Deck 8: Ionic Versus Covalent Bonding
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/88
العب
ملء الشاشة (f)
Deck 8: Ionic Versus Covalent Bonding
1
The _____ is a balance between the repulsive interactions between electrons on adjacent ions and the attractive interactions between ions with opposite charges.
total energy of the system
2
Each chemical bond is characterized by a particular optimal internuclear distance called the _____.
bond distance
3
Explain the three features of chemical bonding.
All models of chemical bonding have three features in common:
Atoms interact with one another to form aggregates such as molecules, compounds, and crystals because doing so lowers the total energy of the system; that is, the aggregates are more stable than the isolated atoms.
Energy is required to dissociate bonded atoms or ions into isolated atoms or ions. For ionic solids, in which the ions form a three-dimensional array called a lattice, this energy is called the lattice energy (U), the enthalpy change that occurs when a solid ionic compound is transformed into gaseous ions. For covalent compounds, this energy is called the bond energy, which is the enthalpy change that occurs when a given bond in a gaseous molecule is broken.
Each chemical bond is characterized by a particular optimal internuclear distance called the bond distance (r0).
Atoms interact with one another to form aggregates such as molecules, compounds, and crystals because doing so lowers the total energy of the system; that is, the aggregates are more stable than the isolated atoms.
Energy is required to dissociate bonded atoms or ions into isolated atoms or ions. For ionic solids, in which the ions form a three-dimensional array called a lattice, this energy is called the lattice energy (U), the enthalpy change that occurs when a solid ionic compound is transformed into gaseous ions. For covalent compounds, this energy is called the bond energy, which is the enthalpy change that occurs when a given bond in a gaseous molecule is broken.
Each chemical bond is characterized by a particular optimal internuclear distance called the bond distance (r0).
4
Energy of the electrostatic attraction (E), a measure of the force's strength, is inversely proportional to the _____between the charged particles, where each ion's charge is represented by the symbol Q.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
5
Covalent bonding signifies that positively and negatively charged ions are held together by electrostatic forces.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which of the following best describes lattice energy?
A) It is the enthalpy change that occurs when a solid ionic compound is transformed into gaseous ions.
B) It is the enthalpy change that occurs in covalent compounds when a given bond in a gaseous molecule is broken.
C) It is the enthalpy change that occurs when a specified amount of solute is dissolved in a given quantity of solvent.
D) It is the enthalpy change that occurs when 1 mol of a substance is melted.
E) It is the enthalpy change that occurs during a combustion reaction.
A) It is the enthalpy change that occurs when a solid ionic compound is transformed into gaseous ions.
B) It is the enthalpy change that occurs in covalent compounds when a given bond in a gaseous molecule is broken.
C) It is the enthalpy change that occurs when a specified amount of solute is dissolved in a given quantity of solvent.
D) It is the enthalpy change that occurs when 1 mol of a substance is melted.
E) It is the enthalpy change that occurs during a combustion reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
7
Ions cannot be infinitely close together.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which of the following best describes covalent bonding?
A) It is a type of chemical bonding exhibited in compounds that contain positively and negatively charged ions.
B) It is a type of chemical bonding exhibited in compounds that dissolve in aqueous solutions and conduct electricity.
C) It is a type of chemical bonding exhibited in nonvolatile compounds.
D) It is a type of chemical bonding in which electrons are shared between atoms in a molecule or a polyatomic ion.
E) It is a type of chemical bonding in which the charged particles are held together by the electromagnetic forces.
A) It is a type of chemical bonding exhibited in compounds that contain positively and negatively charged ions.
B) It is a type of chemical bonding exhibited in compounds that dissolve in aqueous solutions and conduct electricity.
C) It is a type of chemical bonding exhibited in nonvolatile compounds.
D) It is a type of chemical bonding in which electrons are shared between atoms in a molecule or a polyatomic ion.
E) It is a type of chemical bonding in which the charged particles are held together by the electromagnetic forces.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
9
Explain the significance of the energy of the electrostatic attraction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
10
The enthalpy change that occurs when a given bond in a gaseous molecule is broken is known as bond _____.
A) strength
B) distance
C) order
D) energy
E) length
A) strength
B) distance
C) order
D) energy
E) length

