Deck 7: How Cells Harvest Energy From Food
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/11
العب
ملء الشاشة (f)
Deck 7: How Cells Harvest Energy From Food
1
After glycolysis, the pyruvate molecules go to the
A) nucleus of the cell and provide energy.
B) membranes of the cell and are broken down in the presence of CO2 to make more ATP.
C) mitochondria of the cell and are broken down in the presence of O2 to make more ATP.
D) Golgi bodies and are packaged and stored until needed.
The enzyme pyruvate dehydrogenase acts to
A) oxidize pyruvate to acetyl-CoA.
B) decarboxylate pyruvate.
C) reduce NAD +.
D) All of the above.
A) nucleus of the cell and provide energy.
B) membranes of the cell and are broken down in the presence of CO2 to make more ATP.
C) mitochondria of the cell and are broken down in the presence of O2 to make more ATP.
D) Golgi bodies and are packaged and stored until needed.
The enzyme pyruvate dehydrogenase acts to
A) oxidize pyruvate to acetyl-CoA.
B) decarboxylate pyruvate.
C) reduce NAD +.
D) All of the above.
In this question, we discuss the link between glycolysis and the Krebs cycle.
Glycolysis is the initial processing of one molecule of glucose into two molecules of pyruvate, generating a small amount of ATP through substrate-level phosphorylation and a few reduced electron carriers for the respiratory chain. The pyruvate is a central metabolite - it can feed a number of different anabolic processes, but it is also the precursor molecule that feeds into the citric acid cycle.
The Citric Acid Cycle, also known as the Krebs cycle or the tricarboxylic acid cycle, first converts pyruvate into acetyl-CoA, and then uses this molecule to generate ATP and reduced electron carriers from the respiratory chain. This process takes place in the mitochondria.
The cycle is an eight-step process; for each molecule of acetyl-CoA that enters the cycle, four molecules of NADH (reduced electron carrier) are generated, one molecule of FADH2 (another reduced electron carrier) is generated, and one molecule of ATP is generated. These electron carriers will be used in the respiratory chain to generate further ATP.
a) Option C is correct - the mitochondria are the site of further processing of pyruvate.
Option A is incorrect - energy processes do not occur in the nucleus.
Option B is incorrect - this is true in bacteria, but not in eukaryotes.
Option D is incorrect - while the Golgi apparatus completes packaging of proteins, pyruvate is not in the category.
b) Option D is correct - all three options are correct.
Option A is incorrect - this is a true statement, but it is not the only true statement.
Option B is incorrect - this is a true statement, but it is not the only true statement.
Option C is incorrect - this is a true statement, but it is not the only true statement.
Glycolysis is the initial processing of one molecule of glucose into two molecules of pyruvate, generating a small amount of ATP through substrate-level phosphorylation and a few reduced electron carriers for the respiratory chain. The pyruvate is a central metabolite - it can feed a number of different anabolic processes, but it is also the precursor molecule that feeds into the citric acid cycle.
The Citric Acid Cycle, also known as the Krebs cycle or the tricarboxylic acid cycle, first converts pyruvate into acetyl-CoA, and then uses this molecule to generate ATP and reduced electron carriers from the respiratory chain. This process takes place in the mitochondria.
The cycle is an eight-step process; for each molecule of acetyl-CoA that enters the cycle, four molecules of NADH (reduced electron carrier) are generated, one molecule of FADH2 (another reduced electron carrier) is generated, and one molecule of ATP is generated. These electron carriers will be used in the respiratory chain to generate further ATP.
a) Option C is correct - the mitochondria are the site of further processing of pyruvate.
Option A is incorrect - energy processes do not occur in the nucleus.
Option B is incorrect - this is true in bacteria, but not in eukaryotes.
Option D is incorrect - while the Golgi apparatus completes packaging of proteins, pyruvate is not in the category.
b) Option D is correct - all three options are correct.
Option A is incorrect - this is a true statement, but it is not the only true statement.
Option B is incorrect - this is a true statement, but it is not the only true statement.
Option C is incorrect - this is a true statement, but it is not the only true statement.
2
The electrons generated from the Krebs cycle are transferred to ____________, which then carries them to _______________.
A) NADH, oxygen
B) NAD + , the electron transport chain
C) ATP, glycolysis
D) pyruvate, the electron transport chain
One of the initial substrates for the Krebs cycle is
A) oxaloacetate, a four-carbon sugar.
B) glyceraldehyde, a three-carbon sugar.
C) glucose, produced from the electron transport chain.
D) ATP, produced from the oxidation of pyruvate.
The electron carrier cytochrome c is one of many different cytochromes, but unlike the others, the sequence of cytochrome c is nearly identical in all species. Among humans, no genetic disorder affecting cytochrome c has ever been reported. Why do you suppose this is so?
Consider the structure of a mitochondrion. If you were to poke a hole in a mitochondrion, could it still perform oxidative respiration? Explain.
A) NADH, oxygen
B) NAD + , the electron transport chain
C) ATP, glycolysis
D) pyruvate, the electron transport chain
One of the initial substrates for the Krebs cycle is
A) oxaloacetate, a four-carbon sugar.
B) glyceraldehyde, a three-carbon sugar.
C) glucose, produced from the electron transport chain.
D) ATP, produced from the oxidation of pyruvate.
