Deck 6: Substituted Alkanes: Alkyl Halides, Alcohols, Amines, Ethers,thiols, and Thioethers
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/68
العب
ملء الشاشة (f)
Deck 6: Substituted Alkanes: Alkyl Halides, Alcohols, Amines, Ethers,thiols, and Thioethers
1
Which of these structures is a tertiary amine?
A)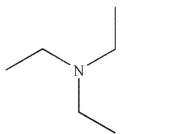
B)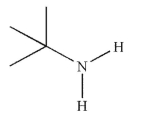
C)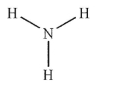
D)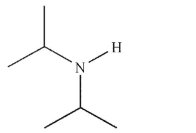
E)None of these structures are tertiary amines.
A)

B)

C)

D)

E)None of these structures are tertiary amines.

2
Which of the following structures is pyrrolidine?
A)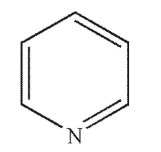
B)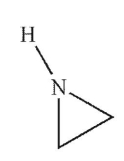
C)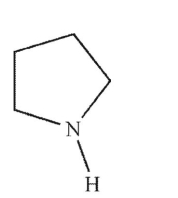
D)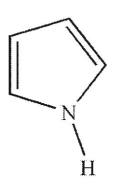
E)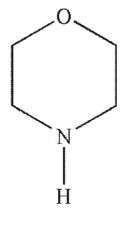
A)

B)

C)

D)

E)


3
Rank the following compounds in order of increasing length of the bond that is shown:
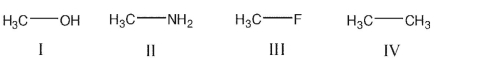
A) I>II>III>IV
B) IV>II>I>III
C) III >I>II>IV
D) II >I>IV>III
E) IV>II>III>I
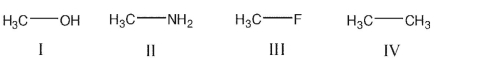
A) I>II>III>IV
B) IV>II>I>III
C) III >I>II>IV
D) II >I>IV>III
E) IV>II>III>I
IV>II>I>III
4
Which of these statements is false?
A)Amines are both Lewis bases and Brønsted bases.
B)In solution or in the gas phase, the trend for amine basicity is identical.
C)The conjugate acid of an amine is an ammonium ion.
D)Tetrabutyl ammonium chloride cannot form hydrogen bonds in solution.
E)Ammonia is a weaker base than dimethylamine, both in solution and in the gas phase.
A)Amines are both Lewis bases and Brønsted bases.
B)In solution or in the gas phase, the trend for amine basicity is identical.
C)The conjugate acid of an amine is an ammonium ion.
D)Tetrabutyl ammonium chloride cannot form hydrogen bonds in solution.
E)Ammonia is a weaker base than dimethylamine, both in solution and in the gas phase.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which of the following alkyl halides contains the longest carbon-halogen bond?
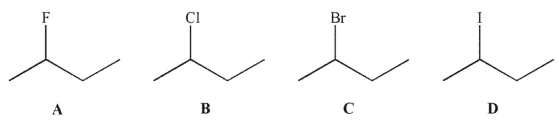
A)A
B)B
C)C
D)D
E)All the carbon-halogen bonds are the same length.

A)A
B)B
C)C
D)D
E)All the carbon-halogen bonds are the same length.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which of the following statements about amines is false?
A)All amines are capable of forming hydrogen bonds.
B)Amines can be classified as primary, secondary, or tertiary.
C)Amines are weak Brønsted bases.
D)Most amines have a strong, unpleasant odor.
E)In the gas phase, the basicity of amines increases with increasing alkyl substitution.
A)All amines are capable of forming hydrogen bonds.
B)Amines can be classified as primary, secondary, or tertiary.
C)Amines are weak Brønsted bases.
D)Most amines have a strong, unpleasant odor.
E)In the gas phase, the basicity of amines increases with increasing alkyl substitution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following is not a diol?
A)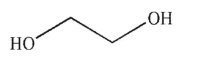
B)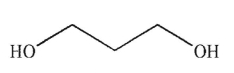
C)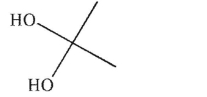
D)
E) All these molecules are diols.
A)

B)

C)

D)

