Deck 23: Special Topic: Reactions Controlled by Orbital Symmetry
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/46
العب
ملء الشاشة (f)
Deck 23: Special Topic: Reactions Controlled by Orbital Symmetry
1
The starting compound undergoes a sigmatropic rearrangement to give the product shown.Which of these terms correctly describes the process that occurred? 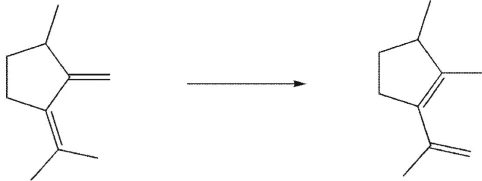
A) 1,3 sigmatropic rearrangement
B) 1,5 sigmatropic rearrangement
C) 2,3 sigmatropic rearrangement
D) 3,3 sigmatropic rearrangement
E) None of these terms describes the process that occurred.

A) 1,3 sigmatropic rearrangement
B) 1,5 sigmatropic rearrangement
C) 2,3 sigmatropic rearrangement
D) 3,3 sigmatropic rearrangement
E) None of these terms describes the process that occurred.
1,5 sigmatropic rearrangement
2
What is the product of the thermal ring-opening electrocyclic reaction of cis-3,4-dimethylcyclobutene?
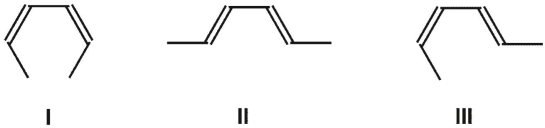
A) predominantly I
B) predominantly II
C) predominantly III
D) mixture of I and II in approximately equal amounts
E) mixture of II and III in approximately equal amounts

A) predominantly I
B) predominantly II
C) predominantly III
D) mixture of I and II in approximately equal amounts
E) mixture of II and III in approximately equal amounts
predominantly III
3
Which of these electrocyclic reactions will occur through a disrotatory process?
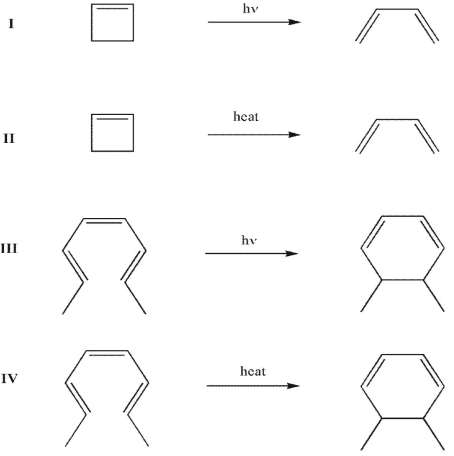
A) I and II
B) I and III
C) II and IV
D) I and IV
E) all