The electron carrier cytochrome c is one of many different cytochromes, but unlike the others, the sequence of cytochrome c is nearly identical in all species. Among humans, no genetic disorder affecting cytochrome c has ever been reported. Why do you suppose this is so?
Consider the structure of a mitochondrion. If you were to poke a hole in a mitochondrion, could it still perform oxidative respiration? Explain.
In this question, we discuss the link between the Krebs cycle and the electron transport chain.
The Citric Acid Cycle, also known as the Krebs cycle or the tricarboxylic acid cycle, first converts pyruvate into acetyl-CoA, and then uses this molecule to generate ATP and reduced electron carriers from the respiratory chain. This process takes place in the mitochondria.
The cycle is an eight-step process; for each molecule of acetyl-CoA that enters the cycle, four molecules of NADH (reduced electron carrier) are generated from NAD+, one molecule of FADH2 (another reduced electron carrier) is generated from FAD+, and one molecule of ATP is generated. These electron carriers will be used in the electron transport chain to generate further ATP.
The respiratory chain, also known as the electron transport chain or oxidative phosphorylation, takes the electron carriers generated in glycolysis and the citric acid cycle and uses their energy to create a proton gradient that can drive an enzyme called ATP synthase to generate large quantities of ATP. The chain itself does not actually generate ATP - just the end enzyme.
There a number of components in the chains of most aerobic organisms - NADH dehydrogenase, flavin mononucleotide (FMN), coenzyme Q, cytochrome b , cytochrome c 1 , cytochrome c , and cytochromes a and a 3 complexed together - that use their actions to pass electrons through chain to move five protons across the mitochondrial membrane or cell membrane against the proton gradient to generate a motive force for making ATP through ATP synthase.
a) Option B is correct - this is the correct combination.
Option A is incorrect - NADH has already received electrons and cannot receive more.
Option C is incorrect - ATP does not accept electrons, and glycolysis would be backwards in the chain.
Option D is incorrect - pyruvate does not accept electrons.
b) Option A is correct - this is the first step of the cycle, which accept acetyl-CoA.
Option B is incorrect - glyceraldehyde is a component of glycolysis and the Calvin cycle.
Option C is incorrect - the electron transport chain does not produce glucose.
Option D is incorrect - pyruvate is not part of the Krebs cycle.
c) Cytochrome c is so important to energy production that cells with malformation of this cytochrome are unable to generate sufficient ATP to survive. This cytochrome is also highly conserved for this reason - its function is vital no matter what cell types are involved.
d) A mitochondria functions based on the creation of a chemiosmotic gradient, which requires an integral membrane. If a hole is punched into the mitochondria, it cannot maintain this gradient and thus cannot generate ATP.
The Citric Acid Cycle, also known as the Krebs cycle or the tricarboxylic acid cycle, first converts pyruvate into acetyl-CoA, and then uses this molecule to generate ATP and reduced electron carriers from the respiratory chain. This process takes place in the mitochondria.
The cycle is an eight-step process; for each molecule of acetyl-CoA that enters the cycle, four molecules of NADH (reduced electron carrier) are generated from NAD+, one molecule of FADH2 (another reduced electron carrier) is generated from FAD+, and one molecule of ATP is generated. These electron carriers will be used in the electron transport chain to generate further ATP.
The respiratory chain, also known as the electron transport chain or oxidative phosphorylation, takes the electron carriers generated in glycolysis and the citric acid cycle and uses their energy to create a proton gradient that can drive an enzyme called ATP synthase to generate large quantities of ATP. The chain itself does not actually generate ATP - just the end enzyme.
There a number of components in the chains of most aerobic organisms - NADH dehydrogenase, flavin mononucleotide (FMN), coenzyme Q, cytochrome b , cytochrome c 1 , cytochrome c , and cytochromes a and a 3 complexed together - that use their actions to pass electrons through chain to move five protons across the mitochondrial membrane or cell membrane against the proton gradient to generate a motive force for making ATP through ATP synthase.
a) Option B is correct - this is the correct combination.
Option A is incorrect - NADH has already received electrons and cannot receive more.
Option C is incorrect - ATP does not accept electrons, and glycolysis would be backwards in the chain.
Option D is incorrect - pyruvate does not accept electrons.
b) Option A is correct - this is the first step of the cycle, which accept acetyl-CoA.
Option B is incorrect - glyceraldehyde is a component of glycolysis and the Calvin cycle.
Option C is incorrect - the electron transport chain does not produce glucose.
Option D is incorrect - pyruvate is not part of the Krebs cycle.
c) Cytochrome c is so important to energy production that cells with malformation of this cytochrome are unable to generate sufficient ATP to survive. This cytochrome is also highly conserved for this reason - its function is vital no matter what cell types are involved.
d) A mitochondria functions based on the creation of a chemiosmotic gradient, which requires an integral membrane. If a hole is punched into the mitochondria, it cannot maintain this gradient and thus cannot generate ATP.
3
The production of the vast majority of the ATP molecules within the cells of your body is powered by electrons harvested
A) during pyruvate oxidation.
B) during glycolysis.
C) during the Krebs cycle.
D) during the electron transport chain.
How much less ATP would be generated in the cells of a person who consumed a diet of pyruvate instead of glucose (use one molecule of each for your calculation)?
