Deck 42: Atomic Physics
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/59
العب
ملء الشاشة (f)
Deck 42: Atomic Physics
1
In the subshell of the Li2+ ion with orbital quantum number 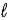 , the allowed values of the magnetic quantum number
, the allowed values of the magnetic quantum number 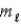 are
are
A) −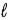 to
to 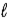
B) −(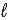 + 1)to(
+ 1)to( 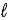 + l)
+ l)
C) − (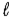 + 2) to (
+ 2) to (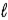 + 2)
+ 2)
D) −(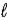 + 3) to (
+ 3) to (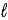 + 3)
+ 3)
E) 0 to n − 1
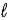 , the allowed values of the magnetic quantum number
, the allowed values of the magnetic quantum number 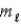 are
areA) −
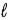 to
to 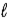
B) −(
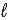 + 1)to(
+ 1)to( 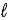 + l)
+ l)C) − (
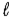 + 2) to (
+ 2) to (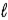 + 2)
+ 2)D) −(
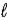 + 3) to (
+ 3) to (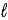 + 3)
+ 3)E) 0 to n − 1
− 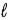 to
to 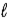
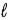 to
to 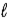
2
Suppose Bohr had chosen the potential energy of the electron in the hydrogen atom to be V = 0 when the electron is in the orbit with n = 1. He could do this by
A) choosing n = 1 for the orbit where the kinetic energy of the electron is zero.
B) adding a constant 13.6 eV to the potential energy for all values of n.
C) adding a constant 27.2 eV to the potential energy for all values of n.
D) subtracting a constant 13.6 eV from the potential energy for all values of n.
E) subtracting a constant 27.2 eV from the potential energy for all values of n.
A) choosing n = 1 for the orbit where the kinetic energy of the electron is zero.
B) adding a constant 13.6 eV to the potential energy for all values of n.
C) adding a constant 27.2 eV to the potential energy for all values of n.
D) subtracting a constant 13.6 eV from the potential energy for all values of n.
E) subtracting a constant 27.2 eV from the potential energy for all values of n.
adding a constant 27.2 eV to the potential energy for all values of n.
3
The energy needed to remove an electron from the first excited state of a Li2+ ion is
A) 53 eV
B) 31 eV
C) 92 eV
D) 122 eV
E) 61 eV
A) 53 eV
B) 31 eV
C) 92 eV
D) 122 eV
E) 61 eV
31 eV
4
In a shell of the hydrogen atom with n = 3, the permitted values of the orbital magnetic quantum number 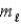 are
are
A) −1, 0, 1
B) 2, 1, 0
C) 2, 1, 0, −1, −2
D) 0
E) 3, 2, 1, 0, −1, −2, −3
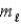 are
areA) −1, 0, 1
B) 2, 1, 0
C) 2, 1, 0, −1, −2
D) 0
E) 3, 2, 1, 0, −1, −2, −3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
5
Of the following states, 5s, 3p, 4f, 5p, 4g, 3d, and 2p, the one which is NOT allowed is
A) 3p
B) 4f
C) 3d
D) 4g
E) 2p
A) 3p
B) 4f
C) 3d
D) 4g
E) 2p

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
6
The allowed values of 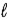 for the n = 3 shell in a Li2+ ion are
for the n = 3 shell in a Li2+ ion are
A) 1, 2
B) 0, 1
C) 0, 1, 2
D) 0, 1, 2, 3
E) 1, 2, 3
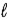 for the n = 3 shell in a Li2+ ion are
for the n = 3 shell in a Li2+ ion areA) 1, 2
B) 0, 1
C) 0, 1, 2
D) 0, 1, 2, 3
E) 1, 2, 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
7
The K, L, M symbols represent values of the quantum number
A) n
B)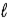
C)
D) ms
E) mj
A) n
B)
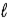
C)

D) ms
E) mj

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
8
What wavelength (in μm) is associated with the Paschen series for n = 4?
(RH = 1.097 × 107 m−1)
A) 320
B) 530
C) 2.7
D) 1.9
E) 0.5
(RH = 1.097 × 107 m−1)
A) 320
B) 530
C) 2.7
D) 1.9
E) 0.5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
9
The allowed values of n for the Li2+ ion are
A) 1 to ∞
B) 2 to ∞
C) 3 to ∞
D) any real number
E) 1 to 10
A) 1 to ∞
B) 2 to ∞
C) 3 to ∞
D) any real number
E) 1 to 10

