Deck 16: Carbonyl Chemistry 1: Addition Reactions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/73
العب
ملء الشاشة (f)
Deck 16: Carbonyl Chemistry 1: Addition Reactions
1
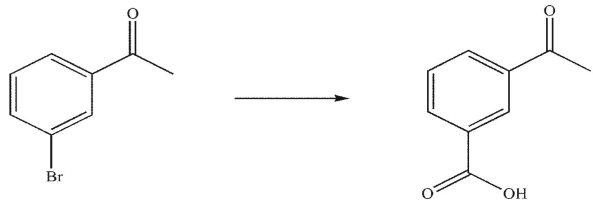
What are the missing reagents in the reaction sequence shown here?
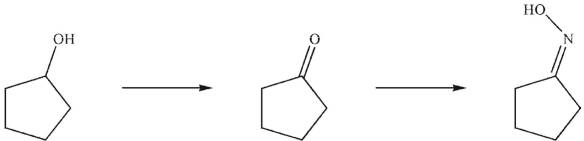
A)1. Na2Cr2O7, H2SO4, H2O
2. NH2OH , trace acid
B)1. LiAlH4
2. H2O
3. NH2OH, trace acid
C)1. NaBH4, MeOH
2. NH3 , then H3O+
D)NH3 , then H3O+
E) None of these

A)1. Na2Cr2O7, H2SO4, H2O
2. NH2OH , trace acid
B)1. LiAlH4
2. H2O
3. NH2OH, trace acid
C)1. NaBH4, MeOH
2. NH3 , then H3O+
D)NH3 , then H3O+
E) None of these
1. Na2Cr2O7, H2SO4, H2O
2. NH2OH , trace acid
2. NH2OH , trace acid
2
Which of the following structures is a hydrate?
A)
B)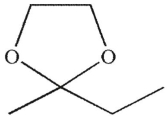
C)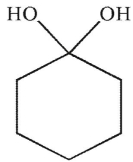
D)
E)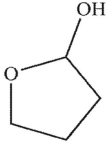
A)

B)

C)

D)

E)


3
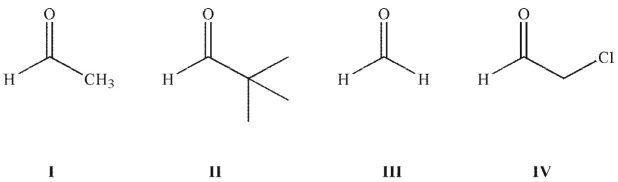
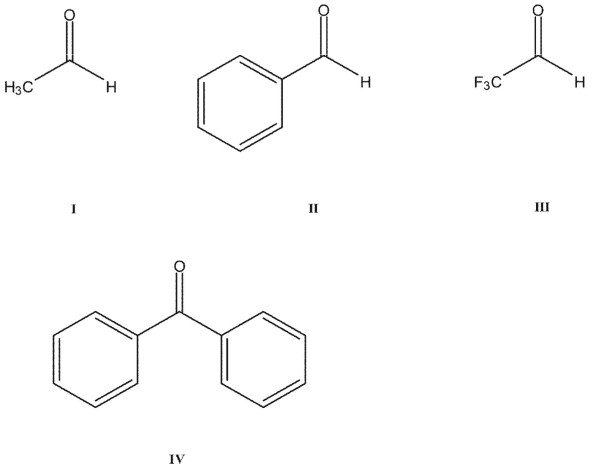
Rank these carbonyl compounds in order of increasing C=O stretching frequency in their IR spectra:
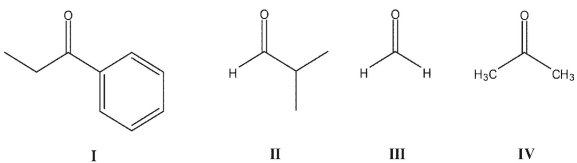
A) I< II < III < IV
B)I< IV < II < III
C)I < II < IV < III
D)III < II < IV < I
E)IV < I < II < III

A) I< II < III < IV
B)I< IV < II < III
C)I < II < IV < III
D)III < II < IV < I
E)IV < I < II < III
I< IV < II < III
4
Which of the following compounds corresponds to the spectrum shown here?
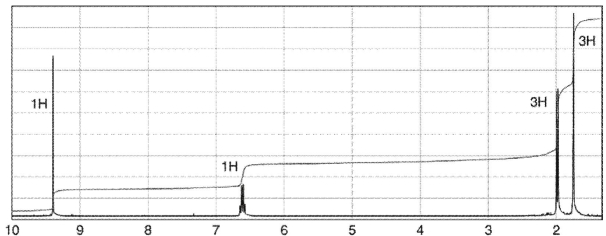
A)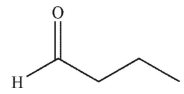
B)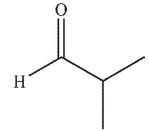
C)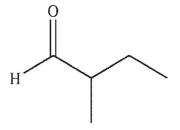
D)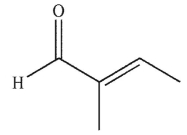
E)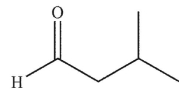