By the end of oxidative metabolism, all six of the carbon atoms of a glucose molecule are gone. Where did they go?
A) carbon dioxide
B) acetyl CoA
C) pyruvate
D) ATP
A) during pyruvate oxidation.
B) during glycolysis.
C) during the Krebs cycle.
D) during the electron transport chain.
How much less ATP would be generated in the cells of a person who consumed a diet of pyruvate instead of glucose (use one molecule of each for your calculation)?
By the end of oxidative metabolism, all six of the carbon atoms of a glucose molecule are gone. Where did they go?
A) carbon dioxide
B) acetyl CoA
C) pyruvate
D) ATP
In this question, we discuss the overall effects of the three parts of cellular respiration.
Three major processes make up catabolism in most organisms:
• Glycolysis - also known as the Embden-Meyerhof-Parnas pathway, this is the initial processing of glucose into pyruvate, generating a small amount of ATP and a few reduced electron carriers for the respiratory chain; this process takes place in the cytoplasm of microbes.
• Citric Acid Cycle - also known as the Krebs cycle or the tricarboxylic acid cycle, this pathway first converts pyruvate into acetyl-CoA, and then uses this molecule to generate ATP and reduced electron carriers from the respiratory chain; the cycle takes place in the mitochondria in eukaryotic microbes and in the cytoplasm of bacteria.
• Respiratory Chain - also known as the electron transport chain or oxidative phosphorylation, this process takes the electron carriers generated in glycolysis and the citric acid cycle and uses their energy to create a proton gradient that can drive an enzyme called ATP synthase to generate large quantities of ATP; this process takes place in the inner cristae membranes of mitochondria in eukaryotic microbes, and in the cell membrane of bacteria.
To understand the total amount of energy derived from one molecule of glucose, we will begin at glycolysis and analyze the energy used up and resulting in each step.
Glycolysis takes in one molecule of glucose, and in turn generates four molecules of ATP, two molecules of NADH, and two molecules of pyruvate. Two ATP are also consumed during the first steps of glycolysis. Thus, the overall energy produced in this step is 2 ATP molecules.
For each run of the citric acid cycle, we consume one pyruvate and generate 1 ATP molecule, 4 NADH molecules, and 1 FADH2 molecule. This happens twice (as we have two pyruvates), so the total for one glucose molecule through the citric acid cycle is 2 ATP.
The respiratory chain thus receives ten NADH molecules and 2 FADH2 molecules. Each NADH molecule can generate 3 ATP, while each FADH2 molecule can generate 2 ATP. Thus, we receive a total of 34 ATP from the respiratory chain.
The grand total is 36-38 ATP from one molecule of glucose.
a) Option D is correct - most ATP is made in this step of cellular respiration.
Option A is incorrect - this generates only a few ATP.
Option B is incorrect - only two ATP are generated in this fashion.
Option C is incorrect - only one ATP is generated in this process.
b) Normal glucose processes generate 36 ATP; one molecule of glucose leads to two molecules of pyruvate. If we do not have glucose available, we would skip glycolysis, and thus only generate 15 ATP.
c) Option A is correct - the breakdown processes of glycolysis and the Krebs cycle generates six molecules of carbon dioxide.
Option B is incorrect - this is an intermediate leading into the Krebs cycle.
Option C is incorrect - pyruvate is a byproduct of glycolysis
Option D is incorrect - ATP is not built directly from the carbon of glucose.
Three major processes make up catabolism in most organisms:
• Glycolysis - also known as the Embden-Meyerhof-Parnas pathway, this is the initial processing of glucose into pyruvate, generating a small amount of ATP and a few reduced electron carriers for the respiratory chain; this process takes place in the cytoplasm of microbes.
• Citric Acid Cycle - also known as the Krebs cycle or the tricarboxylic acid cycle, this pathway first converts pyruvate into acetyl-CoA, and then uses this molecule to generate ATP and reduced electron carriers from the respiratory chain; the cycle takes place in the mitochondria in eukaryotic microbes and in the cytoplasm of bacteria.
• Respiratory Chain - also known as the electron transport chain or oxidative phosphorylation, this process takes the electron carriers generated in glycolysis and the citric acid cycle and uses their energy to create a proton gradient that can drive an enzyme called ATP synthase to generate large quantities of ATP; this process takes place in the inner cristae membranes of mitochondria in eukaryotic microbes, and in the cell membrane of bacteria.
To understand the total amount of energy derived from one molecule of glucose, we will begin at glycolysis and analyze the energy used up and resulting in each step.
Glycolysis takes in one molecule of glucose, and in turn generates four molecules of ATP, two molecules of NADH, and two molecules of pyruvate. Two ATP are also consumed during the first steps of glycolysis. Thus, the overall energy produced in this step is 2 ATP molecules.
For each run of the citric acid cycle, we consume one pyruvate and generate 1 ATP molecule, 4 NADH molecules, and 1 FADH2 molecule. This happens twice (as we have two pyruvates), so the total for one glucose molecule through the citric acid cycle is 2 ATP.
The respiratory chain thus receives ten NADH molecules and 2 FADH2 molecules. Each NADH molecule can generate 3 ATP, while each FADH2 molecule can generate 2 ATP. Thus, we receive a total of 34 ATP from the respiratory chain.
