E)Sr
A)CBr4
B)F2
C)Mn
D)C6H14
E)Sr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
60
Which of the following has the strongest London dispersion forces?
A)CH4
B)CF4
C)CCl4
D)CBr4
E)CI4
A)CH4
B)CF4
C)CCl4
D)CBr4
E)CI4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
61
A solid substance has a high melting point, is hard, brittle, and conducts electricity when molten.This substance is probably a(n)________ solid.
A)metallic
B)ionic
C)network
D)nonpolar molecular
E)polar molecular
A)metallic
B)ionic
C)network
D)nonpolar molecular
E)polar molecular

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
62
Which of the following statements regarding the liquid state is incorrect?
A)Detergents increase the surface tension of water, in order to help it stay in droplet form.
B)If ice were denser than liquid water, lakes would freeze from the bottom up.
C)In the summer, one would use a motor oil with a high viscosity rating to better lubricate the engine at high temperatures.
D)A liquid such as C15H32 would be expected to be more viscous than C6H14.
E)Water rises in a glass capillary tube due to the interactions between the water molecules and the glass.
A)Detergents increase the surface tension of water, in order to help it stay in droplet form.
B)If ice were denser than liquid water, lakes would freeze from the bottom up.
C)In the summer, one would use a motor oil with a high viscosity rating to better lubricate the engine at high temperatures.
D)A liquid such as C15H32 would be expected to be more viscous than C6H14.
E)Water rises in a glass capillary tube due to the interactions between the water molecules and the glass.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
63
Select the pair of substances which has the one with the higher equilibrium vapor pressure at a given temperature listed first.
A)H2O, HCl
B)CH3OH, CH4
C)CH3CH2CH2OH, CH3COCH3
D)AsH3, PH3
E)Cl2, Br2
A)H2O, HCl
B)CH3OH, CH4
C)CH3CH2CH2OH, CH3COCH3
D)AsH3, PH3
E)Cl2, Br2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
64
Which of the following statements regarding the liquid state is incorrect?
A)Liquids are about 1000 times denser than gases.
B)Liquids are normally about 90-95% as dense as their corresponding solid.
C)The viscosity of a liquid is a measure of its resistance to flow.
D)Molecules with high intermolecular forces will have high surface tension.
E)Surface tension increases as temperature increases.
A)Liquids are about 1000 times denser than gases.
B)Liquids are normally about 90-95% as dense as their corresponding solid.
C)The viscosity of a liquid is a measure of its resistance to flow.
D)Molecules with high intermolecular forces will have high surface tension.
E)Surface tension increases as temperature increases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
65
Which choice correctly lists the intermolecular forces present in CH3Br?
A)London forces only
B)London forces and dipole-dipole forces
C)London forces, dipole-dipole forces, and hydrogen bonding
D)dipole-dipole forces and hydrogen bonding
E)London forces and hydrogen bonding
A)London forces only
B)London forces and dipole-dipole forces
C)London forces, dipole-dipole forces, and hydrogen bonding
D)dipole-dipole forces and hydrogen bonding
E)London forces and hydrogen bonding

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
66
Which of the following statements regarding intermolecular forces is incorrect?
A)Hydrogen bonding is the strongest of the intermolecular forces.
B)London dispersion forces increase with increasing molecular size.
C)Dipole-dipole forces are present in all atoms and molecules.
D)In order for a pure substance to participate in hydrogen bonding, it must have hydrogen bonded to F, O, or N.
E)Intermolecular forces are much weaker than bonding forces.
A)Hydrogen bonding is the strongest of the intermolecular forces.
B)London dispersion forces increase with increasing molecular size.
C)Dipole-dipole forces are present in all atoms and molecules.
D)In order for a pure substance to participate in hydrogen bonding, it must have hydrogen bonded to F, O, or N.
E)Intermolecular forces are much weaker than bonding forces.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
67
Which of the following statements regarding the solid state is incorrect?
A)Many metals follow a close-packing arrangement of their atoms, much like the way fruit is stacked in the grocery store.
B)The forces holding the particles together in a solid will affect their properties.
C)Network solids tend to have very high melting points.
D)Nonpolar molecular solids tend to be good conductors of heat and electricity.
E)Ionic solids tend to be brittle.
A)Many metals follow a close-packing arrangement of their atoms, much like the way fruit is stacked in the grocery store.
B)The forces holding the particles together in a solid will affect their properties.
C)Network solids tend to have very high melting points.
D)Nonpolar molecular solids tend to be good conductors of heat and electricity.
E)Ionic solids tend to be brittle.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
68
Select the pair of substances which has the one with the higher equilibrium vapor pressure at a given temperature listed first.
A)H2O, H2S
B)CH3CH2OH, CH3OH
C)CH3COCH3, CH3CH2CH2OH
D)NH3, PH3
E)Br2, Cl2
A)H2O, H2S
B)CH3CH2OH, CH3OH
C)CH3COCH3, CH3CH2CH2OH
D)NH3, PH3
E)Br2, Cl2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
69
A solid substance has a high melting point, is hard, and is a good conductor of both heat and electricity.It is also malleable and ductile.This substance is probably a(n) ________ solid.
A)metallic
B)ionic
C)network
D)nonpolar molecular
E)polar molecular
A)metallic
B)ionic
C)network
D)nonpolar molecular
E)polar molecular