E) All these molecules are diols.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which of these compounds is a secondary amine?
A) CH3NH2
B)(CH3)2NH
C)(CH3)3N
D)CH3CH2NH2
E)(CH3)4N+Cl-
A) CH3NH2
B)(CH3)2NH
C)(CH3)3N
D)CH3CH2NH2
E)(CH3)4N+Cl-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which is the correct name for the molecule shown here?
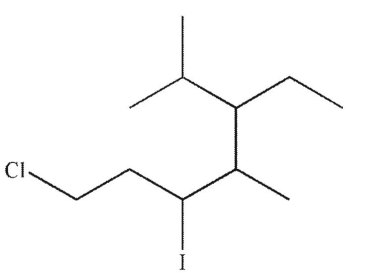
A) 1-chloro-3-iodo-4,6-dimethyl-5-ethylheptane
B) 1-chloro-5-ethyl-3-iodo-4,6-dimethylheptane
C) 1-chloro-3-iodo-5-isopropyl-4-methylheptane
D) 7-chloro-3-ethyl-5-iodo-2,4-dimethylheptane
E) 7 -chloro-5-iodo-3-isopropyl-4-methylheptane

A) 1-chloro-3-iodo-4,6-dimethyl-5-ethylheptane
B) 1-chloro-5-ethyl-3-iodo-4,6-dimethylheptane
C) 1-chloro-3-iodo-5-isopropyl-4-methylheptane
D) 7-chloro-3-ethyl-5-iodo-2,4-dimethylheptane
E) 7 -chloro-5-iodo-3-isopropyl-4-methylheptane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which of the following is the weakest acid in solution?
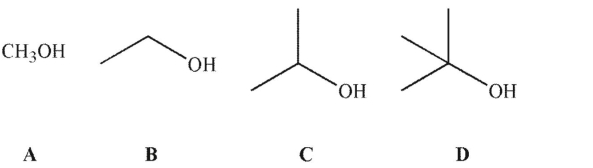
A) A
B) B
C)C
D) D
E) All have the same acidity.

A) A
B) B
C)C
D) D
E) All have the same acidity.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
11
Select the correct name for this molecule.
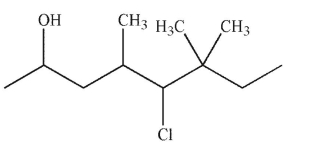
A) 4-chloro-3,3,5-trimethyl-7-octanol
B) 5 -chloro-6-ethyl-4,6-dimethyl-2-heptanol
C) 5 -chloro- 4,6,6 -trimethyl-2-octanol
D) 3-chloro-2-ethyl-2,4-dimethyl-6-heptanol
E) 3 -chloro-2-ethyl-2,4-methylheptanol

A) 4-chloro-3,3,5-trimethyl-7-octanol
B) 5 -chloro-6-ethyl-4,6-dimethyl-2-heptanol
C) 5 -chloro- 4,6,6 -trimethyl-2-octanol
D) 3-chloro-2-ethyl-2,4-dimethyl-6-heptanol
E) 3 -chloro-2-ethyl-2,4-methylheptanol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which of the following structures is/are a secondary alkyl halide? 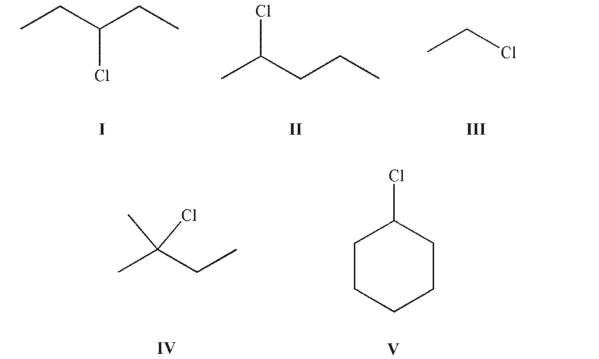
A) I and II
B) II and V
C) I, II, and III
D) IV
E) I, II, and V