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
5
Give the correct IUPAC name for the following carbonyl compound.
![<strong>Give the correct IUPAC name for the following carbonyl compound. </strong> A) bicyclo[2.2.2]oct-7-en-2,5-dione B) tricyclo[2.2.2]oct-2-en-5,7-dione C) bicyclo[2.2.2]oct-5-en-1,4-dione D) bicyclo[2.2.2]oct-7-en-1,4-dione E) tricyclo[2.2.2]hex-2-en-3,5-dione](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7f86_6eed_1932_a1cf_190f2ae76f52_TB34225555_11.jpg)
A) bicyclo[2.2.2]oct-7-en-2,5-dione
B) tricyclo[2.2.2]oct-2-en-5,7-dione
C) bicyclo[2.2.2]oct-5-en-1,4-dione
D) bicyclo[2.2.2]oct-7-en-1,4-dione
E) tricyclo[2.2.2]hex-2-en-3,5-dione
![<strong>Give the correct IUPAC name for the following carbonyl compound. </strong> A) bicyclo[2.2.2]oct-7-en-2,5-dione B) tricyclo[2.2.2]oct-2-en-5,7-dione C) bicyclo[2.2.2]oct-5-en-1,4-dione D) bicyclo[2.2.2]oct-7-en-1,4-dione E) tricyclo[2.2.2]hex-2-en-3,5-dione](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7f86_6eed_1932_a1cf_190f2ae76f52_TB34225555_11.jpg)
A) bicyclo[2.2.2]oct-7-en-2,5-dione
B) tricyclo[2.2.2]oct-2-en-5,7-dione
C) bicyclo[2.2.2]oct-5-en-1,4-dione
D) bicyclo[2.2.2]oct-7-en-1,4-dione
E) tricyclo[2.2.2]hex-2-en-3,5-dione

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which of the hydrates shown is most stable?
A)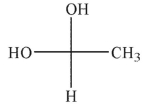
B)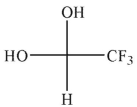
C)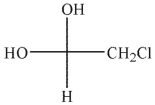
D)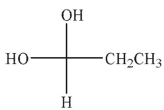
E)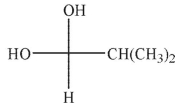
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following compounds cannot be used to form an imine?
A)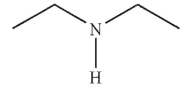
B)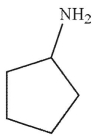
C)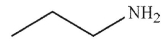
D)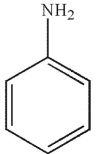
E)NH3
A)

B)

C)

D)

E)NH3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which of these factors is least important in determining the regiochemistry of hydration of carbonyl compounds?
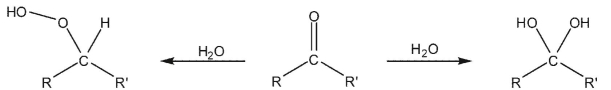
A) the greater strength of the C-O versus the O-O bond.
B) the pH under which the reaction takes place
C) the greater ability of oxygen to accommodate a negative charge in the intermediate
D) the greater orbital overlap at carbon for the π* orbital of the carbonyl
E) All these factors are important in determining the regiochemistry of the addition.

A) the greater strength of the C-O versus the O-O bond.
B) the pH under which the reaction takes place
C) the greater ability of oxygen to accommodate a negative charge in the intermediate
D) the greater orbital overlap at carbon for the π* orbital of the carbonyl
E) All these factors are important in determining the regiochemistry of the addition.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which of these compounds does not contain an acetal group?
A)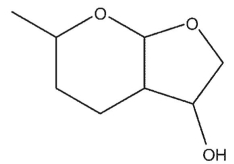
B)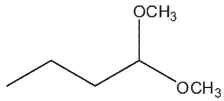
C)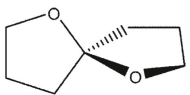
D)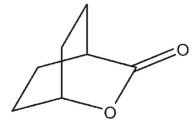
E) All are acetals.
A)

B)

C)

D)

E) All are acetals.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
10
For which of the following compounds do you expect the aldehyde to be more stable than its hydrate?
A) I
B) II
C) III
D) I, II, and IV
E) III and IV

A) I
B) II
C) III
D) I, II, and IV
E) III and IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which of the following is the major product of the reaction conditions shown?
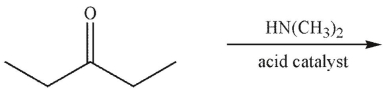
A)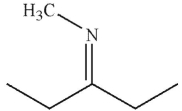
B)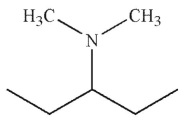
C)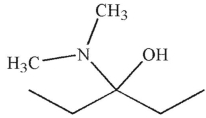
D)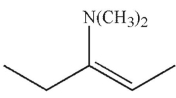
E)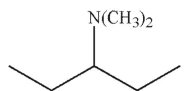
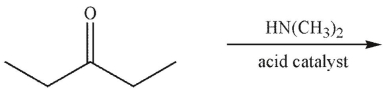
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which is the correct name for the ketone shown here?
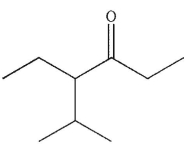
A) 4-ethyl-5-methyl-3-hexanone
B) 3 -ethyl-2-methyl-4-hexanone
C) 3 -isopropyl-4-hexanone
D) 4-ethyl-5,5-dimethyl-3-pentanone
E) 2-ethyl-1,1-dimethyl-3-pentanone