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
70
Which of the following statements regarding the solid state is incorrect?
A)Solids are not very compressible.
B)Solids have rigid shapes and definite sizes.
C)If a material solidifies in a partially disordered state, it is an amorphous solid.
D)Substances which solidify in a very orderly manner form crystalline solids.
E)Glass is an example of a crystalline solid.
A)Solids are not very compressible.
B)Solids have rigid shapes and definite sizes.
C)If a material solidifies in a partially disordered state, it is an amorphous solid.
D)Substances which solidify in a very orderly manner form crystalline solids.
E)Glass is an example of a crystalline solid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
71
Arrange the following substances in order of increasing boiling point: CH3OH, CH4, CH3CH2OH, HOCH2CH2OH
A)CH3OH < CH4 < CH3CH2OH < HOCH2CH2OH
B)CH4 < CH3OH < CH3CH2OH < HOCH2CH2OH
C)CH4 < CH3OH < HOCH2CH2OH < CH3CH2OH
D)CH3OH < CH4 < HOCH2CH2OH < CH3CH2OH
E)HOCH2CH2OH < CH3CH2OH < CH3OH < CH4
A)CH3OH < CH4 < CH3CH2OH < HOCH2CH2OH
B)CH4 < CH3OH < CH3CH2OH < HOCH2CH2OH
C)CH4 < CH3OH < HOCH2CH2OH < CH3CH2OH
D)CH3OH < CH4 < HOCH2CH2OH < CH3CH2OH
E)HOCH2CH2OH < CH3CH2OH < CH3OH < CH4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
72
Arrange the following substances in order of increasing boiling point: N2, H2, NH3, PH3
A)H2 < N2 < PH3 < NH3
B)N2 < H2 < PH3 < NH3
C)H2 < N2 < NH3 < PH3
D)N2 < PH3 < NH3 < H2
E)NH3 < PH3 < N2 < H2
A)H2 < N2 < PH3 < NH3
B)N2 < H2 < PH3 < NH3
C)H2 < N2 < NH3 < PH3
D)N2 < PH3 < NH3 < H2
E)NH3 < PH3 < N2 < H2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
73
Which of the following statements regarding intermolecular forces is incorrect?
A)Hydrogen bonding is the strongest of the intermolecular forces for substances of similar molar mass.
B)London dispersion forces are present in all atoms and molecules.
C)Dipole-dipole forces increase with increasing molecular size.
D)Bonding forces are much stronger than intermolecular forces.
E)Hydrogen bonding is the reason for the abnormally high boiling point of water.
A)Hydrogen bonding is the strongest of the intermolecular forces for substances of similar molar mass.
B)London dispersion forces are present in all atoms and molecules.
C)Dipole-dipole forces increase with increasing molecular size.
D)Bonding forces are much stronger than intermolecular forces.
E)Hydrogen bonding is the reason for the abnormally high boiling point of water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
74
A solid substance has a very high melting point, is very hard, and is a nonconductor whether molten or solid.This substance is probably a(n)________ solid.
A)metallic
B)ionic
C)network
D)nonpolar molecular
E)polar molecular
A)metallic
B)ionic
C)network
D)nonpolar molecular
E)polar molecular

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
75
Which choice correctly lists the intermolecular forces present in CH4?
A)London forces only
B)London forces and dipole-dipole forces
C)London forces, dipole-dipole forces, and hydrogen bonding
D)dipole-dipole forces and hydrogen bonding
E)London forces and hydrogen bonding
A)London forces only
B)London forces and dipole-dipole forces
C)London forces, dipole-dipole forces, and hydrogen bonding
D)dipole-dipole forces and hydrogen bonding
E)London forces and hydrogen bonding

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
76
Which of the following statements regarding the solid state is incorrect?
A)Most polymers, such as rubbers and plastics, are amorphous.
B)Minerals, such as quartz and amethyst, are crystalline solids.
C)Solids normally do not flow.
D)The particles of a solid are held in a rigid, 3-dimensional array.
E)The arrangement of the particles in a crystal lattice is called space packing.
A)Most polymers, such as rubbers and plastics, are amorphous.
B)Minerals, such as quartz and amethyst, are crystalline solids.
C)Solids normally do not flow.
D)The particles of a solid are held in a rigid, 3-dimensional array.
E)The arrangement of the particles in a crystal lattice is called space packing.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
77
Which of the following statements regarding the liquid state is incorrect?
A)Liquid water is denser than ice (solid water).
B)The viscosity of a liquid is related to its intermolecular forces-the stronger the forces, the higher the viscosity.
C)As temperature increases, so will the viscosity of a liquid.
D)Substances with strong intermolecular forces have high surface tension.
E)Detergents interfere with the attractive forces among water molecules to lower the surface tension of the water.
A)Liquid water is denser than ice (solid water).
B)The viscosity of a liquid is related to its intermolecular forces-the stronger the forces, the higher the viscosity.
C)As temperature increases, so will the viscosity of a liquid.
D)Substances with strong intermolecular forces have high surface tension.
E)Detergents interfere with the attractive forces among water molecules to lower the surface tension of the water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
78
Which of the following is not one of the classifications into which solids can be organized?
A)metallic
B)ionic
C)molecular
D)liquid crystals
E)network
A)metallic
B)ionic
C)molecular
D)liquid crystals
E)network

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
79
Which choice correctly lists the intermolecular forces present in CH3NH2?
A)London forces only
B)dipole-dipole forces only
C)London forces, dipole-dipole forces, and hydrogen bonding
D)dipole-dipole forces and hydrogen bonding
E)hydrogen bonding only
A)London forces only
B)dipole-dipole forces only
C)London forces, dipole-dipole forces, and hydrogen bonding
D)dipole-dipole forces and hydrogen bonding
E)hydrogen bonding only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck
80
A solid substance has a moderate melting point, is fairly soft, and is a nonconductor.This substance is probably a(n) ________ solid.
A)metallic
B)ionic
C)network
D)nonpolar molecular
E)polar molecular
A)metallic
B)ionic
C)network
D)nonpolar molecular
E)polar molecular

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 107 في هذه المجموعة.
فتح الحزمة
k this deck








