Deck 3: Alkenes and Alkynes
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/70
العب
ملء الشاشة (f)
Deck 3: Alkenes and Alkynes
1
Which of the following isomers of C7H14 do you expect to be most stable?
A)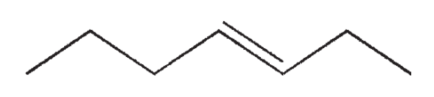
B)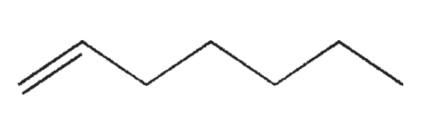
C)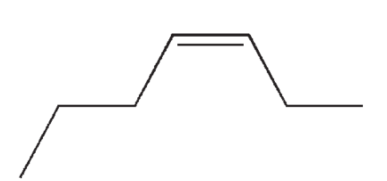
D)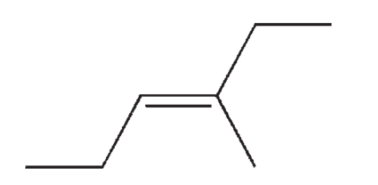
E)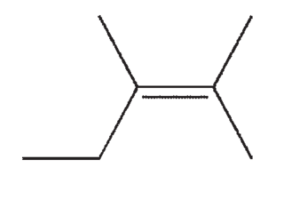
A)

B)

C)

D)

E)


2
What is the approximate value of the indicated bond angle in the following structure?
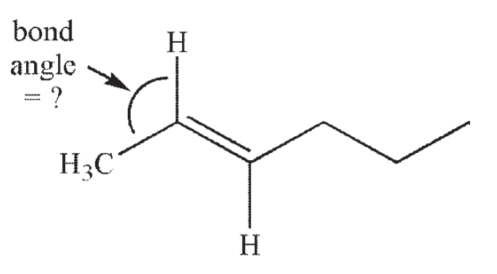
A) 60°
B) 90°
C) 109.5°
D) 120°
E) 180°

A) 60°
B) 90°
C) 109.5°
D) 120°
E) 180°
120°
3
Which alkene is the least stable?
A)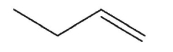
B)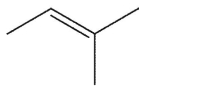
C)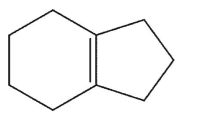
D)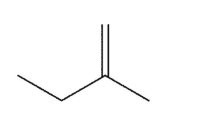
E)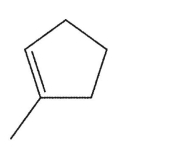
A)

B)

C)

D)

E)


4
How many signals do you expect to see in the 13C NMR spectrum of the structure shown?
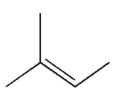
A) 1
B) 3
C) 4
D) 5
E) 10

A) 1
B) 3
C) 4
D) 5
E) 10

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
5
How many σ and π antibonding molecular orbitals are there in acetylene?
A) 2 σ*, 1 π*
B) 3 σ*, 1 π*
C) 3 σ*, 2 π *
D) 1 σ*, 3 π*
E) 1 σ*, 4 π*
A) 2 σ*, 1 π*
B) 3 σ*, 1 π*
C) 3 σ*, 2 π *
D) 1 σ*, 3 π*
E) 1 σ*, 4 π*

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
6
How many σ bonds and how many π bonds are in ethylene, C2H4?
A) 4 σ bonds and 1 π bond
B) 4 σ bonds and 2 π bonds
C) 5 σ bonds and 1 π bond
D) 1 σ bond and 1 π bond
E) 2 σ bonds and 5 π bonds
A) 4 σ bonds and 1 π bond
B) 4 σ bonds and 2 π bonds
C) 5 σ bonds and 1 π bond
D) 1 σ bond and 1 π bond
E) 2 σ bonds and 5 π bonds

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
7
What atomic orbitals are involved in the formation of the σ bond between the two carbons indicated in this structure?
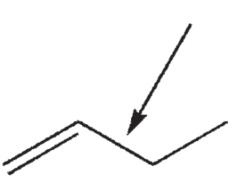
A) C sp2 and C sp2
B) C sp2 and C sp3
C) C sp3 and C sp3
D) C p and C p
E) C p and C sp3