A) 4-ethyl-5-methyl-3-hexanone
B) 3 -ethyl-2-methyl-4-hexanone
C) 3 -isopropyl-4-hexanone
D) 4-ethyl-5,5-dimethyl-3-pentanone
E) 2-ethyl-1,1-dimethyl-3-pentanone

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
13
What is the major product generated on removal of the protecting groups in this molecule using the conditions shown?
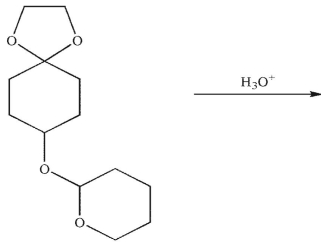
A)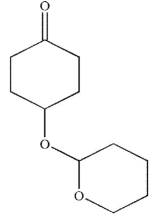
B)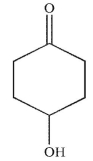
C)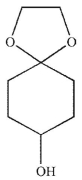
D)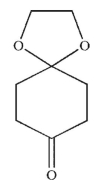
E)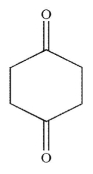

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which carbonyl compounds below has the IUPAC name: (2 R, 3 S)-2,3 -dibromo-4-methylpentanal?
A)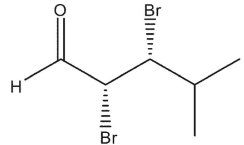
B)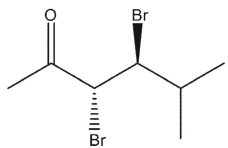
C)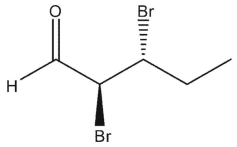
D)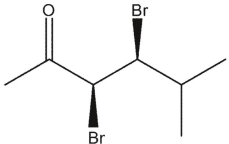
E)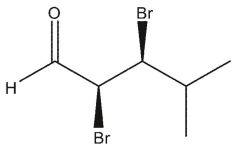
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which of the following statements correctly describes the major product of this reaction?
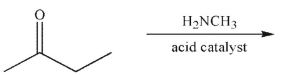
A) The major product is an enamine; imines form only when the amine is secondary.
B) The major product is a carbinolamine.
C) The major product is an enamine; to form an imine, the nitrogen-containing nucleophile must be ammonia.
D) The major product is an imine; imines are more stable than enamines.
E) The major product is an enamine because the carbonyl starting material is a ketone.

A) The major product is an enamine; imines form only when the amine is secondary.
B) The major product is a carbinolamine.
C) The major product is an enamine; to form an imine, the nitrogen-containing nucleophile must be ammonia.
D) The major product is an imine; imines are more stable than enamines.
E) The major product is an enamine because the carbonyl starting material is a ketone.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which of the following reagents is used to install a protecting group for an alcohol?
A)trimethylsiyl chloride
B)dihydropyran
C)ethylene glycol
D)both a and b
E)a, b, and c
A)trimethylsiyl chloride
B)dihydropyran
C)ethylene glycol
D)both a and b
E)a, b, and c

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which of the following compounds, when placed in water, do you expect to have the least amount of hydrate present at equilibrium?
A)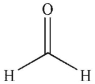
B)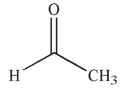
C)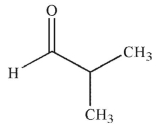
D)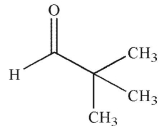
E) All these compounds will have about the same amounts of hydrate at equilibrium.
A)

B)

C)

D)

E) All these compounds will have about the same amounts of hydrate at equilibrium.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which of the following is the major product of the reaction conditions shown?
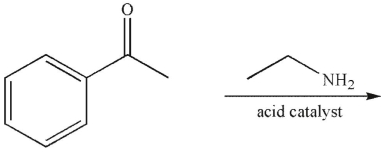
A)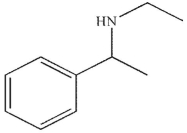
B)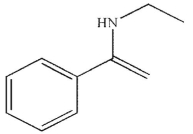
C)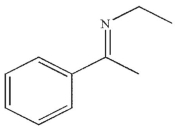
D)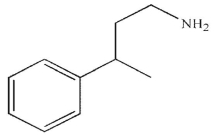
E)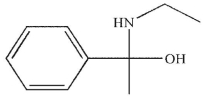

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
19
What sequence of reagents could be used for the following synthetic transformation?

A)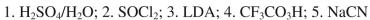
B)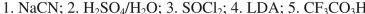
C)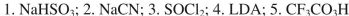
D)
E)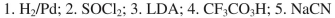

A)
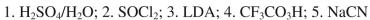
B)
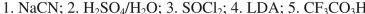
C)
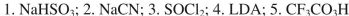
D)

E)
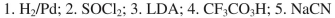

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
20
Rank the following compounds in order of increasing equilibrium constant for conversion to the hydrate.
A) II < IV < III < I
B) III < I < II < IV
C) IV < III < I < II
D) IV < II < I < III
E) I < IV < III < II