A) I and II
B) II and V
C) I, II, and III
D) IV
E) I, II, and V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which of these statements is false?
A)The lone pair on nitrogen provides a fourth group to complete a tetrahedral arrangement.
B)Amines that possess a stereogenic nitrogen atom interconvert through the inversion process.
C)For simple amines, the barrier to inversion is very low.
D)At the transition state for amine inversion, the amine is planar.
E)If amine inversion is fast, enantiomeric amines can be separated from each other.
A)The lone pair on nitrogen provides a fourth group to complete a tetrahedral arrangement.
B)Amines that possess a stereogenic nitrogen atom interconvert through the inversion process.
C)For simple amines, the barrier to inversion is very low.
D)At the transition state for amine inversion, the amine is planar.
E)If amine inversion is fast, enantiomeric amines can be separated from each other.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of the following nitrogen-containing compounds is/are chiral at room temperature?
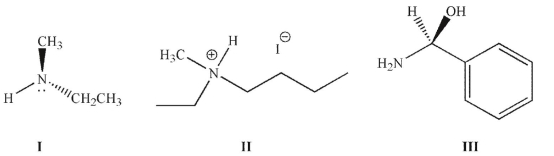
A) I
B) II
C) III
D) II and III
E) I, II, and III

A) I
B) II
C) III
D) II and III
E) I, II, and III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which of the following molecules is the strongest acid?
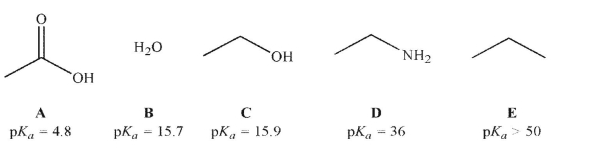
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
16
For which of the following reactions would you expect Keq>1?
A)
B)
C)
D)
E)
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which of the following can protonate ethanol to a significant extent? The pKa of CH3CH2OH2+ is -2.2 .
A) H2O, pKa=15.7
B) CH3CH2OH, pKa=15.9
C) NH3, pKa=36
D) HCl, pKa=-7
E) Propanoic acid, pKa=4.9
A) H2O, pKa=15.7
B) CH3CH2OH, pKa=15.9
C) NH3, pKa=36
D) HCl, pKa=-7
E) Propanoic acid, pKa=4.9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
18
Select the correct name for this molecule.
![<strong>Select the correct name for this molecule. </strong> A) (1 S, 6 R) -bicyclo[6.6.0]dec-3-en-1,6-diol B) (5 S, 6 R) -bicyclo[4.4.0]dec-2-en-5,6-diol C) (1 S, 6 R) -bicyclo[4.4.0]dec-3-en-1,6-diol D) (1 S, 6 R) -bicyclo[4.4.0]dec-8-en-1,6-diol E) (1 S, 6 R) -bicyclo[4.4.0]oct-2-en-1,6-diol](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7c41_5b98_ed6a_b10d_29869ee870a0_TB34225555_11.jpg)
A) (1 S, 6 R) -bicyclo[6.6.0]dec-3-en-1,6-diol
B) (5 S, 6 R) -bicyclo[4.4.0]dec-2-en-5,6-diol
C) (1 S, 6 R) -bicyclo[4.4.0]dec-3-en-1,6-diol
D) (1 S, 6 R) -bicyclo[4.4.0]dec-8-en-1,6-diol
E) (1 S, 6 R) -bicyclo[4.4.0]oct-2-en-1,6-diol
![<strong>Select the correct name for this molecule. </strong> A) (1 S, 6 R) -bicyclo[6.6.0]dec-3-en-1,6-diol B) (5 S, 6 R) -bicyclo[4.4.0]dec-2-en-5,6-diol C) (1 S, 6 R) -bicyclo[4.4.0]dec-3-en-1,6-diol D) (1 S, 6 R) -bicyclo[4.4.0]dec-8-en-1,6-diol E) (1 S, 6 R) -bicyclo[4.4.0]oct-2-en-1,6-diol](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7c41_5b98_ed6a_b10d_29869ee870a0_TB34225555_11.jpg)
A) (1 S, 6 R) -bicyclo[6.6.0]dec-3-en-1,6-diol
B) (5 S, 6 R) -bicyclo[4.4.0]dec-2-en-5,6-diol
C) (1 S, 6 R) -bicyclo[4.4.0]dec-3-en-1,6-diol
D) (1 S, 6 R) -bicyclo[4.4.0]dec-8-en-1,6-diol
E) (1 S, 6 R) -bicyclo[4.4.0]oct-2-en-1,6-diol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which of these ammonium ions has the strongest conjugate base?
A)
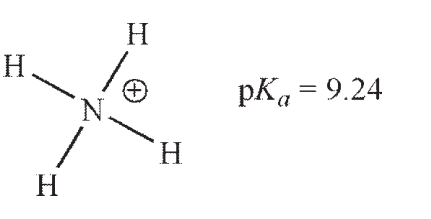
B)
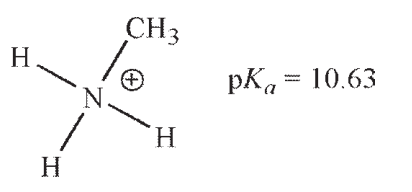
C)
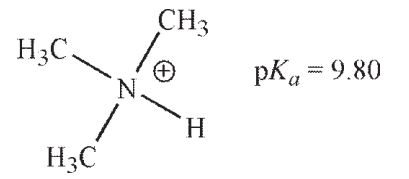
D)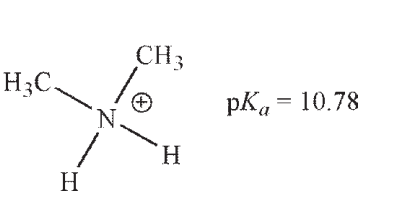
E) The conjugate bases of all these ammonium ions are equally basic.
A)
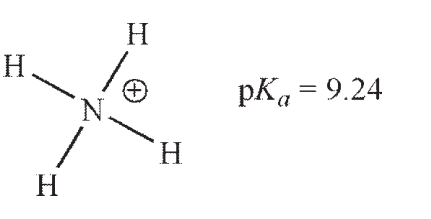
B)