A) C sp2 and C sp2
B) C sp2 and C sp3
C) C sp3 and C sp3
D) C p and C p
E) C p and C sp3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which of the following structures is allyl alcohol?
A)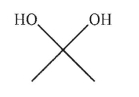
B)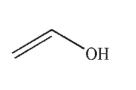
C)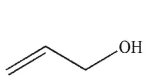
D)
E)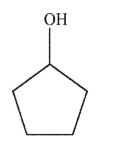
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which of the following structures is vinyl bromide?
A)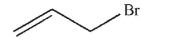
B)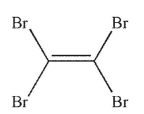
C)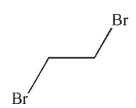
D)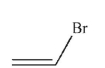
E)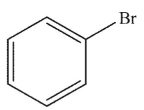
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
10
According to Bredt's rule, which of the following cycloalkenes is unlikely to exist?
A)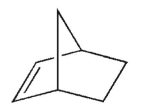
B)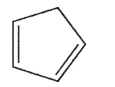
C)
D)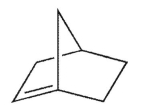
E)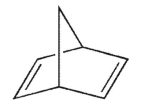
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which of the following structures correctly represents trans-3-heptene?
A)
B)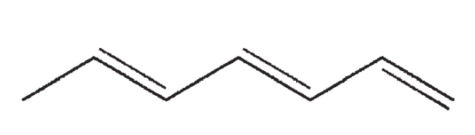
C)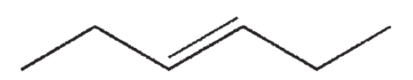
D)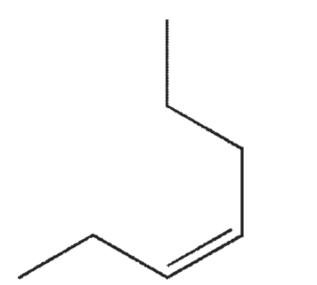
E)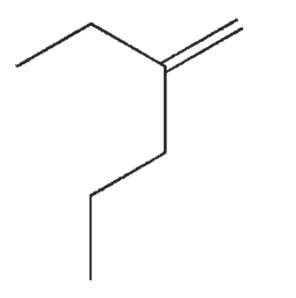
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
12
How many σ bonds and how many π bonds are present in propyne?
A) 2 σ bonds, 1 π bond
B) 5 σ bonds, 2 π bonds
C) 6 σ bonds, 2 π bonds
D) 7 σ bonds, 1 π bond
E) 7 σ bonds, 2 π bonds
A) 2 σ bonds, 1 π bond
B) 5 σ bonds, 2 π bonds
C) 6 σ bonds, 2 π bonds
D) 7 σ bonds, 1 π bond
E) 7 σ bonds, 2 π bonds

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
13
What is the indicated bond angle in the following structure? 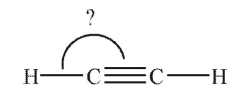
A) 60°
B) 90°
C) 109.5°
D) 120°
E) 180°
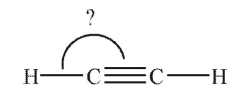
A) 60°
B) 90°
C) 109.5°
D) 120°
E) 180°

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of these compounds would you expect to be stable? 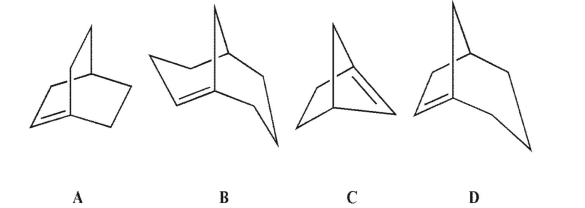
A) A
B) B
C) C
D) D
E) None of these