A) II < IV < III < I
B) III < I < II < IV
C) IV < III < I < II
D) IV < II < I < III
E) I < IV < III < II

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
21
What is the product of the following reaction?
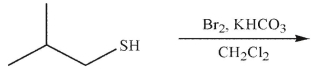
A)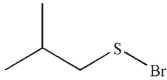
B)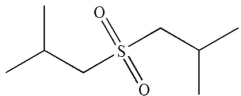
C)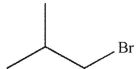
D)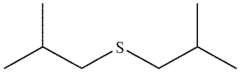
E)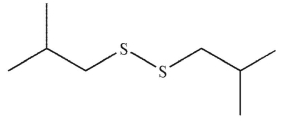
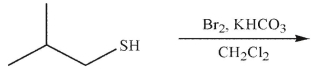
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
22
Rank these carbonyl compounds in order of increasing C=O dipole moment.
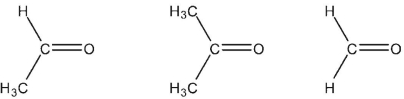


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
23
The following compound
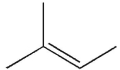 was treated with the following sequence of reagents
was treated with the following sequence of reagents
1. HBr, H2O2
2. Li, ether
3. Formaldehyde, followed by H3O+
4. CrO3, pyridine
5. Ph3P=CH2
Which of the following absorptions would you not expect to see in the IR spectrum of the final product of this multistep synthesis?
A) C=O stretch
B) C-O stretch
C) C=C stretch
D) C-H stretch
E) a and b
 was treated with the following sequence of reagents
was treated with the following sequence of reagents1. HBr, H2O2
2. Li, ether
3. Formaldehyde, followed by H3O+
4. CrO3, pyridine
5. Ph3P=CH2
Which of the following absorptions would you not expect to see in the IR spectrum of the final product of this multistep synthesis?
A) C=O stretch
B) C-O stretch
C) C=C stretch
D) C-H stretch
E) a and b

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
24
In the multistep synthesis shown here, what would be the first step?
A) Mg, ether
B) CO2
C) H3O+
D) KMnO4
E)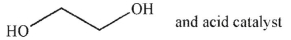

A) Mg, ether
B) CO2
C) H3O+
D) KMnO4
E)
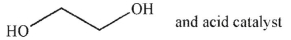

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which is a mechanistic intermediate in the following transformation?
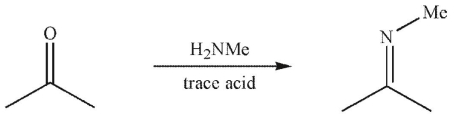
A)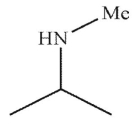
B)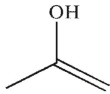
C)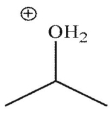
D)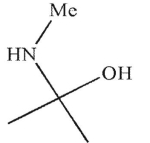
E)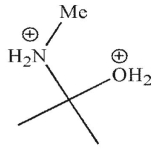

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
26
Draw the following compounds with correct stereochemistry.
A) (Z)-4-oxo-2-pentenal
B) (R)-2-\mathrm{hydroxy}-4 -heptanone
C) (S)-4 -bromocyclopent-2-en-1-one
A) (Z)-4-oxo-2-pentenal
B) (R)-2-\mathrm{hydroxy}-4 -heptanone
C) (S)-4 -bromocyclopent-2-en-1-one

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
27
What is the product of this reaction?
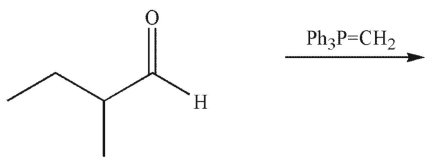
A)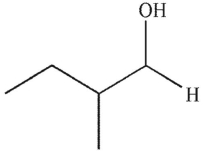
B)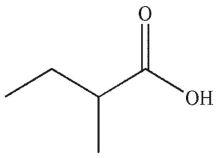
C)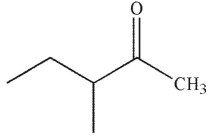
D)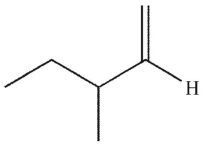
E) No reaction occurs.

A)

B)

C)

D)

E) No reaction occurs.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which of the following statements about the biological oxidation of alcohols is false?
A) Alcohol dehydrogenase is an enzyme that binds both ethyl alcohol and nicotinamide adenine dinucleotide NAD+
B) NAD+ is a pyridinium ion and a strong Lewis acid.
C) In the reaction catalyzed by alcohol dehydrogenase, ethyl alcohol is reduced and NAD+ is oxidized.
D) Alcohol dehydrogenase catalyzes a hydride transfer.
E) One of the products of the reaction catalyzed by alcohol dehydrogenase is an aldehyde.
A) Alcohol dehydrogenase is an enzyme that binds both ethyl alcohol and nicotinamide adenine dinucleotide NAD+
B) NAD+ is a pyridinium ion and a strong Lewis acid.
C) In the reaction catalyzed by alcohol dehydrogenase, ethyl alcohol is reduced and NAD+ is oxidized.
D) Alcohol dehydrogenase catalyzes a hydride transfer.
E) One of the products of the reaction catalyzed by alcohol dehydrogenase is an aldehyde.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which statement about this reaction is correct? 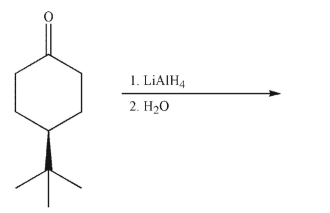
A)The products are enantiomers, formed in a racemic mixture.
B)The products are diastereomers, formed in equal amounts.
C)The products are enantiomers, formed in unequal amounts.
D)The products are diastereomers, formed in unequal amounts.
E)Not enough information is given to predict the products.