C)

D)

E) The conjugate bases of all these ammonium ions are equally basic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which of these structures is ethylene glycol?
A)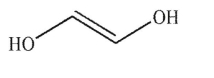
B)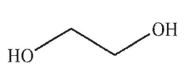
C)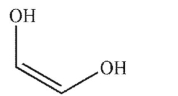
D)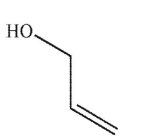
E) None of these structures.
A)

B)

C)

D)

E) None of these structures.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
21
Provide the IUPAC name for the molecule shown here with correct stereochemistry. 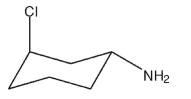


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
22
Place the following molecules in order of decreasing acidity.
A) A>B>C
B) A>C>B
C) B>C>A
D) B>A>C
E) C>A>B

A) A>B>C
B) A>C>B
C) B>C>A
D) B>A>C
E) C>A>B

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
23
Provide the IUPAC name for this molecule, including stereochemical designations of stereogenic
carbons.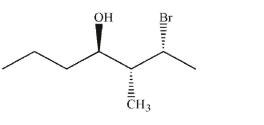
carbons.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
24
Which of the following statements about organolithium reagents is false?
A)They are strongly basic.
B)They are strongly acidic.
C)They are formed from the reaction of an alkyl halide and lithium metal.
D)They react with water to produce alkanes.
E)They are formed in a radical (one electron transfer) reaction.
A)They are strongly basic.
B)They are strongly acidic.
C)They are formed from the reaction of an alkyl halide and lithium metal.
D)They react with water to produce alkanes.
E)They are formed in a radical (one electron transfer) reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
25
Boiling point increases as shown in the following series of amines:
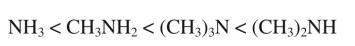
Why is the boiling point of (CH3)3N lower than that of (CH3)2NH?
A) (CH3)2NH has a higher molar mass than (CH3)3N .
B) (CH3)2NH can form hydrogen bonds, but (CH3)3 N cannot.
C) (CH3)3 N can form hydrogen bonds, but (CH3)2NH cannot.
D) (CH3)2NH is polar, but (CH3)3 N is not.
E) (CH3)3N is polar, but (CH3)2NH is not.
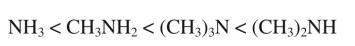
Why is the boiling point of (CH3)3N lower than that of (CH3)2NH?
A) (CH3)2NH has a higher molar mass than (CH3)3N .
B) (CH3)2NH can form hydrogen bonds, but (CH3)3 N cannot.
C) (CH3)3 N can form hydrogen bonds, but (CH3)2NH cannot.
D) (CH3)2NH is polar, but (CH3)3 N is not.
E) (CH3)3N is polar, but (CH3)2NH is not.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
26
Provide a common name for the molecule shown here. 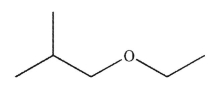