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
11
_____ is a type of bonding in which positively and negatively charged ions are held together by electrostatic forces.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
12
The energy of the electrostatic attraction (E), a measure of the force's strength, is inversely proportional to _____.
A) the internuclear distance between the charged particles
B) the sum of the charges on the ions
C) the product of the charges on the ions
D) Avogadro's number
E) the difference of the charges on the ions
A) the internuclear distance between the charged particles
B) the sum of the charges on the ions
C) the product of the charges on the ions
D) Avogadro's number
E) the difference of the charges on the ions

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
13
What is the amount of energy released when 1 mol of a gaseous compound with charges of +1.00 on the cation and -1.00 on the anion is formed? Assume the internuclear distance in the gas phase as 185 pm.
A) 845 kJ/mol
B) 693 kJ/mol
C) 548 kJ/mol
D) 752 kJ/mol
E) 476 kJ/mol
A) 845 kJ/mol
B) 693 kJ/mol
C) 548 kJ/mol
D) 752 kJ/mol
E) 476 kJ/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
14
The energy of the electrostatic attraction between two is inversely proportional to their respective charges.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
15
What is the amount of energy released when 1 mol of a gaseous compound with charges of +3.000 on the cation and -1.000 on the anion is formed? Assume the internuclear distance in the gas phase as 215.0 pm.
A) 1574 kJ/mol
B) 1941 kJ/mol
C) 1894 kJ/mol
D) 1688 kJ/mol
E) 1736 kJ/mol
A) 1574 kJ/mol
B) 1941 kJ/mol
C) 1894 kJ/mol
D) 1688 kJ/mol
E) 1736 kJ/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
16
Sugar and salt are white crystalline compounds with very different bonding characteristics. How can the two compounds be differentiated based on the type of bonds they contain?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
17
A system consisting of separate ion pairs is more stable than an ionic lattice.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
18
Energy is required to disassociate bonded atoms or ions into isolated atoms or ions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
19
Energy is absorbed when a bond is formed.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
20
Isolated atoms are more stable than the aggregated atoms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
21
Lattice energy is directly proportional to the size of the ions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
22
Lattice energies are the lowest for substances with small, highly charged ions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
23
The position of the atoms is different in the various resonance structures of a compound.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
24
Hydrogen, with only two valence electrons, obeys the octet rule.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
25
_____ is the temperature at which the individual ions in a lattice or the individual molecules in a covalent compound have enough kinetic energy to overcome the attractive forces that hold them in place in the solid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
26
Explain the relationship between lattice energies and physical properties.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which of the following compounds has the lowest lattice energy?
A) Ca(OH)2
B) KBr
C) InAs
D) ZnS
E) SrSe
A) Ca(OH)2
B) KBr
C) InAs
D) ZnS
E) SrSe

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
28
The tendency for atoms to lose, gain, or share electrons to reach a total of eight valence electrons is known as the _____.
A) Frenkel defect
B) octet rule
C) band theory
D) aufbau principle
E) Meissner effect
A) Frenkel defect
B) octet rule
C) band theory
D) aufbau principle
E) Meissner effect

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
29
The number of dots in the Lewis dot symbol is the same as the number of valence electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
30
If an atom has the number of bonds typical for that atom, its formal charge is zero.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which of the following feature is true of lattice energy?
A) It depends on the product of the charges on the ions.
B) It depends on the difference of the charges on the ions.
C) It is directly proportional to the internuclear distance of the ions.
D) It is directly proportional to the size of the ions.
E) It is inversely proportional to the sum of the charges of the ions.
A) It depends on the product of the charges on the ions.
B) It depends on the difference of the charges on the ions.
C) It is directly proportional to the internuclear distance of the ions.
D) It is directly proportional to the size of the ions.
E) It is inversely proportional to the sum of the charges of the ions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
32
A thermochemical cycle that describes the process in which an ionic solid is conceptually formed from its component elements in a stepwise manner is known as the _____ cycle.
A) Tyndall
B) Graham
C) Born-Haber
D) Boyle
E) Henry
A) Tyndall
B) Graham
C) Born-Haber
D) Boyle
E) Henry