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
10
An electron is moving at a speed of 2.1 × 106 m/s in the first Bohr orbit. Determine its de Broglie wavelength.
A) 0.30 × 10−10 m
B) 1.7 × 10−10 m
C) 0.50 × 10−10 m
D) 3.5 × 10−10 m
E) 1.5 × 10−10 m
A) 0.30 × 10−10 m
B) 1.7 × 10−10 m
C) 0.50 × 10−10 m
D) 3.5 × 10−10 m
E) 1.5 × 10−10 m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
11
How fast is the electron moving in the first Bohr orbit?
A) 3.3 × 106 m/s
B) 2.2 × 106 m/s
C) 4.4 × 106 m/s
D) 5.5 × 106 m/s
E) 5.5 × 1015 m/s
A) 3.3 × 106 m/s
B) 2.2 × 106 m/s
C) 4.4 × 106 m/s
D) 5.5 × 106 m/s
E) 5.5 × 1015 m/s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
12
An energy of 13.6 eV is needed to ionize an electron from the ground state of a hydrogen atom. Selecting the longest wavelength that will work from the those given below, what wavelength is needed if a photon accomplishes this task?
A) 60 nm
B) 80 nm
C) 70 nm
D) 90 nm
E) 40 nm
A) 60 nm
B) 80 nm
C) 70 nm
D) 90 nm
E) 40 nm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
13
What value of wavelength is associated with the Lyman series for n = 2?
(RH = 1.097 × 107 m−1)
A) 8.2 × 106 m
B) 1.2 × 10−7 m
C) 2.7 × 106 m
D) 3.6 × 10−7 m
E) 8.8 × 10−7 m
(RH = 1.097 × 107 m−1)
A) 8.2 × 106 m
B) 1.2 × 10−7 m
C) 2.7 × 106 m
D) 3.6 × 10−7 m
E) 8.8 × 10−7 m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
14
An electron in a hydrogen atom makes a transition from the n = 4 to the n = 3 energy state. Determine the energy (in eV) of the emitted photon.
A) 0.54
B) 0.66
C) 0.85
D) 1.51
E) 10.2
A) 0.54
B) 0.66
C) 0.85
D) 1.51
E) 10.2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
15
An electron in a hydrogen atom makes a transition from the n = 3 to the n = 1 energy state. Determine the wavelength of the emitted photon (in nm).
A) 1006
B) 209
C) 306
D) 103
E) 821
A) 1006
B) 209
C) 306
D) 103
E) 821

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
16
One of the main problems with the Bohr model of the hydrogen atom when compared with the results of the methods of quantum mechanics used to describe atoms, was that the Bohr model predicted
A) the ground state angular momentum was L = 1
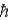 .
.
B) the frequency of the radiation emitted when an electron "jumps" from one allowed orbit to another was hf = Ei − Ef.
C) the potential energy function for the hydrogen atom was given by V(r) = −ke2/r.
D) the energy of the ground state of the hydrogen atom was En = −13.6 eV.
A) the ground state angular momentum was L = 1
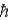 .
.B) the frequency of the radiation emitted when an electron "jumps" from one allowed orbit to another was hf = Ei − Ef.
C) the potential energy function for the hydrogen atom was given by V(r) = −ke2/r.
D) the energy of the ground state of the hydrogen atom was En = −13.6 eV.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
17
The s, p, d, f, symbols represent values of the quantum number
A) ms
B) n
C)
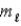
D)
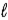
E) mj
A) ms
B) n
C)
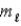
D)
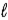
E) mj

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
18
A hydrogen atom is in its first excited state (n = 2). The linear momentum of the electron is (in kg ⋅ m/s)
A) 3 × 10−24
B) 2 × 10−24
C) 1 × 10−24
D) 4 × 10−24
E) 3 × 10−15
A) 3 × 10−24
B) 2 × 10−24
C) 1 × 10−24
D) 4 × 10−24
E) 3 × 10−15

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
19
The number of states in the He+ ion corresponding to the principle quantum number n = 5 are
A) 18
B) 25
C) 50
D) 9
E) 11
A) 18
B) 25
C) 50
D) 9
E) 11

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
20
Light is emitted by hydrogen atoms in the visible range for a hydrogen atom. Its wavelength is 656 nm. What value of n is associated with the light? (RH = 1.097 × 107 m−1)
A) 5
B) 2
C) 4
D) 3
E) 6
A) 5
B) 2
C) 4
D) 3
E) 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
21
Characteristic x-rays can be produced by bombarding targets with electrons. These x-rays occur when
A) electrons from higher shells fill the vacant lower shell
B) electrons fill the vacant valence shell
C) photons are emitted with energies on the order of 103 eV
D) photons are emitted with wavelengths on the order of 103 nm
A) electrons from higher shells fill the vacant lower shell
B) electrons fill the vacant valence shell
C) photons are emitted with energies on the order of 103 eV
D) photons are emitted with wavelengths on the order of 103 nm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
22
The energy needed to change a He+ ion in the ground state into a He2+ ion is
A) 13.6 eV
B) 54.4 eV
C) 112.4 eV
D) 92.9 eV
E) 27.2 eV
A) 13.6 eV
B) 54.4 eV
C) 112.4 eV
D) 92.9 eV
E) 27.2 eV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
23
A hydrogen atom in the 4f state has a total angular momentum (in terms of 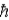 ) of magnitude
) of magnitude
A)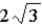
B) 3
C) 6
D) 12
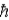 ) of magnitude
) of magnitudeA)
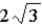
B) 3
C) 6
D) 12