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which of the following compounds do you expect to have the least solubility in water at 25°C?
A) CH3OH
B)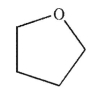
C) CH3CH2OH
D) acetone
E)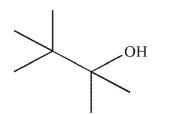
A) CH3OH
B)

C) CH3CH2OH
D) acetone
E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
28
Draw a correct structure for morpholine.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
29
Provide the IUPAC name for the structure shown here. 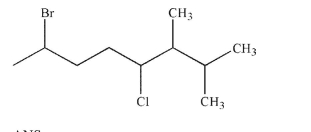


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which of these types of compounds is not able either to form or to accept hydrogen bonds?
A)haloalkanes
B)ethers
C)alcohols
D)primary amines
E)tertiary amines
A)haloalkanes
B)ethers
C)alcohols
D)primary amines
E)tertiary amines

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
31
Provide the IUPAC name for the molecule shown here with correct stereochemistry. 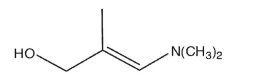


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
32
Draw a correct structure for 4-chloro-2,3-dimethylpentamine.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
33
Rank the following compounds from least soluble in water to most soluble.
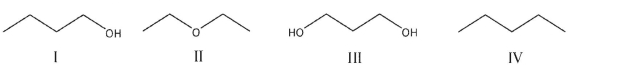
A) I< II < III
B) IV < II < III < I
C) IV < III < II < I
D) IV < I < II < III
E) IV < II < I < III

A) I< II < III
B) IV < II < III < I
C) IV < III < II < I
D) IV < I < II < III
E) IV < II < I < III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which of the following applications would a crown ether typically not be used for?
A)as a chelating agent to remove heavy metals from water
B)to capture minute amounts of precious metals from seawater
C)as a means to indirectly bring reactive anions into nonpolar solvent media
D)to study the chemistry of "host-guest" complexation
E)to stabilize Grignard and organolithium reagents
A)as a chelating agent to remove heavy metals from water
B)to capture minute amounts of precious metals from seawater
C)as a means to indirectly bring reactive anions into nonpolar solvent media
D)to study the chemistry of "host-guest" complexation
E)to stabilize Grignard and organolithium reagents

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
35
Draw a correct structure for 3-chloro-6-fluoro-5-isopropyl-2-methyloctane.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which of the following solvents is aprotic?
A)acetic acid
B)acetone
C)dimethylamine
D)methanol
E)water
A)acetic acid
B)acetone
C)dimethylamine
D)methanol
E)water

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which of the following solvents is/are protic? 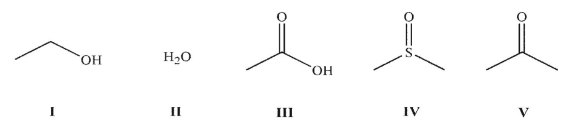
A) I and II
B) I and III
C) I, II, and III
D) I, II, and IV
E) I, II, III, and IV

A) I and II
B) I and III
C) I, II, and III
D) I, II, and IV
E) I, II, III, and IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
38
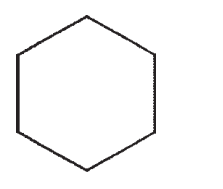
Predict the product of the following reaction:
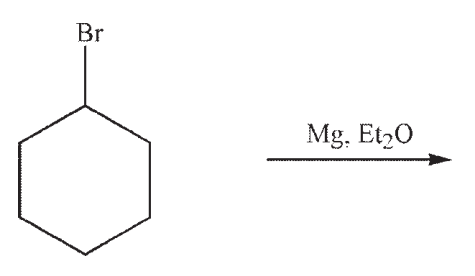
A)
B)
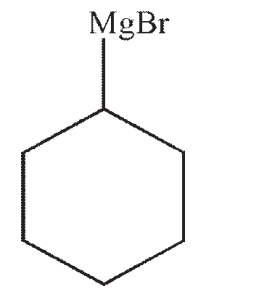
C)
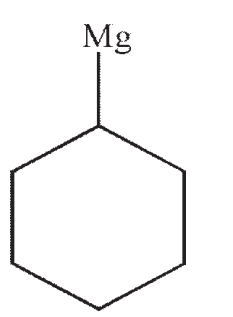
D)
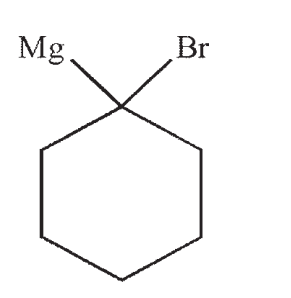
E)No reaction occurs between the starting material and reagents shown.