The grand total is 36-38 ATP from one molecule of glucose.
a) Option D is correct - most ATP is made in this step of cellular respiration.
Option A is incorrect - this generates only a few ATP.
Option B is incorrect - only two ATP are generated in this fashion.
Option C is incorrect - only one ATP is generated in this process.
b) Normal glucose processes generate 36 ATP; one molecule of glucose leads to two molecules of pyruvate. If we do not have glucose available, we would skip glycolysis, and thus only generate 15 ATP.
c) Option A is correct - the breakdown processes of glycolysis and the Krebs cycle generates six molecules of carbon dioxide.
Option B is incorrect - this is an intermediate leading into the Krebs cycle.
Option C is incorrect - pyruvate is a byproduct of glycolysis
Option D is incorrect - ATP is not built directly from the carbon of glucose.
4
Soft drinks are artificially carbonated, which is what causes them to fizz. Beer and sparkling wines are naturally carbonated. How does this natural carbonation occur?
The final electron acceptor in lactic acid fermentation is
A) pyruvate.
B) NAD +.
C) lactic acid.
D) O2.
The final electron acceptor in ethanol fermentation is
A) pyruvate.
B) NAD +.
C) ethanol.
D) acetaldehyde.
NAD + is regenerated during
A) glycolysis.
B) fermentation.
C) the Krebs cycle.
D) the formation of acetyl-CoA.
The final electron acceptor in lactic acid fermentation is
A) pyruvate.
B) NAD +.
C) lactic acid.
D) O2.
The final electron acceptor in ethanol fermentation is
A) pyruvate.
B) NAD +.
C) ethanol.
D) acetaldehyde.
NAD + is regenerated during
A) glycolysis.
B) fermentation.
C) the Krebs cycle.
D) the formation of acetyl-CoA.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 11 في هذه المجموعة.
فتح الحزمة
k this deck
5
Cells can extract energy from foodstuffs other than glucose because
A) proteins, fatty acids, and nucleic acids get converted to glucose and then enter oxidative respiration.
B) each type of macromolecule has its own oxidative respiration pathway.
C) each type of macromolecule is broken down into its subunits, which enter the oxidative respiration pathway.
D) they can all enter the glycolytic pathway.
Your friend wants to go on a low-carbohydrate diet so that he can lose some of the "baby fat" he's still carrying. He asks your advice; what do you tell him?
Which of the following food molecules would generate the most ATP molecules, assuming that glycolysis, the Krebs cycle, and the electron transport chain were all functioning and that the foods were consumed in equal amounts: carbohydrates, proteins, or fats? Explain your answer.
A) proteins, fatty acids, and nucleic acids get converted to glucose and then enter oxidative respiration.
B) each type of macromolecule has its own oxidative respiration pathway.
C) each type of macromolecule is broken down into its subunits, which enter the oxidative respiration pathway.
D) they can all enter the glycolytic pathway.
Your friend wants to go on a low-carbohydrate diet so that he can lose some of the "baby fat" he's still carrying. He asks your advice; what do you tell him?
Which of the following food molecules would generate the most ATP molecules, assuming that glycolysis, the Krebs cycle, and the electron transport chain were all functioning and that the foods were consumed in equal amounts: carbohydrates, proteins, or fats? Explain your answer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 11 في هذه المجموعة.
فتح الحزمة
k this deck
6
How Do Swimming Fish Avoid Low Blood pH?
Animals that live in oxygen-poor environments, like worms living in the oxygen-free mud at the bottom of lakes, are not able to obtain the energy required for muscle movement from the Krebs cycle. Their cells lack the oxygen needed to accept the electrons stripped from food molecules. Instead, these animals rely on glycolysis to obtain ATP, donating the electron to pyruvate, forming lactic acid. While much less efficient than the Krebs cycle, glycolysis does not require oxygen. Even when oxygen is plentiful, the muscles of an active animal may use up oxygen more quickly than it can be supplied by the bloodstream and so be forced to temporarily rely on glycolysis to generate the ATP for continued contraction.
This presents a particular problem for fish. Fish blood is much lower in carbon dioxide than yours is, and as a consequence, the amount of sodium bicarbonate acting as a buffer in fish blood is also quite low. Now imagine you are a trout, and need to suddenly swim very fast to catch a mayfly for dinner. The vigorous swimming will cause your muscles to release large amounts of lactic acid into your poorly-buffered blood; this could severely disturb the blood's acid-base balance and so impede contraction of your swimming muscles before the prey is captured.
The graph to the right presents the results of an experiment designed to explore how a trout solves this dilemma. In the experiment, the trout was made to swim vigorously for 15 minutes in a laboratory tank, and then allowed a day's recovery. The lactic acid concentration in its blood was monitored periodically during swimming and recovery phases.
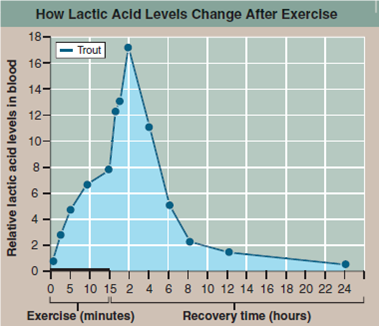
Applying Concepts
a. Variable. What is the dependent variable?
b. Recording Data. Lactic acid levels are presented for both swimming and recovery periods. In what time units are the swimming data presented? The recovery data?