A)The products are enantiomers, formed in a racemic mixture.
B)The products are diastereomers, formed in equal amounts.
C)The products are enantiomers, formed in unequal amounts.
D)The products are diastereomers, formed in unequal amounts.
E)Not enough information is given to predict the products.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
30
Provide a name for the following compound.
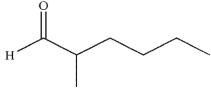


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
31
What is the product of the following reaction?
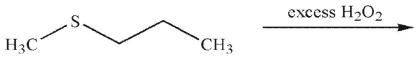
A)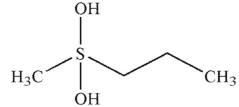
B)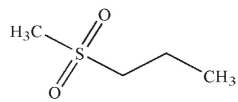
C)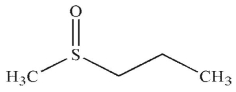
D)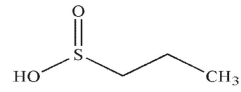
E)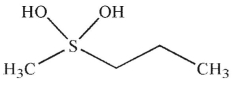

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
32
What is the final product of this reaction? 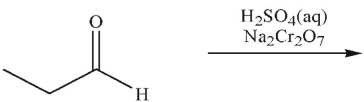
A)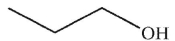
B)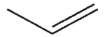
C)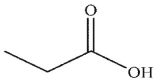
D)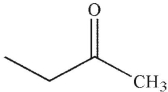
E)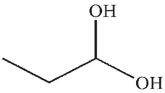
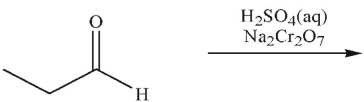
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following compounds will not react with CrO3/pyridine to give a carbonyl compound?
A)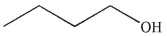
B)
C)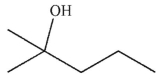
D)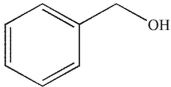
E)
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which reagent would you use to effect this transformation? 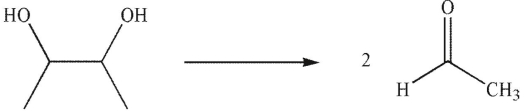
A)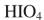
B)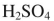
C)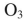
D)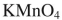
E)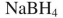

A)
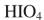
B)
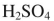
C)
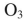
D)
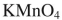
E)
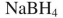

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
35
Which of the following alcohols cannot be made through the reduction of a carbonyl compound using a nucleophilic hydride reagent?
A)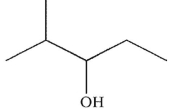
B)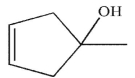
C)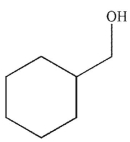
D)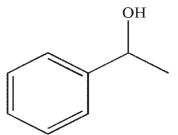
E)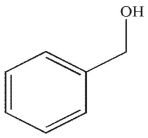
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which of the following compounds would you use, with an acid catalyst, to convert the starting material to the product shown?
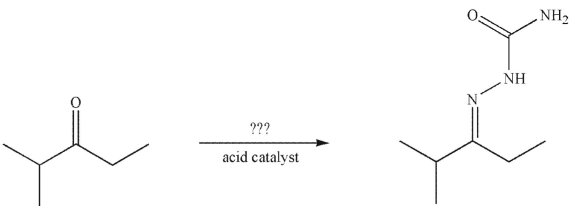
A)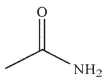
B)
C)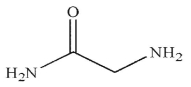
D)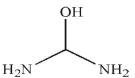
E)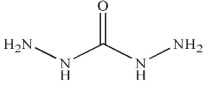

A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which reagents would you use to accomplish the following transformation?
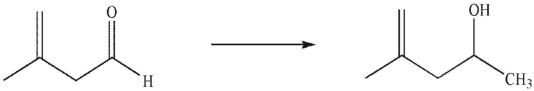
A) LiAlH4, then H3O+
B) NaBH4 in methanol
C)1. CH3MgBr,
2. H3O+
D)H2,Pd /C
E) CH3MgBr and H3O+

A) LiAlH4, then H3O+
B) NaBH4 in methanol
C)1. CH3MgBr,
2. H3O+
D)H2,Pd /C
E) CH3MgBr and H3O+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which of the following products could be made from cyclohexanone plus other reagents?
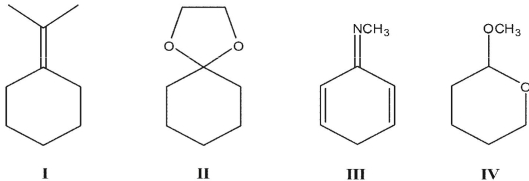
A) I
B) II
C) III
D) I and II
E) II and IV

A) I
B) II
C) III
D) I and II
E) II and IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
39
Which of the following reagents could be used to effect the organic transformation shown here?
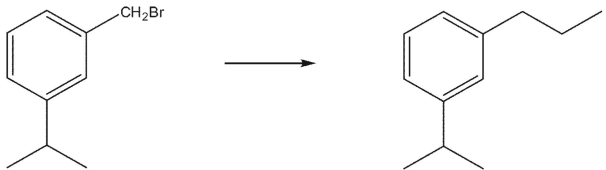
A) CH3CH2Li
B) CH3CH2MgBr
C) (CH3)2CuLi
D) CH3Li
E) (CH3CH2)2CuLi

A) CH3CH2Li
B) CH3CH2MgBr
C) (CH3)2CuLi
D) CH3Li
E) (CH3CH2)2CuLi

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which reagent would you use to accomplish the transformation shown?