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
24
In 1921, Stern and Gerlach performed an experiment that first demonstrated
A) orbital angular momentum quantization
B) energy quantization
C) space quantization
D) magnetic orbital quantization
E) that particles behave like waves
A) orbital angular momentum quantization
B) energy quantization
C) space quantization
D) magnetic orbital quantization
E) that particles behave like waves

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which of the following statements is true?
A)
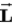 can never be perpendicular to
can never be perpendicular to
 .
.
B)
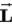 can be aligned parallel to
can be aligned parallel to
 .
.
C)
 must be perpendicular to
must be perpendicular to
 .
.
D)
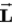 can never be aligned parallel to
can never be aligned parallel to
 .
.
A)
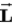 can never be perpendicular to
can never be perpendicular to .
.B)
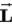 can be aligned parallel to
can be aligned parallel to .
.C)
 must be perpendicular to
must be perpendicular to .
.D)
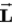 can never be aligned parallel to
can never be aligned parallel to .
.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
26
When using the Pauli Exclusion Principle, we assume the particle's spin angular momentum is of magnitude
A)
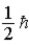
B)
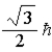
C)

D) ±
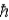
E)
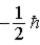
A)
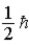
B)
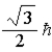
C)

D) ±
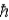
E)
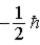

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
27
The radial portion of the de Broglie wavefunction for an electron in the ground state of the hydrogen atom is Ψ1s(r) = 1/( 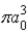 )1/2 exp(−r/a0) where a0 is the Bohr radius. The probability of finding the electron is
)1/2 exp(−r/a0) where a0 is the Bohr radius. The probability of finding the electron is
A)
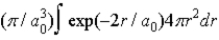
B)
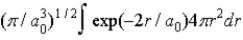
C)
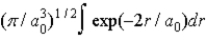
D)
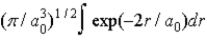
E)
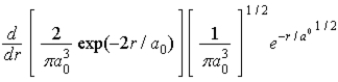
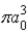 )1/2 exp(−r/a0) where a0 is the Bohr radius. The probability of finding the electron is
)1/2 exp(−r/a0) where a0 is the Bohr radius. The probability of finding the electron isA)
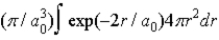
B)
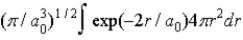
C)
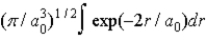
D)
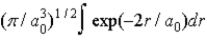
E)
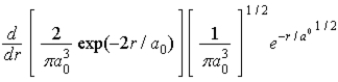

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
28
A Li2+ ion undergoes a transition from the n = 4 to the n = 3 state. The energy of the emitted photon is
A) 4.5 eV
B) 10.2 eV
C) 5.95 eV
D) 2.6 eV
E) 0.66 eV
A) 4.5 eV
B) 10.2 eV
C) 5.95 eV
D) 2.6 eV
E) 0.66 eV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
29
The probability density for the 1s state is given by |Ψ1s|2. The probability of finding the particle somewhere in space is
A)
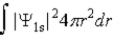
B)
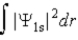
C)
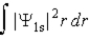
D)
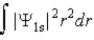
E)
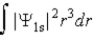
A)
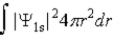
B)
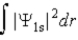
C)
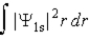
D)
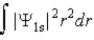
E)
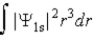

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
30
Rubidium (Z = 37) and potassium (Z = 19) are similar to sodium in that they have ____ electron(s) in the outermost shell.
A) five p
B) three p
C) two s
D) one d
E) one s
A) five p
B) three p
C) two s
D) one d
E) one s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
31
If P(r) is the radial probability density function for an electron in the ground state of a hydrogen atom, the most probable value for r can be found from
A) dP/dt
B) dP/dr
C)
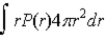
D)
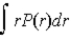
E) d2P/dr2
A) dP/dt
B) dP/dr
C)
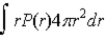
D)
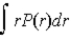
E) d2P/dr2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
32
The probability density of a particle at a distance r from the nucleus is essentially the
A) probability of finding the particle within a small volume about r.
B) probability per unit area of finding the particle within a unit area centered on r.
C) probability per unit length of finding the particle within a unit length of r.
D) probability per unit volume of finding the particle within a small volume about r.
E)
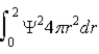
A) probability of finding the particle within a small volume about r.
B) probability per unit area of finding the particle within a unit area centered on r.
C) probability per unit length of finding the particle within a unit length of r.
D) probability per unit volume of finding the particle within a small volume about r.
E)
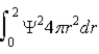