A) I and II
B) I and III
C) II and IV
D) I and IV
E) all
I and IV
4
What is the product of a [1,3] -sigmatropic shift in the following compound?
![<strong>What is the product of a [1,3] -sigmatropic shift in the following compound? </strong> A) B) C) The compound shown cannot undergo a [1,3]-sigmatropic shift. D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e93_2fd6_04e3_9927_7946c44dd2ab_TB34225555_11.jpg)
A)![<strong>What is the product of a [1,3] -sigmatropic shift in the following compound? </strong> A) B) C) The compound shown cannot undergo a [1,3]-sigmatropic shift. D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e93_2fd8_9cf4_9927_3ff7f589c197_TB34225555_11.jpg)
B)![<strong>What is the product of a [1,3] -sigmatropic shift in the following compound? </strong> A) B) C) The compound shown cannot undergo a [1,3]-sigmatropic shift. D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e93_2fda_bfd5_9927_8dbf83b7211f_TB34225555_11.jpg)
C) The compound shown cannot undergo a [1,3]-sigmatropic shift.
D)![<strong>What is the product of a [1,3] -sigmatropic shift in the following compound? </strong> A) B) C) The compound shown cannot undergo a [1,3]-sigmatropic shift. D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e93_2fdc_6d86_9927_fdaf471ec26a_TB34225555_11.jpg)
E)![<strong>What is the product of a [1,3] -sigmatropic shift in the following compound? </strong> A) B) C) The compound shown cannot undergo a [1,3]-sigmatropic shift. D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e93_2fde_6957_9927_c520f6ff0385_TB34225555_11.jpg)
![<strong>What is the product of a [1,3] -sigmatropic shift in the following compound? </strong> A) B) C) The compound shown cannot undergo a [1,3]-sigmatropic shift. D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e93_2fd6_04e3_9927_7946c44dd2ab_TB34225555_11.jpg)
A)
![<strong>What is the product of a [1,3] -sigmatropic shift in the following compound? </strong> A) B) C) The compound shown cannot undergo a [1,3]-sigmatropic shift. D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e93_2fd8_9cf4_9927_3ff7f589c197_TB34225555_11.jpg)
B)
![<strong>What is the product of a [1,3] -sigmatropic shift in the following compound? </strong> A) B) C) The compound shown cannot undergo a [1,3]-sigmatropic shift. D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e93_2fda_bfd5_9927_8dbf83b7211f_TB34225555_11.jpg)
C) The compound shown cannot undergo a [1,3]-sigmatropic shift.
D)
![<strong>What is the product of a [1,3] -sigmatropic shift in the following compound? </strong> A) B) C) The compound shown cannot undergo a [1,3]-sigmatropic shift. D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e93_2fdc_6d86_9927_fdaf471ec26a_TB34225555_11.jpg)
E)
![<strong>What is the product of a [1,3] -sigmatropic shift in the following compound? </strong> A) B) C) The compound shown cannot undergo a [1,3]-sigmatropic shift. D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e93_2fde_6957_9927_c520f6ff0385_TB34225555_11.jpg)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which of the following statements best describes the chemical events leading from starting material to product? ![<strong>Which of the following statements best describes the chemical events leading from starting material to product? </strong> A) a [3,3] sigmatropic rearrangement, then an intramolecular Diels-Alder reaction B) a [1,5] hydrogen shift/sigmatropic rearrangement, followed by a [3,3] sigmatropic rearrangement C) two successive Diels-Alder reactions D) two successive [3,3] sigmatropic rearrangements E) an intramolecular Diels-Alder reaction followed by a [2,3] sigmatropic rearrangement](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e92_e4a9_c96e_9927_2d34f1e95ff4_TB34225555_11.jpg)
A) a [3,3] sigmatropic rearrangement, then an intramolecular Diels-Alder reaction
B) a [1,5] hydrogen shift/sigmatropic rearrangement, followed by a [3,3] sigmatropic rearrangement
C) two successive Diels-Alder reactions
D) two successive [3,3] sigmatropic rearrangements
E) an intramolecular Diels-Alder reaction followed by a [2,3] sigmatropic rearrangement
![<strong>Which of the following statements best describes the chemical events leading from starting material to product? </strong> A) a [3,3] sigmatropic rearrangement, then an intramolecular Diels-Alder reaction B) a [1,5] hydrogen shift/sigmatropic rearrangement, followed by a [3,3] sigmatropic rearrangement C) two successive Diels-Alder reactions D) two successive [3,3] sigmatropic rearrangements E) an intramolecular Diels-Alder reaction followed by a [2,3] sigmatropic rearrangement](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e92_e4a9_c96e_9927_2d34f1e95ff4_TB34225555_11.jpg)
A) a [3,3] sigmatropic rearrangement, then an intramolecular Diels-Alder reaction
B) a [1,5] hydrogen shift/sigmatropic rearrangement, followed by a [3,3] sigmatropic rearrangement
C) two successive Diels-Alder reactions
D) two successive [3,3] sigmatropic rearrangements
E) an intramolecular Diels-Alder reaction followed by a [2,3] sigmatropic rearrangement

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which statement is true regarding the favored process in this thermal electrocyclic reaction? 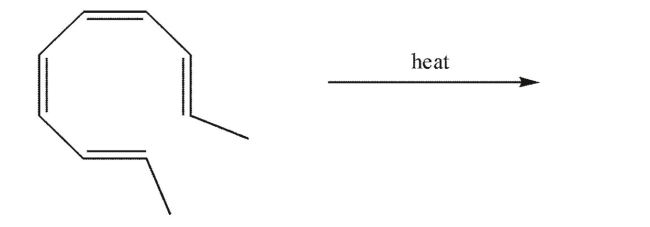
A)Conrotatory ring closure produces a racemic mixture.
B)Disrotatory ring closure produces a racemic mixture.
C)Conrotatory ring closure produces a single achiral product.
D)Disrotatory ring closure produces a single achiral product.
E)both a and c

A)Conrotatory ring closure produces a racemic mixture.
B)Disrotatory ring closure produces a racemic mixture.
C)Conrotatory ring closure produces a single achiral product.
D)Disrotatory ring closure produces a single achiral product.
E)both a and c