Animals that live in oxygen-poor environments, like worms living in the oxygen-free mud at the bottom of lakes, are not able to obtain the energy required for muscle movement from the Krebs cycle. Their cells lack the oxygen needed to accept the electrons stripped from food molecules. Instead, these animals rely on glycolysis to obtain ATP, donating the electron to pyruvate, forming lactic acid. While much less efficient than the Krebs cycle, glycolysis does not require oxygen. Even when oxygen is plentiful, the muscles of an active animal may use up oxygen more quickly than it can be supplied by the bloodstream and so be forced to temporarily rely on glycolysis to generate the ATP for continued contraction.
This presents a particular problem for fish. Fish blood is much lower in carbon dioxide than yours is, and as a consequence, the amount of sodium bicarbonate acting as a buffer in fish blood is also quite low. Now imagine you are a trout, and need to suddenly swim very fast to catch a mayfly for dinner. The vigorous swimming will cause your muscles to release large amounts of lactic acid into your poorly-buffered blood; this could severely disturb the blood's acid-base balance and so impede contraction of your swimming muscles before the prey is captured.
The graph to the right presents the results of an experiment designed to explore how a trout solves this dilemma. In the experiment, the trout was made to swim vigorously for 15 minutes in a laboratory tank, and then allowed a day's recovery. The lactic acid concentration in its blood was monitored periodically during swimming and recovery phases.


Applying Concepts
a. Variable. What is the dependent variable?
b. Recording Data. Lactic acid levels are presented for both swimming and recovery periods. In what time units are the swimming data presented? The recovery data?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 11 في هذه المجموعة.
فتح الحزمة
k this deck
7
How Do Swimming Fish Avoid Low Blood pH?
Animals that live in oxygen-poor environments, like worms living in the oxygen-free mud at the bottom of lakes, are not able to obtain the energy required for muscle movement from the Krebs cycle. Their cells lack the oxygen needed to accept the electrons stripped from food molecules. Instead, these animals rely on glycolysis to obtain ATP, donating the electron to pyruvate, forming lactic acid. While much less efficient than the Krebs cycle, glycolysis does not require oxygen. Even when oxygen is plentiful, the muscles of an active animal may use up oxygen more quickly than it can be supplied by the bloodstream and so be forced to temporarily rely on glycolysis to generate the ATP for continued contraction.
This presents a particular problem for fish. Fish blood is much lower in carbon dioxide than yours is, and as a consequence, the amount of sodium bicarbonate acting as a buffer in fish blood is also quite low. Now imagine you are a trout, and need to suddenly swim very fast to catch a mayfly for dinner. The vigorous swimming will cause your muscles to release large amounts of lactic acid into your poorly-buffered blood; this could severely disturb the blood's acid-base balance and so impede contraction of your swimming muscles before the prey is captured.
The graph to the right presents the results of an experiment designed to explore how a trout solves this dilemma. In the experiment, the trout was made to swim vigorously for 15 minutes in a laboratory tank, and then allowed a day's recovery. The lactic acid concentration in its blood was monitored periodically during swimming and recovery phases.
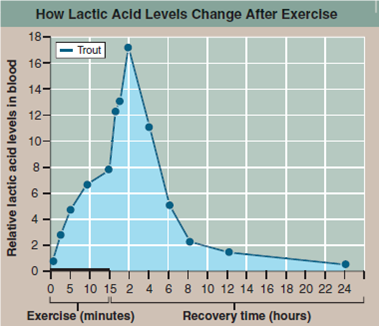
Interpreting Data
a. What is the effect of exercise on the level of lactic acid in the trout's blood?
b. Does the level of lactic acid change after exercise stops? How?
Animals that live in oxygen-poor environments, like worms living in the oxygen-free mud at the bottom of lakes, are not able to obtain the energy required for muscle movement from the Krebs cycle. Their cells lack the oxygen needed to accept the electrons stripped from food molecules. Instead, these animals rely on glycolysis to obtain ATP, donating the electron to pyruvate, forming lactic acid. While much less efficient than the Krebs cycle, glycolysis does not require oxygen. Even when oxygen is plentiful, the muscles of an active animal may use up oxygen more quickly than it can be supplied by the bloodstream and so be forced to temporarily rely on glycolysis to generate the ATP for continued contraction.
This presents a particular problem for fish. Fish blood is much lower in carbon dioxide than yours is, and as a consequence, the amount of sodium bicarbonate acting as a buffer in fish blood is also quite low. Now imagine you are a trout, and need to suddenly swim very fast to catch a mayfly for dinner. The vigorous swimming will cause your muscles to release large amounts of lactic acid into your poorly-buffered blood; this could severely disturb the blood's acid-base balance and so impede contraction of your swimming muscles before the prey is captured.
The graph to the right presents the results of an experiment designed to explore how a trout solves this dilemma. In the experiment, the trout was made to swim vigorously for 15 minutes in a laboratory tank, and then allowed a day's recovery. The lactic acid concentration in its blood was monitored periodically during swimming and recovery phases.


Interpreting Data
a. What is the effect of exercise on the level of lactic acid in the trout's blood?
b. Does the level of lactic acid change after exercise stops? How?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 11 في هذه المجموعة.
