Deck 2: The Origin and Chemistry of Life
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/15
العب
ملء الشاشة (f)
Deck 2: The Origin and Chemistry of Life
1
Explain each of these properties of water, and describe how each is conferred by the dipolar nature of a water molecule: high specific heat capacity; high heat of vaporization; unique density behavior; high surface tension; good solvent for ions of salts.
The dipole nature of the H2O molecule confers the following properties:
• High specific heat capacity:
The partial negative and the partial positive charge of the water molecule cause attraction between water molecules. It causes the formation of hydrogen bonds between water molecules. These hydrogen bonds need a high amount of heat to be broken. This high amount of heat is known as high specific heat capacity of water. As water has a high specific heat capacity, it is able to absorb heat, without increasing much in temperature.
• High heat of vaporization:
The hydrogen bonds formed between water molecules are a result of the partial positive and negative charges on the molecule. These hydrogen bonds need a huge amount of energy to be broken. During vaporization, the water molecule must break all the hydrogen bonds before it is able to escape into the air as water vapor. As water needs a huge amount of energy to evaporate, this indicates that water has a high rate of vaporization.
• Unique density behavior:
Water is unique among common substances in that its density decreases when it freezes. When water turns to ice, it floats on water as it is lighter than water. At , the partial positive charge on a water molecule interact with the partial negative charge on the adjacent water molecule. The hydrogen bonds between the molecules form a crystal lattice structure. The molecules of ice are also farther apart than in water. This makes ice lighter than water.
, the partial positive charge on a water molecule interact with the partial negative charge on the adjacent water molecule. The hydrogen bonds between the molecules form a crystal lattice structure. The molecules of ice are also farther apart than in water. This makes ice lighter than water.
• High surface tension:
The hydrogen bonds present in water are responsible for the cohesive nature of the surface of water. These hydrogen bonds give water a high surface tension. The mutual attraction of water molecules allows water to form thin and continuous films.
• Good solvent for ions of salts:
When a substance like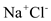 is dissolved in water, the partial positive charge of the water molecule attracts the negative ions while the partial negative charge attracts the positive ions. In this way, the water molecules pull the salt crystal apart which results in the total dissolving of the salt in the solvent.
is dissolved in water, the partial positive charge of the water molecule attracts the negative ions while the partial negative charge attracts the positive ions. In this way, the water molecules pull the salt crystal apart which results in the total dissolving of the salt in the solvent.
• High specific heat capacity:
The partial negative and the partial positive charge of the water molecule cause attraction between water molecules. It causes the formation of hydrogen bonds between water molecules. These hydrogen bonds need a high amount of heat to be broken. This high amount of heat is known as high specific heat capacity of water. As water has a high specific heat capacity, it is able to absorb heat, without increasing much in temperature.
• High heat of vaporization:
The hydrogen bonds formed between water molecules are a result of the partial positive and negative charges on the molecule. These hydrogen bonds need a huge amount of energy to be broken. During vaporization, the water molecule must break all the hydrogen bonds before it is able to escape into the air as water vapor. As water needs a huge amount of energy to evaporate, this indicates that water has a high rate of vaporization.
• Unique density behavior:
Water is unique among common substances in that its density decreases when it freezes. When water turns to ice, it floats on water as it is lighter than water. At
 , the partial positive charge on a water molecule interact with the partial negative charge on the adjacent water molecule. The hydrogen bonds between the molecules form a crystal lattice structure. The molecules of ice are also farther apart than in water. This makes ice lighter than water.
, the partial positive charge on a water molecule interact with the partial negative charge on the adjacent water molecule. The hydrogen bonds between the molecules form a crystal lattice structure. The molecules of ice are also farther apart than in water. This makes ice lighter than water. • High surface tension:
The hydrogen bonds present in water are responsible for the cohesive nature of the surface of water. These hydrogen bonds give water a high surface tension. The mutual attraction of water molecules allows water to form thin and continuous films.
• Good solvent for ions of salts:
When a substance like
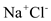 is dissolved in water, the partial positive charge of the water molecule attracts the negative ions while the partial negative charge attracts the positive ions. In this way, the water molecules pull the salt crystal apart which results in the total dissolving of the salt in the solvent.
is dissolved in water, the partial positive charge of the water molecule attracts the negative ions while the partial negative charge attracts the positive ions. In this way, the water molecules pull the salt crystal apart which results in the total dissolving of the salt in the solvent. 2
What was the composition of the earth's atmosphere at the time of the origin of life, and how did it differ from the atmosphere of today?