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
7
Consider the cycloaddition reaction below.Fill in the blanks: this reaction is ________ and is ________allowed ![<strong>Consider the cycloaddition reaction below.Fill in the blanks: this reaction is ________ and is ________allowed </strong> A) [6+4] ; thermally B) [6+4] ; photochemically C) [4+4] ; thermally D) [4+4] ; photochemically E) [4+2] ; thermally](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e94_6ef0_ff9c_9927_2124826f2707_TB34225555_11.jpg)
A) [6+4] ; thermally
B) [6+4] ; photochemically
C) [4+4] ; thermally
D) [4+4] ; photochemically
E) [4+2] ; thermally
![<strong>Consider the cycloaddition reaction below.Fill in the blanks: this reaction is ________ and is ________allowed </strong> A) [6+4] ; thermally B) [6+4] ; photochemically C) [4+4] ; thermally D) [4+4] ; photochemically E) [4+2] ; thermally](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e94_6ef0_ff9c_9927_2124826f2707_TB34225555_11.jpg)
A) [6+4] ; thermally
B) [6+4] ; photochemically
C) [4+4] ; thermally
D) [4+4] ; photochemically
E) [4+2] ; thermally

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which type of sigmatropic rearrangement is involved in this process? 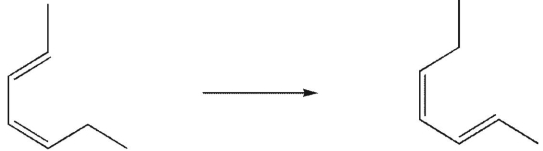
A) 1,3
B) 1,5
C) 1,7
D) 2,2
E) 4,2

A) 1,3
B) 1,5
C) 1,7
D) 2,2
E) 4,2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
9
What is the product of the thermal ring-closing electrocyclic reaction of (E,E) isomer of hexa-2,4-diene 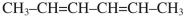 ?
?
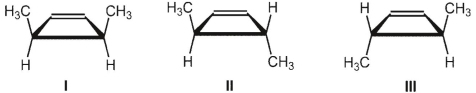
A) I
B) II
C) III
D) mixture of I and II in equal amounts
E) mixture of II and III in equal amounts
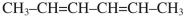 ?
?
A) I
B) II
C) III
D) mixture of I and II in equal amounts
E) mixture of II and III in equal amounts

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which type of sigmatropic rearrangement is involved in this process?
A) 1,3
B) 1,5
C) 1,7
D) 2,2
E) 2,3

A) 1,3
B) 1,5
C) 1,7
D) 2,2
E) 2,3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which type of sigmatropic rearrangement is involved in this process? 
A) 1,3
B) 1,5
C) 1,7
D) 2,3
E) 3,3