فتح الحزمة
k this deck
8
How Do Swimming Fish Avoid Low Blood pH?
Animals that live in oxygen-poor environments, like worms living in the oxygen-free mud at the bottom of lakes, are not able to obtain the energy required for muscle movement from the Krebs cycle. Their cells lack the oxygen needed to accept the electrons stripped from food molecules. Instead, these animals rely on glycolysis to obtain ATP, donating the electron to pyruvate, forming lactic acid. While much less efficient than the Krebs cycle, glycolysis does not require oxygen. Even when oxygen is plentiful, the muscles of an active animal may use up oxygen more quickly than it can be supplied by the bloodstream and so be forced to temporarily rely on glycolysis to generate the ATP for continued contraction.
This presents a particular problem for fish. Fish blood is much lower in carbon dioxide than yours is, and as a consequence, the amount of sodium bicarbonate acting as a buffer in fish blood is also quite low. Now imagine you are a trout and need to suddenly swim very fast to catch a mayfly for dinner. The vigorous swimming will cause your muscles to release large amounts of lactic acid into your poorly buffered blood; this could severely disturb the blood's acid-base balance and so impede contraction of your swimming muscles before the prey is captured.
The graph to the right presents the results of an experiment designed to explore how a trout solves this dilemma. In the experiment, the trout was made to swim vigorously for 15 minutes in a laboratory tank and then allowed a day's recovery. The lactic acid concentration in its blood was monitored periodically during swimming and recovery phases.![How Do Swimming Fish Avoid Low Blood pH? Animals that live in oxygen-poor environments, like worms living in the oxygen-free mud at the bottom of lakes, are not able to obtain the energy required for muscle movement from the Krebs cycle. Their cells lack the oxygen needed to accept the electrons stripped from food molecules. Instead, these animals rely on glycolysis to obtain ATP, donating the electron to pyruvate, forming lactic acid. While much less efficient than the Krebs cycle, glycolysis does not require oxygen. Even when oxygen is plentiful, the muscles of an active animal may use up oxygen more quickly than it can be supplied by the bloodstream and so be forced to temporarily rely on glycolysis to generate the ATP for continued contraction. This presents a particular problem for fish. Fish blood is much lower in carbon dioxide than yours is, and as a consequence, the amount of sodium bicarbonate acting as a buffer in fish blood is also quite low. Now imagine you are a trout and need to suddenly swim very fast to catch a mayfly for dinner. The vigorous swimming will cause your muscles to release large amounts of lactic acid into your poorly buffered blood; this could severely disturb the blood's acid-base balance and so impede contraction of your swimming muscles before the prey is captured. The graph to the right presents the results of an experiment designed to explore how a trout solves this dilemma. In the experiment, the trout was made to swim vigorously for 15 minutes in a laboratory tank and then allowed a day's recovery. The lactic acid concentration in its blood was monitored periodically during swimming and recovery phases. Making Inferences About how much of the total lactic acid created by vigorous swimming is released after this exercise stops? [Hint: notice the x axis scale changes from minutes to hours, so replot all points to minutes and compare areas under curve.]](https://d2lvgg3v3hfg70.cloudfront.net/SM1396/11eb5dbb_bb59_20bc_9b68_e3a360ac1bcf_SM1396_00.jpg)
![How Do Swimming Fish Avoid Low Blood pH? Animals that live in oxygen-poor environments, like worms living in the oxygen-free mud at the bottom of lakes, are not able to obtain the energy required for muscle movement from the Krebs cycle. Their cells lack the oxygen needed to accept the electrons stripped from food molecules. Instead, these animals rely on glycolysis to obtain ATP, donating the electron to pyruvate, forming lactic acid. While much less efficient than the Krebs cycle, glycolysis does not require oxygen. Even when oxygen is plentiful, the muscles of an active animal may use up oxygen more quickly than it can be supplied by the bloodstream and so be forced to temporarily rely on glycolysis to generate the ATP for continued contraction. This presents a particular problem for fish. Fish blood is much lower in carbon dioxide than yours is, and as a consequence, the amount of sodium bicarbonate acting as a buffer in fish blood is also quite low. Now imagine you are a trout and need to suddenly swim very fast to catch a mayfly for dinner. The vigorous swimming will cause your muscles to release large amounts of lactic acid into your poorly buffered blood; this could severely disturb the blood's acid-base balance and so impede contraction of your swimming muscles before the prey is captured. The graph to the right presents the results of an experiment designed to explore how a trout solves this dilemma. In the experiment, the trout was made to swim vigorously for 15 minutes in a laboratory tank and then allowed a day's recovery. The lactic acid concentration in its blood was monitored periodically during swimming and recovery phases. Making Inferences About how much of the total lactic acid created by vigorous swimming is released after this exercise stops? [Hint: notice the x axis scale changes from minutes to hours, so replot all points to minutes and compare areas under curve.]](https://d2lvgg3v3hfg70.cloudfront.net/SM1396/11eb5dbb_bb59_20bd_9b68_a1a40834d9ea_SM1396_00.jpg)
Making Inferences About how much of the total lactic acid created by vigorous swimming is released after this exercise stops? [Hint: notice the x axis scale changes from minutes to hours, so replot all points to minutes and compare areas under curve.]