A) A
B) B
C) C
D) D
E) None of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which of the following structures represents a trans alkene?
A)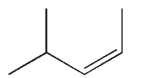
B)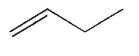
C)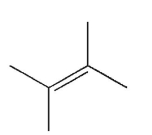
D)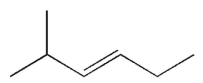
E)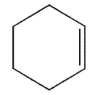
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which of the following structures is properly designated as a Z alkene?
A)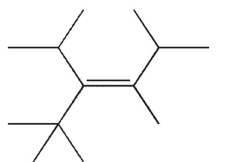
B)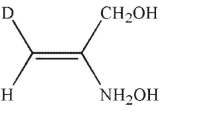
C)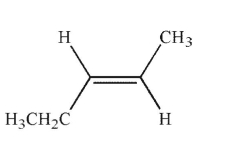
D)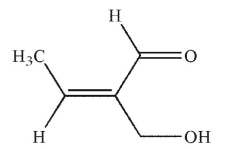
E)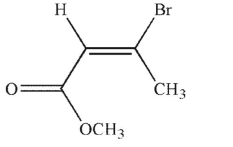
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which conformational equilibrium would you expect to be slow at room temperature?
A)
B)
C)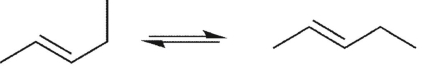
D)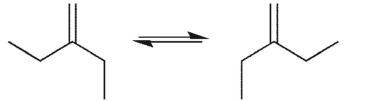
E)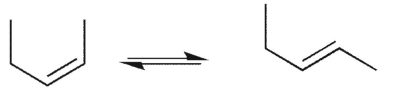
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which of the following structures is properly designated as an E alkene?
A)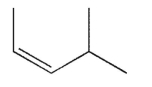
B)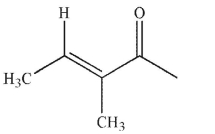
C)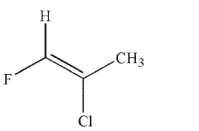
D)
E)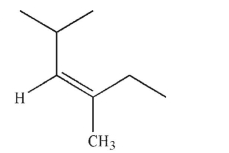
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which set of atomic orbitals could be used to make an sp2 hybrid on carbon?
A) C 2 pz , C 2 px and C 2 py
B) H 1s, C 2 py, and C 2 px
C) C 2 s, 2 py , and C 2 pz
D) C 1 s , C 2 py, and C 2 pz
E) C 2 s , H 2 px and C 2 pz
A) C 2 pz , C 2 px and C 2 py
B) H 1s, C 2 py, and C 2 px
C) C 2 s, 2 py , and C 2 pz
D) C 1 s , C 2 py, and C 2 pz
E) C 2 s , H 2 px and C 2 pz

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
20
How many σ bonds and how many π bonds are in the following structure?
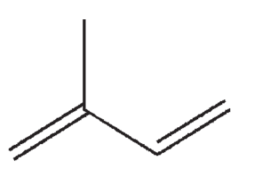
A) 2 σ bonds and 4 π bonds
B) 4 σ bonds and 2 π bonds
C) 7 σ bonds and 2 π bonds
D) 8 σ bonds and 2 π bonds
E) 12 σ bonds and 2π bonds

A) 2 σ bonds and 4 π bonds
B) 4 σ bonds and 2 π bonds
C) 7 σ bonds and 2 π bonds
D) 8 σ bonds and 2 π bonds
E) 12 σ bonds and 2π bonds

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
21
In the first step of the reaction between HBr and 2-methylpropene shown here, identify the HOMO and the LUMO. 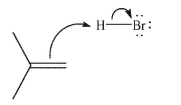
A) HOMO} is C-C π, is H-Br σ.
B) HOMO is C-C π, LUMO is H-Br σ*.
C) HOMO is C-C σ, LUMO is H-Br σ.
D) HOMO is C-C π * , LUMO is H-Br σ.
E) HOMO is H-Br σ and LUMO is C-C π .

A) HOMO} is C-C π, is H-Br σ.
B) HOMO is C-C π, LUMO is H-Br σ*.
C) HOMO is C-C σ, LUMO is H-Br σ.
D) HOMO is C-C π * , LUMO is H-Br σ.
E) HOMO is H-Br σ and LUMO is C-C π .