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
33
The number of dots in an element's Lewis dot symbol is the same as the number of its _____.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which of the following compounds has the highest lattice energy?
A) GaP
B) CaO
C) BaS
D) RbCl
E) HCl
A) GaP
B) CaO
C) BaS
D) RbCl
E) HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
35
The Lewis structure with the set of formal charges closest to zero is the most unstable.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
36
Explain the Lewis electron dot symbols.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
37
_____ are used for predicting the number of bonds formed by most elements in their compounds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which of the following is used to predict the number of bonds formed by most elements in their compounds?
A) Van der Waals forces
B) Aufbau principle
C) Boltzmann distribution
D) Lewis electron dot symbols
E) Bragg equation
A) Van der Waals forces
B) Aufbau principle
C) Boltzmann distribution
D) Lewis electron dot symbols
E) Bragg equation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
39
The octet rule indicates that atoms tend to lose, gain, or share electrons to reach a total of six valence electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
40
Lattice energy is the most important factor in determining the stability of a(n) _____ compound.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
41
Mention the exceptions to the octet rule.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
42
All Brønsted-Lowry bases are electron-pair donors.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
43
The electron pair being shared by atoms is called a(n) _____ pair.
A) homogenous
B) bonding
C) ionic
D) lone
E) neutral
A) homogenous
B) bonding
C) ionic
D) lone
E) neutral

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
44
Which of the following is true of a formal charge?
A) It represents a true charge on an atom in a covalent bond.
B) If an atom has the number of bonds typical for that atom, its formal charge is greater than one.
C) The sum of the formal charges on the atoms within a molecule or an ion must be less than the overall charge on the molecule or ion.
D) The Lewis structure with the set of formal charges closest to zero is usually the most unstable.
E) It predicts the most likely structure when a compound has more than one valid Lewis structure.
A) It represents a true charge on an atom in a covalent bond.
B) If an atom has the number of bonds typical for that atom, its formal charge is greater than one.
C) The sum of the formal charges on the atoms within a molecule or an ion must be less than the overall charge on the molecule or ion.
D) The Lewis structure with the set of formal charges closest to zero is usually the most unstable.
E) It predicts the most likely structure when a compound has more than one valid Lewis structure.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
45
In Lewis electron structures, _____ pairs are not shared between atoms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
46
A(n) _____ is a Lewis electron structure that has different arrangements of electrons around atoms whose positions do not change.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
47
Electron-deficient molecules are Lewis bases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
48
_____ molecules are those which have less than an octet of electrons around one atom.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
49
BCl3 follows the octet rule.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
50
Electron-deficient compounds have a strong tendency to gain electrons in their reactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
51
Explain formal charges.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
52
A Lewis structure cannot be drawn for NO because it:
A) is made up of s-block and p-block elements.
B) is made up of only s-block elements.
C) it has an odd number of valence electrons.
D) shares more than two electron pairs.
E) has an even number of valence electrons.
A) is made up of s-block and p-block elements.
B) is made up of only s-block elements.
C) it has an odd number of valence electrons.
D) shares more than two electron pairs.
E) has an even number of valence electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
53
In the oxoanions of the heavier p-block elements, the central atom often has an expanded valence shell.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
54
Compounds with more than an octet of electrons around an atom are called _____.
A) polar covalent bonds
B) amphoteric bonds
C) adducts
D) expanded-valence molecules
E) resonance structures
A) polar covalent bonds
B) amphoteric bonds
C) adducts
D) expanded-valence molecules
E) resonance structures

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
55
Which of the following is the Lewis structure of CO2?
A)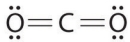
B)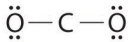
C)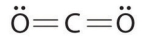
D)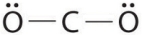
E)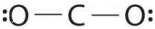
A)
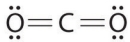
B)
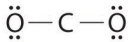
C)
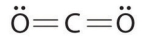
D)
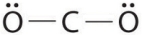
E)
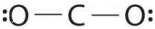

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
56
Which of the following is used to compute a formal charge?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
57
Which of the following does not have a single Lewis structure?
A) H2O
B) Cl2
C) NH4+
D) CH2O
E) O3
A) H2O
B) Cl2
C) NH4+
D) CH2O
E) O3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
58
Which of the following compounds is an exception to the octet rule?
A) H2O
B) CO2
C) NH3
D) CH2O
E) SF6
A) H2O
B) CO2
C) NH3
D) CH2O
E) SF6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
59
Given: The Lewis structure of BeCl2. 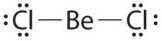 The structure shown above is an exception to the octet rule because the arrangement:
The structure shown above is an exception to the octet rule because the arrangement:
A) does not contain any double bonds.
B) gives each chlorine atom only eight electrons.
C) contains a beryllium atom.
D) contains only two chlorine atoms.
E) gives beryllium only four electrons.
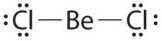 The structure shown above is an exception to the octet rule because the arrangement:
The structure shown above is an exception to the octet rule because the arrangement:A) does not contain any double bonds.
B) gives each chlorine atom only eight electrons.
C) contains a beryllium atom.
D) contains only two chlorine atoms.
E) gives beryllium only four electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
60
What is the formal charge of boron in BH3?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
61
If the bonds in the products are stronger than those in the reactants, the reaction is exothermic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
62
Which of the following has the lowest bond strength?
A) F-F
B) Br-Br
C) Cl-Cl
D) I-I
E) At-At
A) F-F
B) Br-Br
C) Cl-Cl
D) I-I
E) At-At