A)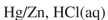
B)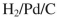
C)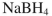
D)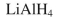
E)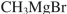

A)
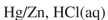
B)
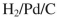
C)
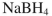
D)
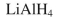
E)
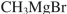

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
41
Predict the product of the following reaction and draw an arrow-pushing mechanism to rationalize its formation.Include all necessary lone pairs, curved arrows, and nonzero formal charges. 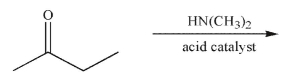


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
42
What product would be recovered from the following reaction? 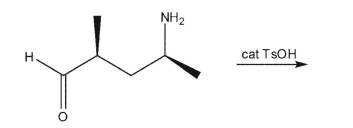


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
43
Draw a mechanism to illustrate the removal of the acetal under the conditions shown.Include all necessary lone pairs, curved arrows, and nonzero formal charges. 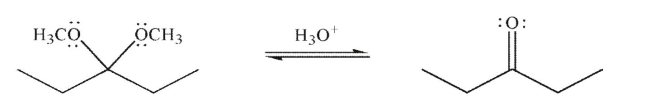


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
44
Draw a mechanism for the following transformation.Include all necessary lone pairs, curved arrows, and nonzero formal charges. 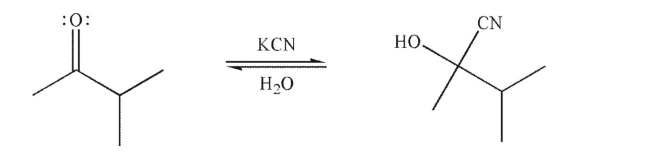


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
45
Draw a mechanism for the following transformation.Include all necessary lone pairs, curved arrows, and nonzero formal charges. 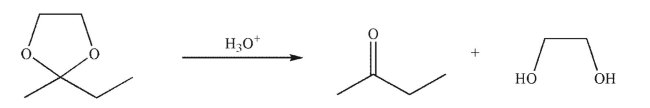


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
46
Predict the product of the following reaction. 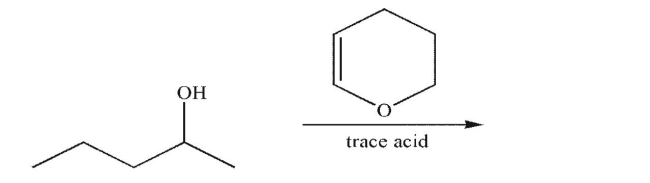


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
47
For each structure, state whether it is an acetal, a hemiacetal, or neither. 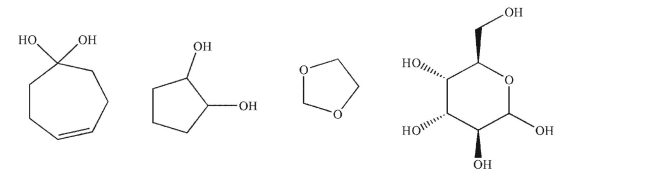


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
48
Draw the structure of the compound with molecular formula C4H6O that has the following 1H NMR and IR spectra.
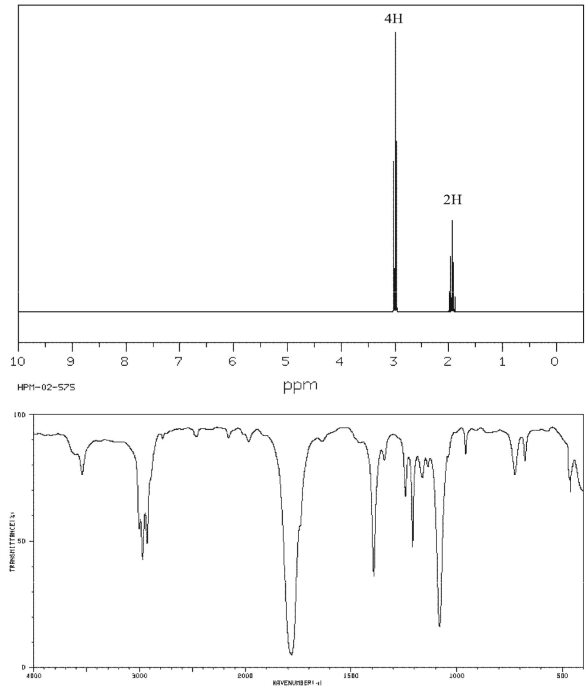


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
49
Draw the product of the following reaction, showing stereochemistry. 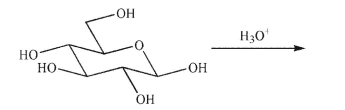