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
22
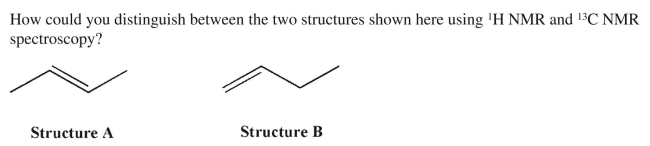

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
23
What is the major product of the reaction shown here? 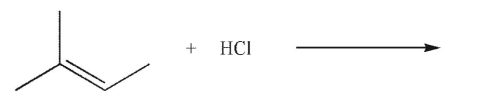
A)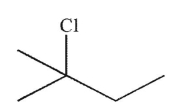
B)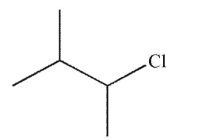
C)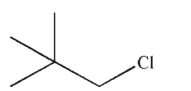
D)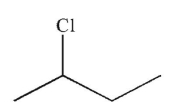
E)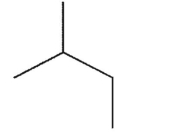
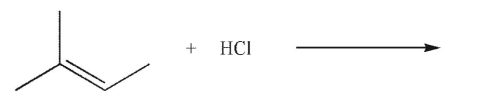
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
24
Draw all geometric isomers of 2-methyl-3-hexene.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which of the following choices is the correct name for the structure shown here?
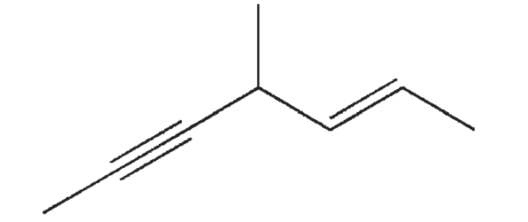
A) 4-methylhept- (E)-2 -en-5-yne
B) 4-methylhept-(Z)-2-en-5-yne
C) 4-methylhept- (E)-5
D) 4-methylhept-(Z)-5-en-2-yne
E) 4-methylheptyne-en-2-yne

A) 4-methylhept- (E)-2 -en-5-yne
B) 4-methylhept-(Z)-2-en-5-yne
C) 4-methylhept- (E)-5
D) 4-methylhept-(Z)-5-en-2-yne
E) 4-methylheptyne-en-2-yne

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which of the following is the most stable carbocation?
A)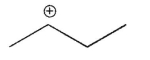
B)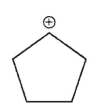
C)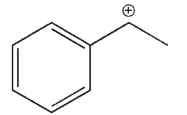
D)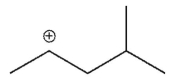
E) All arecequaly sable.
A)

B)

C)

D)

E) All arecequaly sable.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
27
Provide an acceptable IUPAC name for the structure shown here. 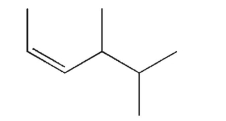


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
28
Calculate the degree of unsaturation for a compound with molecular formula C9H14BrCl.
A) 0
B) 1
C) 2
D) 3
E) Not enough information is given to answer the question.
A) 0
B) 1
C) 2
D) 3
E) Not enough information is given to answer the question.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
29
Draw a line structure for trans-4-methyl-2-pentene.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
30
For ethylene, C2H4, indicate the hybridization of each carbon atom in the molecule, the types of bonds each carbon forms, the orbitals involved in forming each of these bonds, and all bond angles.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
31
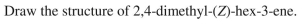

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
32
Which molecule would not have an unsaturation number of 5?
A)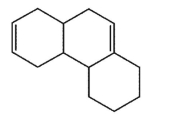
B)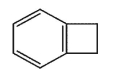
C)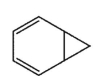
D)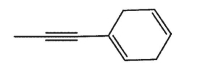
E)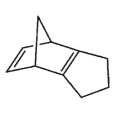
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of these bases can deprotonate acetylene (pKa 26)? The pKa values for the conjugate acids are shown in parentheses.
A) NaOH(15.7)
B) Butyllithium (50-60)
C) NaNH2(36)
D) NaHCO3(6.3)
E) Both b and c
A) NaOH(15.7)
B) Butyllithium (50-60)
C) NaNH2(36)
D) NaHCO3(6.3)
E) Both b and c

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
34
How many signals do you expect to find in the 1H NMR and 13C NMR spectra for the following molecule?