A)

B)

C)

D)

E)No reaction occurs between the starting material and reagents shown.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
39
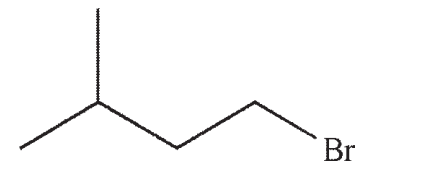
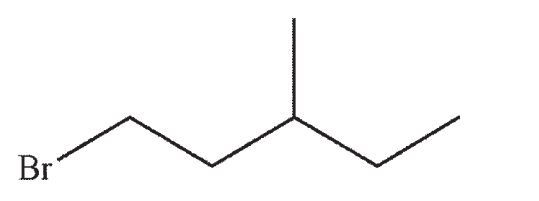
Which of the structures shown below is not a possible starting material for this reaction sequence?
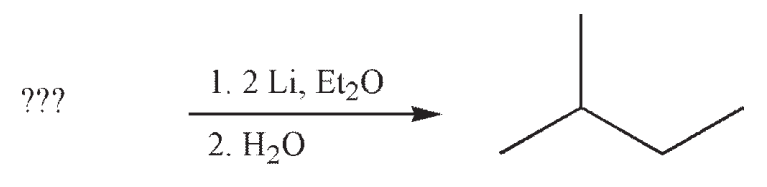
A)
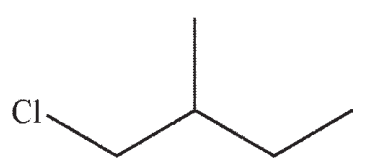
B)
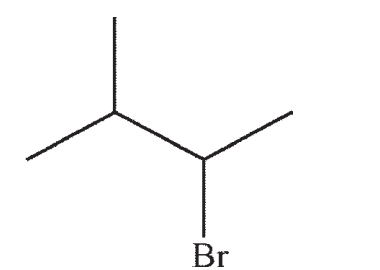
C)
D)
E) Any of these choices is an appropriate starting material for the transformation shown.

A)

B)

C)

D)

E) Any of these choices is an appropriate starting material for the transformation shown.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which reagent would you use to accomplish the following transformation?
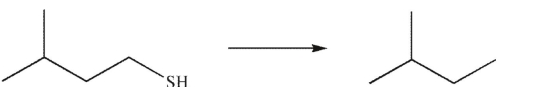
A) H2O
B) Mg, Et2O
C) NH3
D) H2/ Raney Ni
E) Any of these reagents could be used.

A) H2O
B) Mg, Et2O
C) NH3
D) H2/ Raney Ni
E) Any of these reagents could be used.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
41
Why is ethanol a stronger acid than tert-butanol in solution?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
42
Which of these compounds is more acidic, and why? 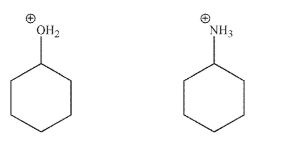


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
43
Which has a higher boiling point, ethanol or propane? Explain.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
44
Draw the mechanism showing the Brønsted acid/base reaction between propanoic acid and
methylamine.Show all curved arrows, lone pairs, and nonzero formal charges.Predict the side of
the reaction that will be favored at equilibrium.
methylamine.Show all curved arrows, lone pairs, and nonzero formal charges.Predict the side of
the reaction that will be favored at equilibrium.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
45
What is the common name of this molecule? 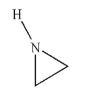


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
46
In the gas phase, the order of acidity of the alcohols shown here is reversed. Explain the difference in acidity trends between the gas phase and solution.
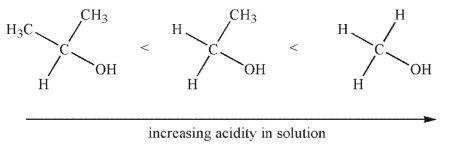


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
47
Predict the product of the following reaction sequence. 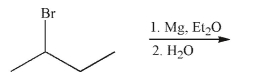
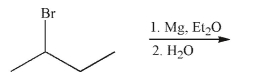