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
63
There is a direct correlation between electronegativity and bond polarity.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
64
Bonds between hydrogen and atoms in the same column of the periodic table decrease in strength as we go down the column. Thus, an H-F bond is _____ than an H-I bond.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
65
In the reaction (CH3)2O + BF3 → (CH3)2O:BF3, the Lewis acid is _____.
A) O:BF3
B) (CH3)2O
C) (CH3)2O:BF3
D) (CH3)2
E) BF3
A) O:BF3
B) (CH3)2O
C) (CH3)2O:BF3
D) (CH3)2
E) BF3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
66
In a polar covalent bond, the:
A) ions are held together by electrostatic forces.
B) electrons are shared unequally between the atoms.
C) protons hold the compound's component elements together.
D) number of protons exceed the number of electrons.
E) atomic numbers of component elements exceed their own mass numbers.
A) ions are held together by electrostatic forces.
B) electrons are shared unequally between the atoms.
C) protons hold the compound's component elements together.
D) number of protons exceed the number of electrons.
E) atomic numbers of component elements exceed their own mass numbers.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
67
Triple bonds between like atoms are longer than double bonds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
68
The asymmetrical charge distribution in a polar substance produces a dipole moment.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
69
Which of the following has the greatest bond strength?
A) H-I
B) H-At
C) H-Br
D) H-F
E) H-Cl
A) H-I
B) H-At
C) H-Br
D) H-F
E) H-Cl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
70
Differentiate between a Lewis acid and a Lewis base. Explain how it differs from the Bronsted-Lowry concept of acids and bases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
71
The bond formed between a Lewis acid and a Lewis base is a coordinate covalent bond.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
72
As bond orders increase, bond lengths generally decrease.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
73
In the reaction H2O + SO3 → H2SO4, the Lewis base is _____.
A) H2SO4
B) SO3
C) H2O
D) SO42-
E) H+
A) H2SO4
B) SO3
C) H2O
D) SO42-
E) H+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
74
What is an adduct?
A) It is the optimal internuclear distance between two bonded atoms.
B) It is the product of reaction between a Lewis acid and a Lewis base with a coordinate covalent bond.
C) It is any species that can donate a pair of electrons.
D) It is any species that can accept a pair of electrons.
E) It is the enthalpy change that occurs when a solid ionic compound is transformed into gaseous ions.
A) It is the optimal internuclear distance between two bonded atoms.
B) It is the product of reaction between a Lewis acid and a Lewis base with a coordinate covalent bond.
C) It is any species that can donate a pair of electrons.
D) It is any species that can accept a pair of electrons.
E) It is the enthalpy change that occurs when a solid ionic compound is transformed into gaseous ions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
75
A bond is polar if the bonded atoms have equal electronegativities.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
76
Any species that can accept a pair of electrons are known as _____.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
77
Bonds between like atoms usually become weaker as we go down a column. Thus, the C-C single bond is _____ than the Si-Si single bond.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
78
Explain the relationship between bond order and bond energy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
79
The bond energy of a C-H single bond is the same in all organic compounds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck
80
Which of the following is true of bond polarity?
A) A bond is nonpolar if the electronegativities of the bonded atoms are unequal.
B) The bond polarity decreases with an increasing difference in electronegativity.
C) A bond is polar if the bonded atoms have equal electronegativities.
D) The ionic character increases with an increasing difference in electronegativity.
E) There is no direct correlation between electronegativity and bond polarity.
A) A bond is nonpolar if the electronegativities of the bonded atoms are unequal.
B) The bond polarity decreases with an increasing difference in electronegativity.
C) A bond is polar if the bonded atoms have equal electronegativities.
D) The ionic character increases with an increasing difference in electronegativity.
E) There is no direct correlation between electronegativity and bond polarity.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 88 في هذه المجموعة.
فتح الحزمة
k this deck