A) 2 signals in the 1H NMR and 3 signals in the 13C NMR
B) 3 signals in the 1H NMR and 3 signals in the 13C NMR
C) 3 signals in the 1H NMR and 4 signals in the 13 C NMR
D) 5 signals in the 1H NMR and 5 signals in the 13 C NMR
E) 8 signals in the 1H NMR and 5 signals in the 13 C NMR

A) 2 signals in the 1H NMR and 3 signals in the 13C NMR
B) 3 signals in the 1H NMR and 3 signals in the 13C NMR
C) 3 signals in the 1H NMR and 4 signals in the 13 C NMR
D) 5 signals in the 1H NMR and 5 signals in the 13 C NMR
E) 8 signals in the 1H NMR and 5 signals in the 13 C NMR

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
35
Which reaction is the most endothermic?
A)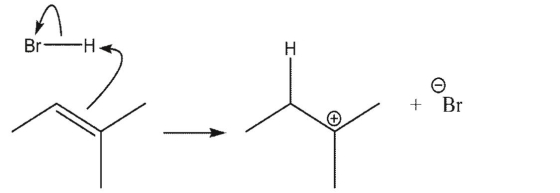
B)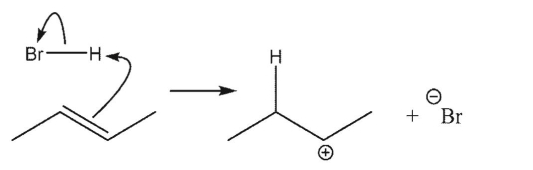
C)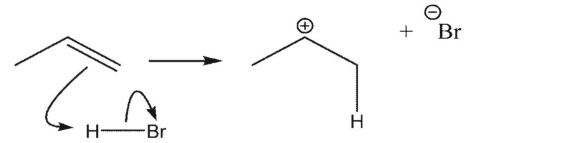
D)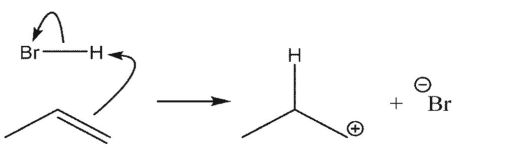
E)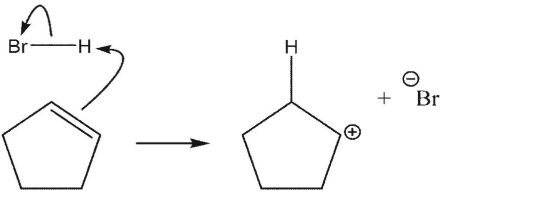
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which of the following is the most stable carbocation?
A)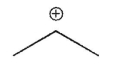
B)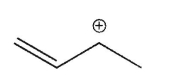
C)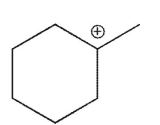
D)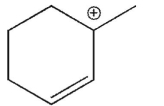
E)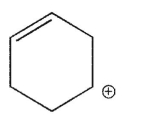
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which of the following is the most acidic hydrocarbon?
A)
B)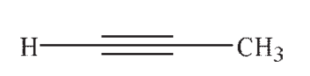
C)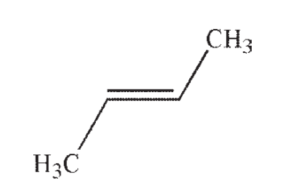
D)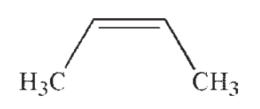
E)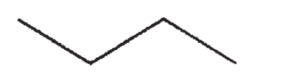
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which of the following compounds do not have three degrees of unsaturation? 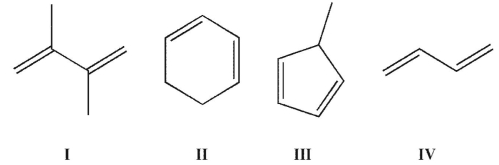
A) I, II, and III
B) II and III
C) I, II, III, and IV
D) I and III
E) I and IV