A) 1,3
B) 1,5
C) 1,7
D) 2,3
E) 3,3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
12
What is the product of the following photochemical [2+2] cycloaddition reaction? (OAc is acetoxy group ![<strong>What is the product of the following photochemical [2+2] cycloaddition reaction? (OAc is acetoxy group </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e94_f991_47ae_9927_05dedecc5838_TB34225555_11.jpg)
![<strong>What is the product of the following photochemical [2+2] cycloaddition reaction? (OAc is acetoxy group </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e94_f992_591f_9927_71c62e872bdf_TB34225555_11.jpg)
A)![<strong>What is the product of the following photochemical [2+2] cycloaddition reaction? (OAc is acetoxy group </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e94_f994_06d0_9927_cfa55b891073_TB34225555_11.jpg)
B)![<strong>What is the product of the following photochemical [2+2] cycloaddition reaction? (OAc is acetoxy group </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e94_f995_db91_9927_4119f0ad997d_TB34225555_11.jpg)
C)![<strong>What is the product of the following photochemical [2+2] cycloaddition reaction? (OAc is acetoxy group </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e94_f997_fe72_9927_ab85854789bb_TB34225555_11.jpg)
D)![<strong>What is the product of the following photochemical [2+2] cycloaddition reaction? (OAc is acetoxy group </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e94_f999_fa43_9927_cb45499f1fe3_TB34225555_11.jpg)
E)![<strong>What is the product of the following photochemical [2+2] cycloaddition reaction? (OAc is acetoxy group </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e94_f99b_a7f4_9927_67b122741521_TB34225555_11.jpg)
![<strong>What is the product of the following photochemical [2+2] cycloaddition reaction? (OAc is acetoxy group </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e94_f991_47ae_9927_05dedecc5838_TB34225555_11.jpg)
![<strong>What is the product of the following photochemical [2+2] cycloaddition reaction? (OAc is acetoxy group </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e94_f992_591f_9927_71c62e872bdf_TB34225555_11.jpg)
A)
![<strong>What is the product of the following photochemical [2+2] cycloaddition reaction? (OAc is acetoxy group </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e94_f994_06d0_9927_cfa55b891073_TB34225555_11.jpg)
B)
![<strong>What is the product of the following photochemical [2+2] cycloaddition reaction? (OAc is acetoxy group </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e94_f995_db91_9927_4119f0ad997d_TB34225555_11.jpg)
C)
![<strong>What is the product of the following photochemical [2+2] cycloaddition reaction? (OAc is acetoxy group </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e94_f997_fe72_9927_ab85854789bb_TB34225555_11.jpg)
D)
![<strong>What is the product of the following photochemical [2+2] cycloaddition reaction? (OAc is acetoxy group </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e94_f999_fa43_9927_cb45499f1fe3_TB34225555_11.jpg)
E)
![<strong>What is the product of the following photochemical [2+2] cycloaddition reaction? (OAc is acetoxy group </strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e94_f99b_a7f4_9927_67b122741521_TB34225555_11.jpg)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which of these processes are thermally allowed? 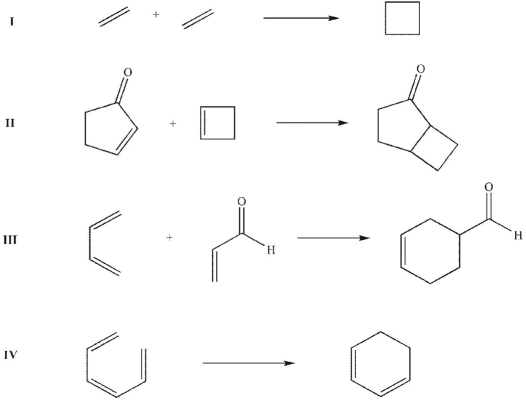
A) I and II
B) II
C) III
D) II, III, and IV
E) III and IV

A) I and II
B) II
C) III
D) II, III, and IV
E) III and IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of these is the "thermal" HOMO for penta-2,4-dien-1-yl radical, 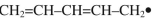
A)
B)
C)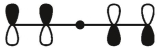
D)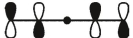
E)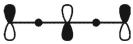
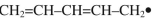
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
15
which of these is the "photochemical" номо for ally radical?
A)
B)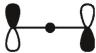
C)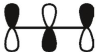
D)
E)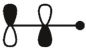
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which of these is the "photochemical" номо for buta-1,-3-diene?
A)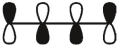
B)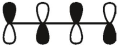
C)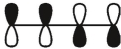
D)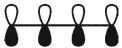
E)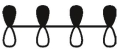
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which of these is the "thermal" номо for allyl radical?
A)
B)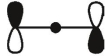
C)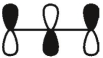
D)
E)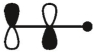
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which of these is the "thermal" номо for hexa-1,3,5-triene?
A)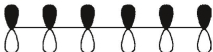
B)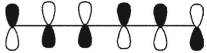
C)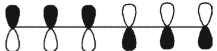
D)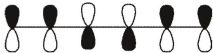
E)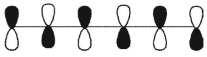
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
19
In which of the following systems will an antarafacial shift be impossible?
A)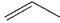
B)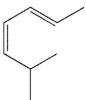
C)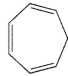
D)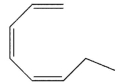
E) None of these systems can undergo an antarafacial shift.
A)

B)

C)

D)