Animals that live in oxygen-poor environments, like worms living in the oxygen-free mud at the bottom of lakes, are not able to obtain the energy required for muscle movement from the Krebs cycle. Their cells lack the oxygen needed to accept the electrons stripped from food molecules. Instead, these animals rely on glycolysis to obtain ATP, donating the electron to pyruvate, forming lactic acid. While much less efficient than the Krebs cycle, glycolysis does not require oxygen. Even when oxygen is plentiful, the muscles of an active animal may use up oxygen more quickly than it can be supplied by the bloodstream and so be forced to temporarily rely on glycolysis to generate the ATP for continued contraction.
This presents a particular problem for fish. Fish blood is much lower in carbon dioxide than yours is, and as a consequence, the amount of sodium bicarbonate acting as a buffer in fish blood is also quite low. Now imagine you are a trout and need to suddenly swim very fast to catch a mayfly for dinner. The vigorous swimming will cause your muscles to release large amounts of lactic acid into your poorly buffered blood; this could severely disturb the blood's acid-base balance and so impede contraction of your swimming muscles before the prey is captured.
The graph to the right presents the results of an experiment designed to explore how a trout solves this dilemma. In the experiment, the trout was made to swim vigorously for 15 minutes in a laboratory tank and then allowed a day's recovery. The lactic acid concentration in its blood was monitored periodically during swimming and recovery phases.
![How Do Swimming Fish Avoid Low Blood pH? Animals that live in oxygen-poor environments, like worms living in the oxygen-free mud at the bottom of lakes, are not able to obtain the energy required for muscle movement from the Krebs cycle. Their cells lack the oxygen needed to accept the electrons stripped from food molecules. Instead, these animals rely on glycolysis to obtain ATP, donating the electron to pyruvate, forming lactic acid. While much less efficient than the Krebs cycle, glycolysis does not require oxygen. Even when oxygen is plentiful, the muscles of an active animal may use up oxygen more quickly than it can be supplied by the bloodstream and so be forced to temporarily rely on glycolysis to generate the ATP for continued contraction. This presents a particular problem for fish. Fish blood is much lower in carbon dioxide than yours is, and as a consequence, the amount of sodium bicarbonate acting as a buffer in fish blood is also quite low. Now imagine you are a trout and need to suddenly swim very fast to catch a mayfly for dinner. The vigorous swimming will cause your muscles to release large amounts of lactic acid into your poorly buffered blood; this could severely disturb the blood's acid-base balance and so impede contraction of your swimming muscles before the prey is captured. The graph to the right presents the results of an experiment designed to explore how a trout solves this dilemma. In the experiment, the trout was made to swim vigorously for 15 minutes in a laboratory tank and then allowed a day's recovery. The lactic acid concentration in its blood was monitored periodically during swimming and recovery phases. Making Inferences About how much of the total lactic acid created by vigorous swimming is released after this exercise stops? [Hint: notice the x axis scale changes from minutes to hours, so replot all points to minutes and compare areas under curve.]](https://d2lvgg3v3hfg70.cloudfront.net/SM1396/11eb5dbb_bb59_20bc_9b68_e3a360ac1bcf_SM1396_00.jpg)
![How Do Swimming Fish Avoid Low Blood pH? Animals that live in oxygen-poor environments, like worms living in the oxygen-free mud at the bottom of lakes, are not able to obtain the energy required for muscle movement from the Krebs cycle. Their cells lack the oxygen needed to accept the electrons stripped from food molecules. Instead, these animals rely on glycolysis to obtain ATP, donating the electron to pyruvate, forming lactic acid. While much less efficient than the Krebs cycle, glycolysis does not require oxygen. Even when oxygen is plentiful, the muscles of an active animal may use up oxygen more quickly than it can be supplied by the bloodstream and so be forced to temporarily rely on glycolysis to generate the ATP for continued contraction. This presents a particular problem for fish. Fish blood is much lower in carbon dioxide than yours is, and as a consequence, the amount of sodium bicarbonate acting as a buffer in fish blood is also quite low. Now imagine you are a trout and need to suddenly swim very fast to catch a mayfly for dinner. The vigorous swimming will cause your muscles to release large amounts of lactic acid into your poorly buffered blood; this could severely disturb the blood's acid-base balance and so impede contraction of your swimming muscles before the prey is captured. The graph to the right presents the results of an experiment designed to explore how a trout solves this dilemma. In the experiment, the trout was made to swim vigorously for 15 minutes in a laboratory tank and then allowed a day's recovery. The lactic acid concentration in its blood was monitored periodically during swimming and recovery phases. Making Inferences About how much of the total lactic acid created by vigorous swimming is released after this exercise stops? [Hint: notice the x axis scale changes from minutes to hours, so replot all points to minutes and compare areas under curve.]](https://d2lvgg3v3hfg70.cloudfront.net/SM1396/11eb5dbb_bb59_20bd_9b68_a1a40834d9ea_SM1396_00.jpg)
Making Inferences About how much of the total lactic acid created by vigorous swimming is released after this exercise stops? [Hint: notice the x axis scale changes from minutes to hours, so replot all points to minutes and compare areas under curve.]

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 11 في هذه المجموعة.
فتح الحزمة
k this deck
9
How Do Swimming Fish Avoid Low Blood pH?