A) I, II, and III
B) II and III
C) I, II, III, and IV
D) I and III
E) I and IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
39
Which of the following is a tertiary carbocation?
A)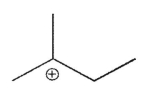
B)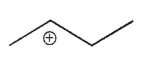
C)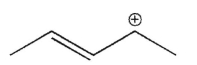
D)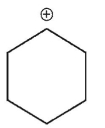
E)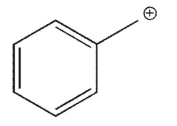
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
40
What is the major product of the reaction shown here? 
A)
B)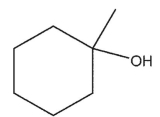
C)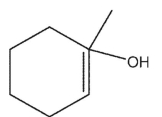
D)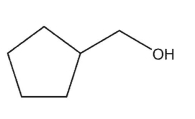
E)

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
41
Using E and Z nomenclature where necessary, draw and name all alkene isomers with the formula C6H12 that can be named as pentenes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
42
How many degrees of unsaturation are there in this compound? 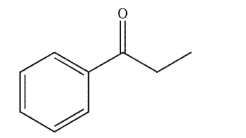


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
43
Estimate the energy change for the reaction shown here. 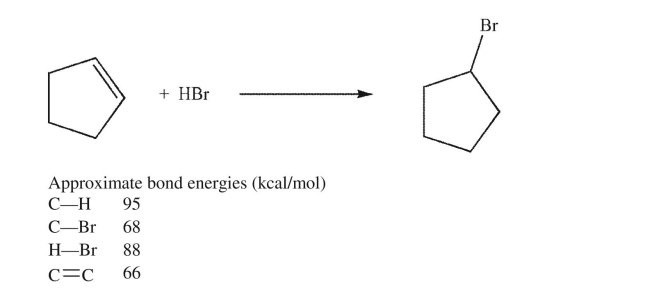


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
44
Draw two possible alkene starting materials that would give the product shown here when treated with HCl.
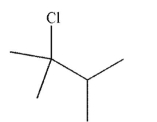


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
45
Draw the structure of 2-methyl-3-hexyne.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
46
Explain why small and medium cycloalkenes are much more stable as cis isomers than as trans
isomers.
isomers.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
47
Draw the structure that results from the mechanistic step shown here. 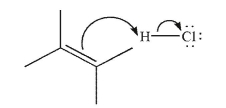


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
48
Calculate the degree of unsaturation and draw six constitutional isomers for the molecular formula C8H12.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
49
Draw the product of the following reaction. 


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
50
What is the product of the following reaction? 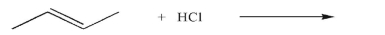
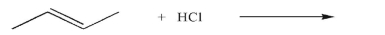

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
51
Arrange in order of acidity (most acidic to least acidic): 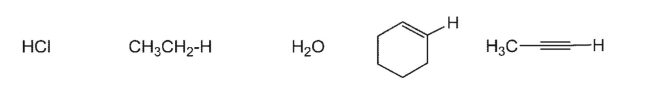


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
52
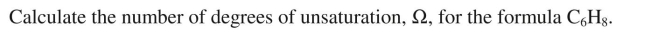

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
53
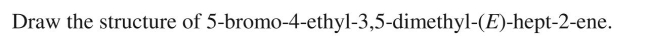

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
54
Predict the product of the following reaction and draw a mechanism to account for its formation.
Include all curved arrows, lone pairs of electrons, and nonzero formal charges.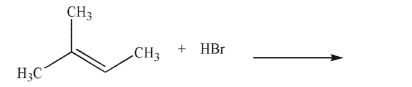
Include all curved arrows, lone pairs of electrons, and nonzero formal charges.
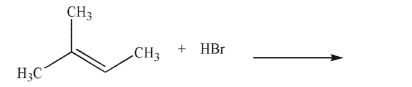