E) None of these systems can undergo an antarafacial shift.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which of these structures is the product of [3,3] sigmatropic rearrangement of this compound? ![<strong>Which of these structures is the product of [3,3] sigmatropic rearrangement of this compound? </strong> A) B) C) D) E) The compound shown cannot undergo a [3,3] sigmatropic rearrangement.](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e93_5ff6_ba78_9927_850d8f3019f7_TB34225555_11.jpg)
A)![<strong>Which of these structures is the product of [3,3] sigmatropic rearrangement of this compound? </strong> A) B) C) D) E) The compound shown cannot undergo a [3,3] sigmatropic rearrangement.](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e93_5ff7_f2f9_9927_d322cbfdc377_TB34225555_11.jpg)
B)![<strong>Which of these structures is the product of [3,3] sigmatropic rearrangement of this compound? </strong> A) B) C) D) E) The compound shown cannot undergo a [3,3] sigmatropic rearrangement.](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e93_5ff8_b64a_9927_4b4674c44bb6_TB34225555_11.jpg)
C)![<strong>Which of these structures is the product of [3,3] sigmatropic rearrangement of this compound? </strong> A) B) C) D) E) The compound shown cannot undergo a [3,3] sigmatropic rearrangement.](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e93_5ff9_799b_9927_155eff248a71_TB34225555_11.jpg)
D)![<strong>Which of these structures is the product of [3,3] sigmatropic rearrangement of this compound? </strong> A) B) C) D) E) The compound shown cannot undergo a [3,3] sigmatropic rearrangement.](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e93_5ffa_63fc_9927_391bb3c375c3_TB34225555_11.jpg)
E) The compound shown cannot undergo a [3,3] sigmatropic rearrangement.
![<strong>Which of these structures is the product of [3,3] sigmatropic rearrangement of this compound? </strong> A) B) C) D) E) The compound shown cannot undergo a [3,3] sigmatropic rearrangement.](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e93_5ff6_ba78_9927_850d8f3019f7_TB34225555_11.jpg)
A)
![<strong>Which of these structures is the product of [3,3] sigmatropic rearrangement of this compound? </strong> A) B) C) D) E) The compound shown cannot undergo a [3,3] sigmatropic rearrangement.](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e93_5ff7_f2f9_9927_d322cbfdc377_TB34225555_11.jpg)
B)
![<strong>Which of these structures is the product of [3,3] sigmatropic rearrangement of this compound? </strong> A) B) C) D) E) The compound shown cannot undergo a [3,3] sigmatropic rearrangement.](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e93_5ff8_b64a_9927_4b4674c44bb6_TB34225555_11.jpg)
C)
![<strong>Which of these structures is the product of [3,3] sigmatropic rearrangement of this compound? </strong> A) B) C) D) E) The compound shown cannot undergo a [3,3] sigmatropic rearrangement.](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e93_5ff9_799b_9927_155eff248a71_TB34225555_11.jpg)
D)
![<strong>Which of these structures is the product of [3,3] sigmatropic rearrangement of this compound? </strong> A) B) C) D) E) The compound shown cannot undergo a [3,3] sigmatropic rearrangement.](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e93_5ffa_63fc_9927_391bb3c375c3_TB34225555_11.jpg)
E) The compound shown cannot undergo a [3,3] sigmatropic rearrangement.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which of these structures is the product of Cope rearrangement of this compound? 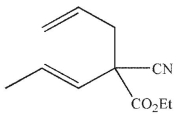
A)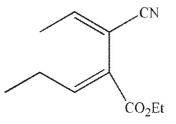
B)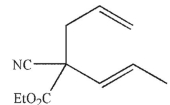
C)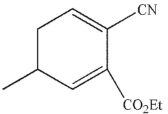
D)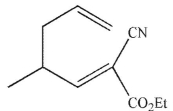
E) None of these is the product of Cope rearrangement of the compound shown.

A)

B)

C)

D)

E) None of these is the product of Cope rearrangement of the compound shown.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
22
What is a major isolated product arising from Cope (Claisen) rearrangement for the following compound? 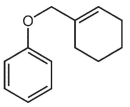
A)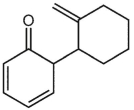
B)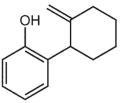
C) This compound does not undergo such rearrangement.
D)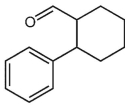
E)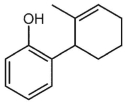

A)

B)