Composition of earth's atmosphere at the time of the origin of life could be a mixture of water, molecular hydrogen, methane and ammonia.
Chemical Composition of Today's atmosphere - Nitrogen (N 2 )- 78%, Oxygen (O2 )- 21%, Carbon Dioxide (CO2 ) - 0.03 %, plus other miscellaneous gases
Chemical Composition of Today's atmosphere - Nitrogen (N 2 )- 78%, Oxygen (O2 )- 21%, Carbon Dioxide (CO2 ) - 0.03 %, plus other miscellaneous gases
3
Regarding the experiments of Miller and Urey described in this chapter, explain what constituted the following in each case: observations, hypothesis, deduction, prediction, data, control. (The scientific method is described on pp. 11-14.)
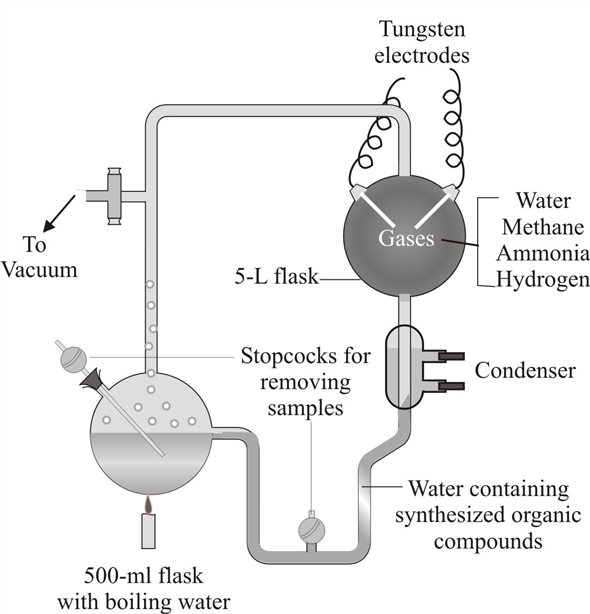 According to Miller and Urey experiments the following are constituted in each case:
According to Miller and Urey experiments the following are constituted in each case:• Observations - Miller and Urey observed that after one week of continuous experiment, 10 to 15% of the carbon within the system was now in the form of organic compounds. Two percent of the carbon had formed amino acids. Sugars, liquids, and some of the building blocks for nucleic acids were also formed.
• Hypothesis - Experiment hypothesized that conditions on primitive Earth favored chemical reactions that synthesized organic compounds from inorganic precursors.
• Deduction - From the experiment they conducted, Miller and Urey came to a conclusion that origin of life is a chemical evolution.
• Prediction - They predicted that primitive Earth consists of reduced atmosphere.
• Data - It represents the synthesis of organic compounds from inorganic precursors.
• Control - It is the same experimental apparatus used by Miller and Urey, but devoid of the energy source.
4
Explain the significance of the Miller-Urey experiments.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 15 في هذه المجموعة.
فتح الحزمة
k this deck
5
Name three different sources of energy that could have powered reactions on early earth to form organic compounds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 15 في هذه المجموعة.
فتح الحزمة
k this deck
6
By what mechanism might organic molecules have been concentrated in a prebiotic world so that further reactions could occur?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 15 في هذه المجموعة.
فتح الحزمة
k this deck
7
Name two simple carbohydrates, two storage carbohydrates, and a structural carbohydrate.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 15 في هذه المجموعة.
فتح الحزمة
k this deck
8
What characteristic differences in molecular structure distinguish lipids and carbohydrates?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 15 في هذه المجموعة.
فتح الحزمة
k this deck
9
Explain the difference between the primary, secondary, tertiary, and quaternary structures of a protein.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 15 في هذه المجموعة.
فتح الحزمة
k this deck
10
What are the important nucleic acids in a cell, and of what units are they constructed?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 15 في هذه المجموعة.
فتح الحزمة
k this deck
11
Distinguish among the following: primary heterotroph, autotroph, secondary heterotroph.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 15 في هذه المجموعة.
فتح الحزمة
k this deck
12
What is the source of oxygen in the present-day atmosphere, and what is its metabolic significance to most organisms living today?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 15 في هذه المجموعة.
فتح الحزمة
k this deck
13
Distinguish prokaryotes from eukaryotes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 15 في هذه المجموعة.
فتح الحزمة
k this deck
14
Describe Margulis's view on the origin of eukaryotes from prokaryotes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 15 في هذه المجموعة.
فتح الحزمة
k this deck
15
What was the "Cambrian explosion" and how might you explain it?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 15 في هذه المجموعة.
فتح الحزمة
k this deck