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
55
At room temperature, cis and trans alkene isomers do not equilibrate; that is, they do not interconvert.Explain why. 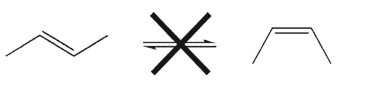


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
56
Name the orbitals that overlap to form each of the indicated bonds in propyne. 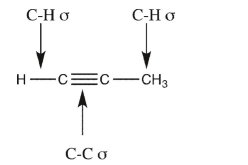


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
57
Place the following alkene isomers in order of increasing stability and indicate which of them has the most negative value for ΔHf°.
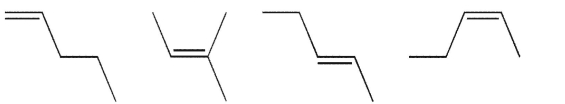


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
58
Is the mechanistic step shown here endothermic or exothermic? Explain your answer briefly. 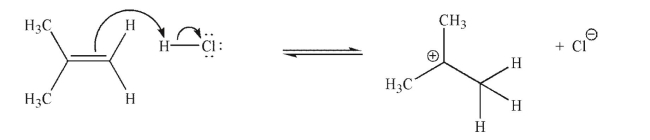


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
59
Draw the structure of (Z)-4, 7-dimethyloct-2-en-5-yne.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
60
Describe all possible combinations of structural units consistent with an unsaturation number of 4.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
61
Write a reaction mechanism for the following reaction. 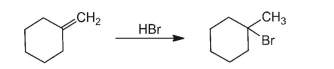


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
62
Provide the product for the reaction below. 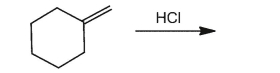


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
63
What is the LUMO in the mechanistic step shown here? 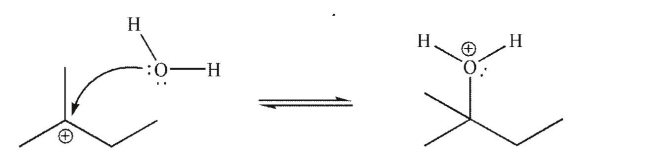


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
64
Draw a reaction coordinate diagram, including the structures of any intermediates, illustrating the
competing reaction pathways below.Indicate the key energy difference on the diagram that makes
path b the predominant one (that is, the one that gives the major product).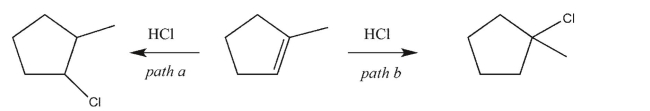
competing reaction pathways below.Indicate the key energy difference on the diagram that makes
path b the predominant one (that is, the one that gives the major product).


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
65
Provide an alkene starting material for the reaction below. 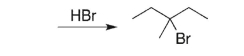


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
66
Predict the reaction product, and provide a mechanism for its formation. 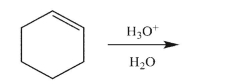


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
67
Which carbocation is the most stable? 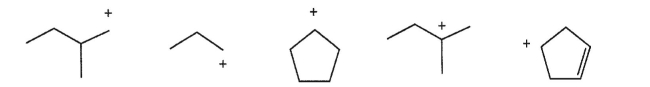


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
68
Predict the product of the following reaction and draw a mechanism to account for its formation.
Include all curved arrows, lone pairs of electrons, and nonzero formal charges.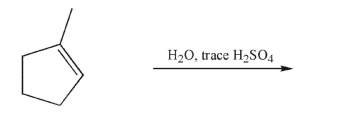
Include all curved arrows, lone pairs of electrons, and nonzero formal charges.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
69
Predict the reaction product. 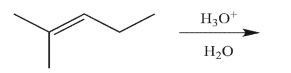


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck
70
Predict the reaction product, and write a mechanism for its formation. 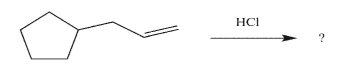


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 70 في هذه المجموعة.
فتح الحزمة
k this deck