C) This compound does not undergo such rearrangement.
D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
23
Rationalize why only the exo product is formed in the following [6+4] cycloaddition reaction. ![Rationalize why only the exo product is formed in the following [6+4] cycloaddition reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB4310/11eb4b6a_e1a4_c2c6_80d3_413f673e8a50_TB4310_00.jpg)
![Rationalize why only the exo product is formed in the following [6+4] cycloaddition reaction.](https://d2lvgg3v3hfg70.cloudfront.net/TB4310/11eb4b6a_e1a4_c2c6_80d3_413f673e8a50_TB4310_00.jpg)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
24
Draw the ground-state ("thermal") 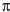 -molecular orbitals for the allyl radical and show electron occupancy on the corresponding energy diagram. Label HOMO and LUMO.
-molecular orbitals for the allyl radical and show electron occupancy on the corresponding energy diagram. Label HOMO and LUMO.
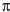 -molecular orbitals for the allyl radical and show electron occupancy on the corresponding energy diagram. Label HOMO and LUMO.
-molecular orbitals for the allyl radical and show electron occupancy on the corresponding energy diagram. Label HOMO and LUMO.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
25
Antarafacial [1,5] -hydride shifts are very rare, and then only occur in cyclic systems, such as the one shown below. What are the reaction conditions required?![Antarafacial [1,5] -hydride shifts are very rare, and then only occur in cyclic systems, such as the one shown below. What are the reaction conditions required?](https://d2lvgg3v3hfg70.cloudfront.net/TB4310/11eb4b6a_e1a5_37fe_80d3_033784baed4b_TB4310_00.jpg)
![Antarafacial [1,5] -hydride shifts are very rare, and then only occur in cyclic systems, such as the one shown below. What are the reaction conditions required?](https://d2lvgg3v3hfg70.cloudfront.net/TB4310/11eb4b6a_e1a5_37fe_80d3_033784baed4b_TB4310_00.jpg)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
26
Draw the products of the electrocyclic reaction that would occur on heating this compound. 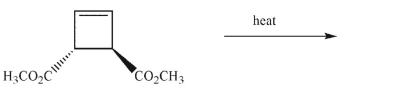
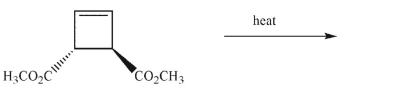

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which of these structures does not undergo Cope rearrangement?
A)
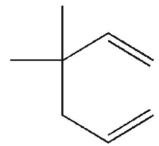
B)
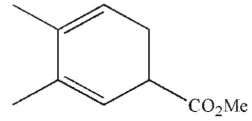
C)
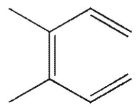
D)
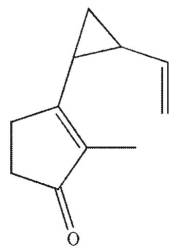
E)
All these compounds can undergo Cope rearrangement.
A)

B)

C)

D)

E)
All these compounds can undergo Cope rearrangement.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
28
Is the process shown conrotatory or disrotatory? 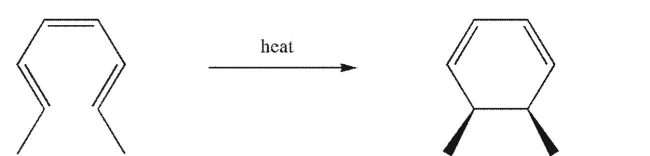


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
29
Is this reaction allowed thermally or photochemically? 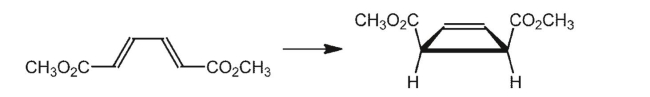