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
50
Propose a mechanism for the removal of the THP ether-protecting group in the following compound under the conditions shown.Include all necessary lone pairs, curved arrows, and nonzero formal charges. 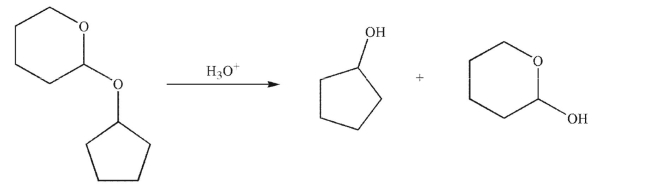


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
51
Predict the product of the following reaction and draw a mechanism to rationalize its formation. Include all necessary lone pairs, curved arrows, and nonzero formal charges. 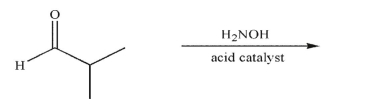


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
52
Draw a mechanism for the following transformation.Include all curved arrows, necessary lone pairs, and nonzero formal charges. 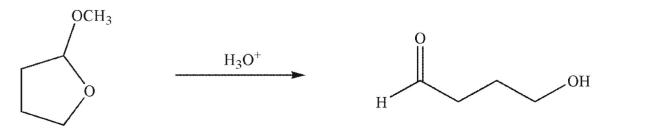


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
53
Match each compound to the appropriate spectrum. 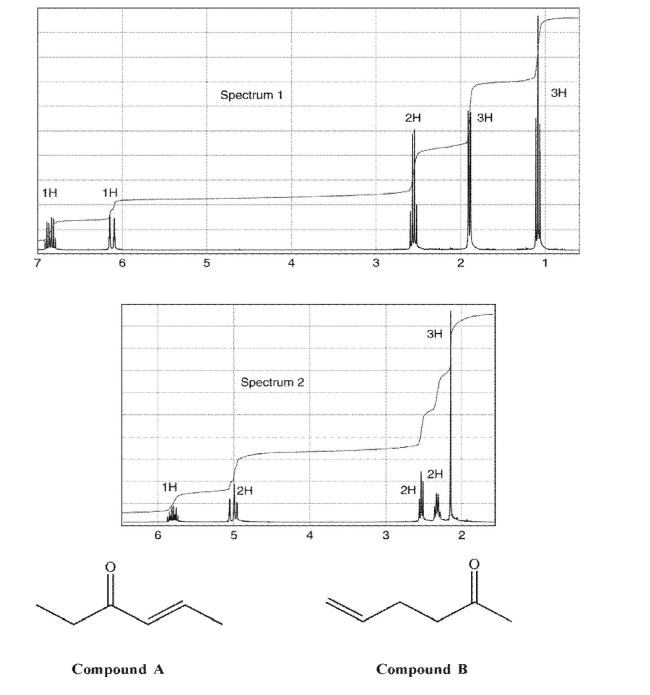


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
54
Predict the product of the following reaction. 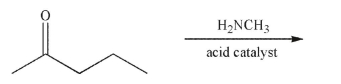


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
55
Draw a mechanism to illustrate the following transformation.Include all necessary lone pairs, curved arrows, and nonzero formal charges. 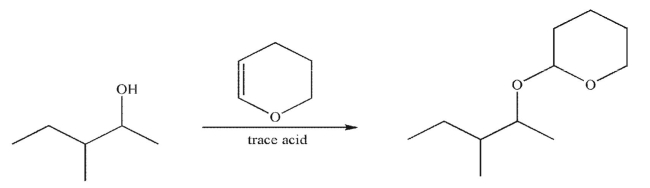


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
56
Draw a mechanism for the hydration of the compound shown here under acidic conditions. Include all lone pairs, curved arrows, and nonzero formal charges. 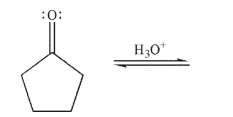


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
57
Draw a mechanism for the hydration of the compound shown here under basic conditions.Include
all lone pairs, curved arrows, and nonzero formal charges.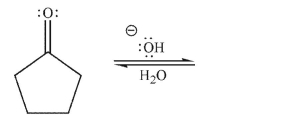
all lone pairs, curved arrows, and nonzero formal charges.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
58
Draw a mechanism to illustrate the formation of an acetal (ketal) under the conditions shown. Include all necessary lone pairs, curved arrows, and nonzero formal charges. 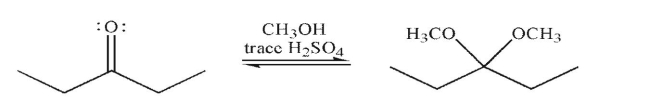
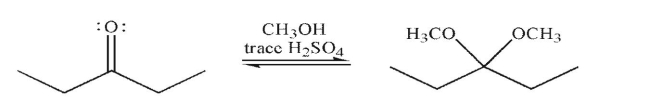