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
48
Distinguish between protic solvents and aprotic solvents and provide at least one example of each.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
49
Draw a mechanism for the Brønsted acid/base reaction of ethanol and hydrochloric acid.Show all
curved arrows, lone pairs, and nonzero formal charges.Which side of the reaction is favored at
equilibrium?
curved arrows, lone pairs, and nonzero formal charges.Which side of the reaction is favored at
equilibrium?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
50
Draw the conjugate acid and the conjugate base of the compound shown. 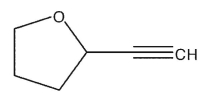


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
51
In the following compounds, identify which proton would be removed first in the presence of
strong base.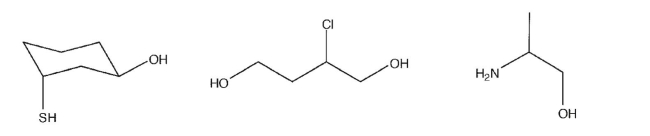
strong base.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
52
Draw a correct structure for isopropyl pentyl ether.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
53
Which of the following acids is stronger? Explain your reasoning. 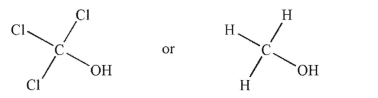
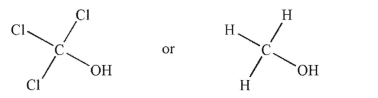

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
54
Predict the product of the following reaction sequence. 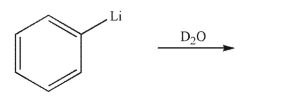


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
55
Draw the conjugate base of the alcohol shown here.Include all lone pairs and nonzero formal
charges.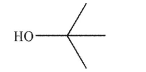
charges.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
56
Draw the conjugate base of methylamine.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
57
The acidity of alcohols in water follows the following order: 
In the gas phase, this order is reversed. Draw an illustration showing how solvent impacts the acidity of the strongest and weakest alcohols in the liquid phase by stabilizing the conjugate base.

In the gas phase, this order is reversed. Draw an illustration showing how solvent impacts the acidity of the strongest and weakest alcohols in the liquid phase by stabilizing the conjugate base.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
58
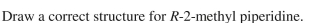

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
59
Draw the two products of the following reaction. 


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
60
Draw all the constitutional isomers of the amine with molecular formula C4H11N. Indicate whether each amine is primary, secondary, or tertiary.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
61
Provide a name for this crown ether. 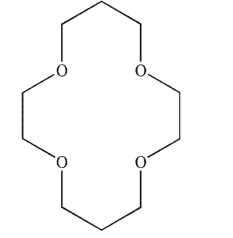


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
62
Which is more acidic: ethanol or ethanethiol? Explain your answer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
63
There are at least two routes to the product shown using reactions from this chapter.Identify the reagents you would use with each starting material to produce the target molecule. 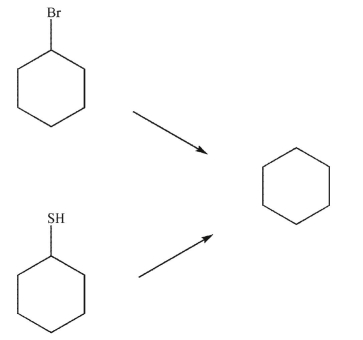


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
64
Propose a synthesis of the target molecule from the starting material shown. Show the reagents needed for each step and the product of each step.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
65
Provide a synthesis of 1-deutero methylcyclohexane from 1-methylcyclohexene.Show the
reagents used in each step and the intermediates in the synthesis.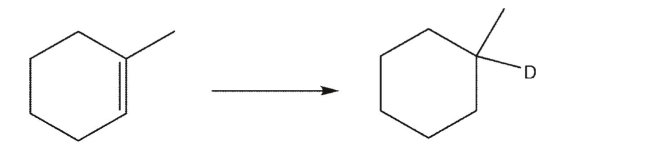
reagents used in each step and the intermediates in the synthesis.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
66
What is the product of the following reaction?
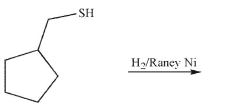


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
67
Outline a synthesis of the target molecule shown from the given starting material.You may use
any organic or inorganic reagents.Show the reagents needed for each step and the product of each
transformation.
any organic or inorganic reagents.Show the reagents needed for each step and the product of each
transformation.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck
68
Provide the IUPAC name for this structure. 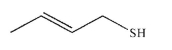


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 68 في هذه المجموعة.
فتح الحزمة
k this deck