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
30
Draw the  -molecular orbitals for buta-1,3-diene and show electron occupancy on the corresponding energy diagram. Label "thermal" HOMO and "photochemical" HOMO.
-molecular orbitals for buta-1,3-diene and show electron occupancy on the corresponding energy diagram. Label "thermal" HOMO and "photochemical" HOMO.
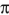 -molecular orbitals for buta-1,3-diene and show electron occupancy on the corresponding energy diagram. Label "thermal" HOMO and "photochemical" HOMO.
-molecular orbitals for buta-1,3-diene and show electron occupancy on the corresponding energy diagram. Label "thermal" HOMO and "photochemical" HOMO.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
31
Ketene '![Ketene ' can dimerize in a [2+2] fashion using the bond of one molecule and the bond of another molecule. Draw the product of this cycloaddition.](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e90_a63e_3deb_9927_69073039f207_TB34225555_11.jpg) can dimerize in a [2+2] fashion using the
can dimerize in a [2+2] fashion using the ![Ketene ' can dimerize in a [2+2] fashion using the bond of one molecule and the bond of another molecule. Draw the product of this cycloaddition.](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e90_a63f_284c_9927_ab594e895d3c_TB34225555_11.jpg) bond of one molecule and the
bond of one molecule and the ![Ketene ' can dimerize in a [2+2] fashion using the bond of one molecule and the bond of another molecule. Draw the product of this cycloaddition.](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e90_a63f_766d_9927_f5ef0ae300af_TB34225555_11.jpg) bond of another molecule. Draw the product of this cycloaddition.
bond of another molecule. Draw the product of this cycloaddition.
![Ketene ' can dimerize in a [2+2] fashion using the bond of one molecule and the bond of another molecule. Draw the product of this cycloaddition.](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e90_a63e_3deb_9927_69073039f207_TB34225555_11.jpg) can dimerize in a [2+2] fashion using the
can dimerize in a [2+2] fashion using the ![Ketene ' can dimerize in a [2+2] fashion using the bond of one molecule and the bond of another molecule. Draw the product of this cycloaddition.](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e90_a63f_284c_9927_ab594e895d3c_TB34225555_11.jpg) bond of one molecule and the
bond of one molecule and the ![Ketene ' can dimerize in a [2+2] fashion using the bond of one molecule and the bond of another molecule. Draw the product of this cycloaddition.](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e90_a63f_766d_9927_f5ef0ae300af_TB34225555_11.jpg) bond of another molecule. Draw the product of this cycloaddition.
bond of another molecule. Draw the product of this cycloaddition.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
32
Draw the ground state ("thermal") and or excited state ("photochemical") highest-occupied 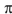 -molecular orbitals (HOMOs) for octa-1,3,5,7-tetraene.
-molecular orbitals (HOMOs) for octa-1,3,5,7-tetraene.
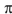 -molecular orbitals (HOMOs) for octa-1,3,5,7-tetraene.
-molecular orbitals (HOMOs) for octa-1,3,5,7-tetraene.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
33
Is the product shown the result of conrotation or disrotation? 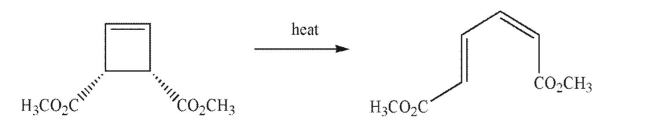


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
34
Explain why thermal dimerization of ethylene is symmetry forbidden.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
35
One of the monomers that makes up the DNA polymer is thymine. 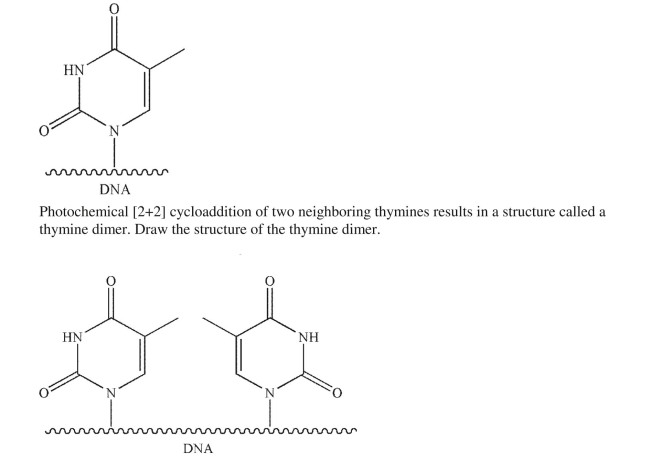


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
36
Draw the product of the conrotatory ring-closing electrocyclic reaction below. 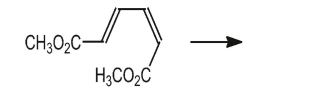


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
37
Is the product shown the result of suprafacial or antarafacial reaction? 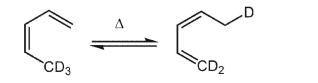


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
38
The following reaction is a sigmatropic shift. Provide the arrow formalism and determine the nature [x, y] of the shift.
![The following reaction is a sigmatropic shift. Provide the arrow formalism and determine the nature [x, y] of the shift.](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e90_71fa_b9a7_9927_810778704ef0_TB34225555_11.jpg)
![The following reaction is a sigmatropic shift. Provide the arrow formalism and determine the nature [x, y] of the shift.](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e90_71fa_b9a7_9927_810778704ef0_TB34225555_11.jpg)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
39
The thermal Diels-Alder reaction can occur through the interaction of the HOMO of the diene and the LUMO of the dienophile. Can the reaction also occur through the interaction of the HOMO of the dienophile and the LUMO of the diene? Using 1,3-butadiene and ethylene as your model system, provide orbital diagrams to support your answer.
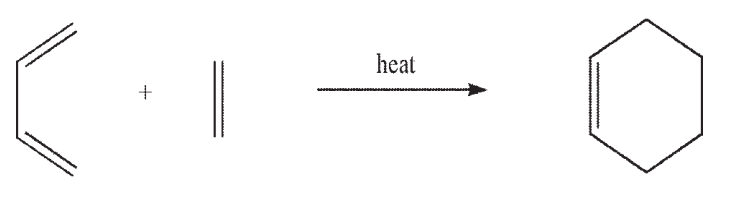