Animals that live in oxygen-poor environments, like worms living in the oxygen-free mud at the bottom of lakes, are not able to obtain the energy required for muscle movement from the Krebs cycle. Their cells lack the oxygen needed to accept the electrons stripped from food molecules. Instead, these animals rely on glycolysis to obtain ATP, donating the electron to pyruvate, forming lactic acid. While much less efficient than the Krebs cycle, glycolysis does not require oxygen. Even when oxygen is plentiful, the muscles of an active animal may use up oxygen more quickly than it can be supplied by the bloodstream and so be forced to temporarily rely on glycolysis to generate the ATP for continued contraction.
This presents a particular problem for fish. Fish blood is much lower in carbon dioxide than yours is, and as a consequence, the amount of sodium bicarbonate acting as a buffer in fish blood is also quite low. Now imagine you are a trout and need to suddenly swim very fast to catch a mayfly for dinner. The vigorous swimming will cause your muscles to release large amounts of lactic acid into your poorly buffered blood; this could severely disturb the blood's acid-base balance and so impede contraction of your swimming muscles before the prey is captured.
The graph to the right presents the results of an experiment designed to explore how a trout solves this dilemma. In the experiment, the trout was made to swim vigorously for 15 minutes in a laboratory tank and then allowed a day's recovery. The lactic acid concentration in its blood was monitored periodically during swimming and recovery phases.
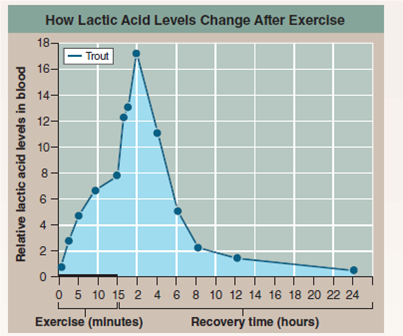
Drawing Conclusions Is this result consistent with the hypothesis that fish maintain blood pH levels by delaying the release of lactic acid from muscles? Why might this be beneficial to the fish?
Animals that live in oxygen-poor environments, like worms living in the oxygen-free mud at the bottom of lakes, are not able to obtain the energy required for muscle movement from the Krebs cycle. Their cells lack the oxygen needed to accept the electrons stripped from food molecules. Instead, these animals rely on glycolysis to obtain ATP, donating the electron to pyruvate, forming lactic acid. While much less efficient than the Krebs cycle, glycolysis does not require oxygen. Even when oxygen is plentiful, the muscles of an active animal may use up oxygen more quickly than it can be supplied by the bloodstream and so be forced to temporarily rely on glycolysis to generate the ATP for continued contraction.
This presents a particular problem for fish. Fish blood is much lower in carbon dioxide than yours is, and as a consequence, the amount of sodium bicarbonate acting as a buffer in fish blood is also quite low. Now imagine you are a trout and need to suddenly swim very fast to catch a mayfly for dinner. The vigorous swimming will cause your muscles to release large amounts of lactic acid into your poorly buffered blood; this could severely disturb the blood's acid-base balance and so impede contraction of your swimming muscles before the prey is captured.
The graph to the right presents the results of an experiment designed to explore how a trout solves this dilemma. In the experiment, the trout was made to swim vigorously for 15 minutes in a laboratory tank and then allowed a day's recovery. The lactic acid concentration in its blood was monitored periodically during swimming and recovery phases.


Drawing Conclusions Is this result consistent with the hypothesis that fish maintain blood pH levels by delaying the release of lactic acid from muscles? Why might this be beneficial to the fish?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 11 في هذه المجموعة.
فتح الحزمة
k this deck
10
The energy for life is obtained by cellular respiration. In animals, this involves
A) breaking down organic molecules that were consumed.
B) capturing photons from plants.
C) obtaining ATP from plants.
D) breaking down CO2 that was produced by plants.
A) breaking down organic molecules that were consumed.
B) capturing photons from plants.
C) obtaining ATP from plants.
D) breaking down CO2 that was produced by plants.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 11 في هذه المجموعة.
فتح الحزمة
k this deck
11
During glycolysis, ATP forms by
A) the breakdown of pyruvate.
B) chemiosmosis.
C) substrate-level phosphorylation.
D) NAD +.
Which of the following processes can occur in the absence of oxygen?
A) the Krebs cycle
B) glycolysis
C) chemiosmosis
D) All of the above.
Every living creature on this planet is capable of carrying out the rather inefficient biochemical process of glycolysis, which
A) makes glucose, using the energy from ATP.
B) makes ATP by splitting glucose and capturing the energy.
C) phosphorylates ATP to make ADP.
D) makes glucose, using oxygen and carbon dioxide and water.
A) the breakdown of pyruvate.
B) chemiosmosis.
C) substrate-level phosphorylation.
D) NAD +.
Which of the following processes can occur in the absence of oxygen?
A) the Krebs cycle
B) glycolysis
C) chemiosmosis
D) All of the above.
Every living creature on this planet is capable of carrying out the rather inefficient biochemical process of glycolysis, which
A) makes glucose, using the energy from ATP.
B) makes ATP by splitting glucose and capturing the energy.
C) phosphorylates ATP to make ADP.
D) makes glucose, using oxygen and carbon dioxide and water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 11 في هذه المجموعة.
فتح الحزمة
k this deck