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
59
An acyclic compound was treated with trace acid to produce this structure. 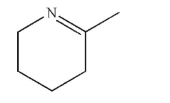 Draw the structure of the acyclic compound.
Draw the structure of the acyclic compound.
 Draw the structure of the acyclic compound.
Draw the structure of the acyclic compound.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
60
For each of the carbonyl compounds shown, qualitatively show the relative free energies (ΔG) of the carbonyl and its hydrate based on Keq for the hydration reaction.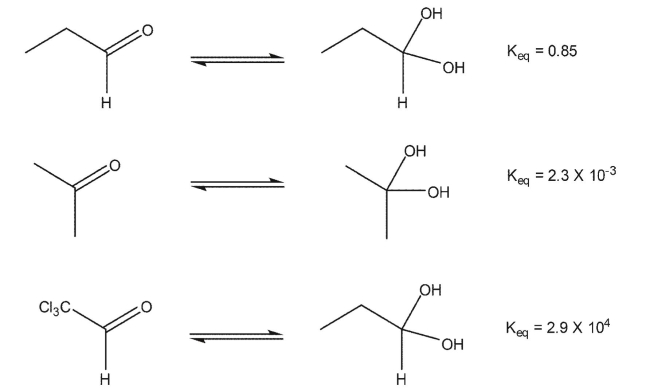


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
61
Write a multistep synthesis for the following transformation. 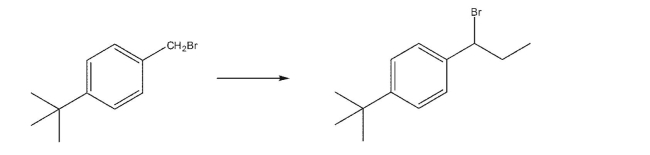


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
62
Predict the product of the following reaction. 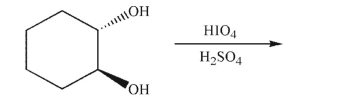


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
63
Draw all combinations of carbonyl compounds and organometallic reagents that would give the alcohol shown. 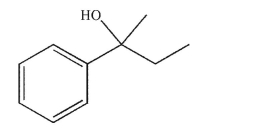


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
64
Predict the product of the following reaction. 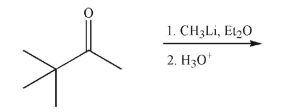


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
65
Which combination of carbonyl compound and organometallic reagent would give the compound shown? 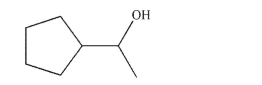


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
66
Design a multistep synthesis for the following transformation.You may use any organic or inorganic reagents.Show the reagents needed for each step and the product of each step. 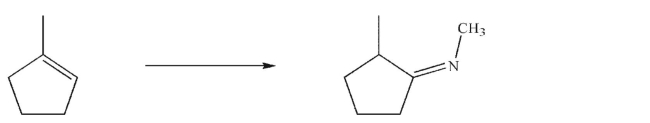


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
67
Use a retrosynthetic analysis to provide five possible precursors to this alcohol. Include the reagents needed to transform each of the precursors to the alcohol. 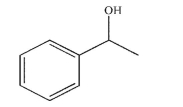


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
68
Design a multistep synthesis for the transformation shown here.You may use any organic or inorganic reagents.Show the reagents needed for each step and the product of each step. 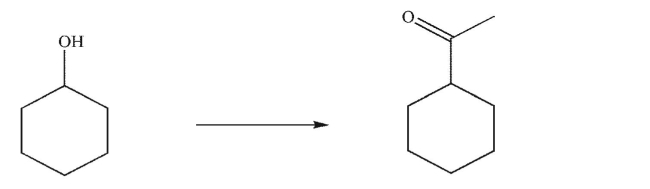


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
69
Which reagent or reagents would you use to accomplish the following transformation? 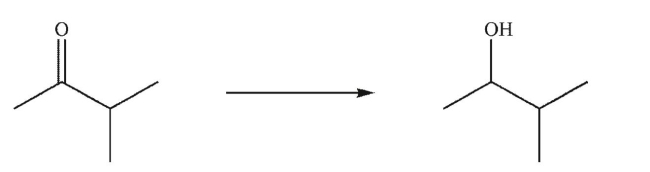


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
70
Predict the product of the following reaction. 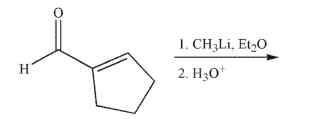


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
71
How would you accomplish the following oxidation reaction? 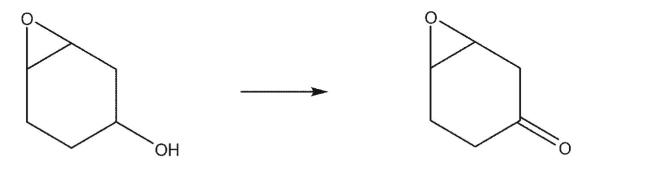


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
72
Predict the product of the following reaction. 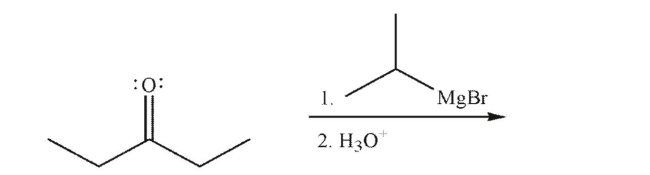


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck
73
Design a multistep synthesis for the following transformation.You may use any organic or inorganic reagents.Show the reagents needed for each step and the product of each step. 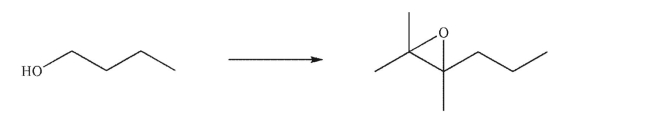


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 73 في هذه المجموعة.
فتح الحزمة
k this deck