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
40
The following reaction involves a sigmatropic shift and another transformation you already know.
Provide arrow formalism for the sigmatropic shift and determine the nature of both steps.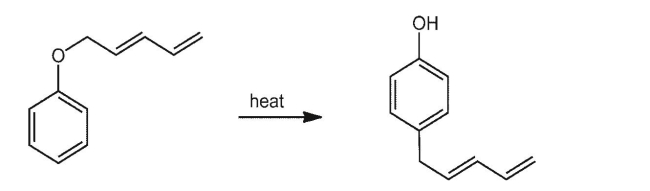
Provide arrow formalism for the sigmatropic shift and determine the nature of both steps.


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
41
Draw the product of thermal Cope rearrangement of this compound. 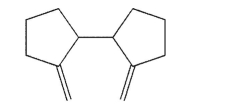


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
42
When both ortho positions on the aromatic ring are blocked, the enolization of the Claisen
rearrangement product is impossible.In this case two sequential [3,3]-shifts occur, affording a
trisubstituted phenol.Starting from the following allyl aryl ether, draw the arrow formalism for
both [3,3] shifts, showing the intermediates and the final product.![When both ortho positions on the aromatic ring are blocked, the enolization of the Claisen rearrangement product is impossible.In this case two sequential [3,3]-shifts occur, affording a trisubstituted phenol.Starting from the following allyl aryl ether, draw the arrow formalism for both [3,3] shifts, showing the intermediates and the final product.](https://d2lvgg3v3hfg70.cloudfront.net/TB4310/11eb4b6a_e1a5_d44b_80d3_d9db32bf292b_TB4310_00.jpg)
rearrangement product is impossible.In this case two sequential [3,3]-shifts occur, affording a
trisubstituted phenol.Starting from the following allyl aryl ether, draw the arrow formalism for
both [3,3] shifts, showing the intermediates and the final product.
![When both ortho positions on the aromatic ring are blocked, the enolization of the Claisen rearrangement product is impossible.In this case two sequential [3,3]-shifts occur, affording a trisubstituted phenol.Starting from the following allyl aryl ether, draw the arrow formalism for both [3,3] shifts, showing the intermediates and the final product.](https://d2lvgg3v3hfg70.cloudfront.net/TB4310/11eb4b6a_e1a5_d44b_80d3_d9db32bf292b_TB4310_00.jpg)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
43
Draw the isolated product arising from thermal Cope rearrangement of this compound. 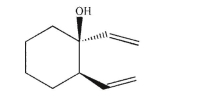


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
44
Predict the product of Claisen rearrangement of this compound. 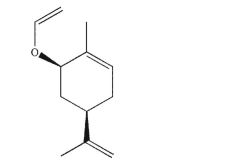


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
45
Draw the product of a [2,3]-sigmatropic shift reaction of the compound below.![Draw the product of a [2,3]-sigmatropic shift reaction of the compound below.](https://d2lvgg3v3hfg70.cloudfront.net/TB4310/11eb4b6a_e1a5_3801_80d3_e9a6437c8c3b_TB4310_00.jpg)
![Draw the product of a [2,3]-sigmatropic shift reaction of the compound below.](https://d2lvgg3v3hfg70.cloudfront.net/TB4310/11eb4b6a_e1a5_3801_80d3_e9a6437c8c3b_TB4310_00.jpg)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck
46
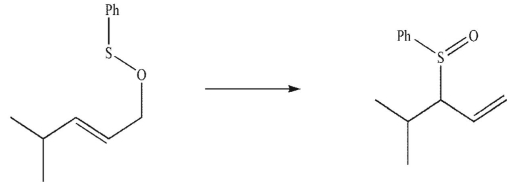
Draw the product of a [2,3] sigmatropic rearrangement of this compound.
![Draw the product of a [2,3] sigmatropic rearrangement of this compound.](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e90_2738_1575_9927_d7b86efbde72_TB34225555_11.jpg)
![Draw the product of a [2,3] sigmatropic rearrangement of this compound.](https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/11ec7e90_2738_1575_9927_d7b86efbde72_TB34225555_11.jpg)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 46 في هذه المجموعة.
فتح الحزمة
k this deck