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
33
The magnitude of the spin angular momentum for an electron is equal to
A)
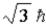
B)
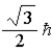
C)
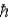 /2
/2
D) ±
 /2
/2
E)
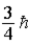
A)
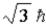
B)
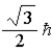
C)
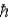 /2
/2D) ±
 /2
/2E)
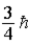

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
34
Forbidden transitions and selection rules suggest that
A) a photon has linear momentum.
B) a photon has energy.
C) a photon has angular momentum.
D) a photon has parity.
E) a photon has mass.
A) a photon has linear momentum.
B) a photon has energy.
C) a photon has angular momentum.
D) a photon has parity.
E) a photon has mass.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
35
When electrons fill a subshell in which the orbitals have equal energy, the order in which the orbitals are filled is such that
A) a minimum number of electrons has unpaired spins.
B) a minimum number of electrons has intrinsic angular momentum.
C) a maximum number of electrons has unpaired spins.
D) a maximum number of electrons first fills the next energy level.
E) the maximum number of electrons has the same set of quantum numbers.
A) a minimum number of electrons has unpaired spins.
B) a minimum number of electrons has intrinsic angular momentum.
C) a maximum number of electrons has unpaired spins.
D) a maximum number of electrons first fills the next energy level.
E) the maximum number of electrons has the same set of quantum numbers.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
36
For the following allowed transitions, which photon would have the largest wavelength when an electron "jumps" from one energy level, characterized by the quantum number n, to another?
A) n = 2 to n = 1
B) n = 3 to n = 2
C) n = 3 to n = 1
D) n = 1 to n = 3
E) n = 4 to n = 1
A) n = 2 to n = 1
B) n = 3 to n = 2
C) n = 3 to n = 1
D) n = 1 to n = 3
E) n = 4 to n = 1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
37
What angle does the orbital angular momentum make with the z axis of a hydrogen atom in the state n = 3, 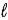 = 2,
= 2, 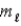 = −1?
= −1?
A) −66°
B) 66°
C) 24°
D) 114°
E) 73°
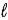 = 2,
= 2, 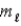 = −1?
= −1?A) −66°
B) 66°
C) 24°
D) 114°
E) 73°

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
38
The ground state configuration of chlorine (Z = 17) is
A) 1s2 2s2 2p5 3s2 3p6
B) 1s2 2s2 2p6 3s2 3p5
C) 1s2 2s2 2p6 3s2 3p4 3d1
D) 1s2 2s2 2p6 3s2 3p5 4s1
E) 1s2 2s2 2p6 3s1 3p7
A) 1s2 2s2 2p5 3s2 3p6
B) 1s2 2s2 2p6 3s2 3p5
C) 1s2 2s2 2p6 3s2 3p4 3d1
D) 1s2 2s2 2p6 3s2 3p5 4s1
E) 1s2 2s2 2p6 3s1 3p7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
39
In a completely filled atomic shell,
A) the intrinsic spin of the electrons does not produce a resultant magnetic moment.
B) the orbital motion of the electrons does not produce a resultant magnetic moment.
C) the atom will be an alkali metal.
D) only (a) and (b) are correct.
E) none of the above are correct.
A) the intrinsic spin of the electrons does not produce a resultant magnetic moment.
B) the orbital motion of the electrons does not produce a resultant magnetic moment.
C) the atom will be an alkali metal.
D) only (a) and (b) are correct.
E) none of the above are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
40
The Pauli Exclusion Principle states
A) no two electrons in the same atom can have the same set of quantum numbers.
B) there is an inherent uncertainty in the position and momentum of a particle.
C) when an atom has orbitals of equal energy, the maximum number of electrons will have unpaired spins.
D) when an atom has orbitals of equal energy, the maximum number of electrons will be paired spins.
E) no two atoms can have the same set of quantum numbers.
A) no two electrons in the same atom can have the same set of quantum numbers.
B) there is an inherent uncertainty in the position and momentum of a particle.
C) when an atom has orbitals of equal energy, the maximum number of electrons will have unpaired spins.
D) when an atom has orbitals of equal energy, the maximum number of electrons will be paired spins.
E) no two atoms can have the same set of quantum numbers.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
41
Which of the following, in which n and m have integer values, is a correct formula for a wavelength emitted by a hydrogen atom?
A)
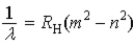
B)
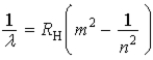
C)
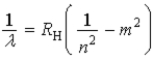
D)
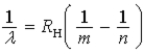
E)
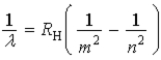
A)
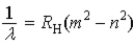
B)
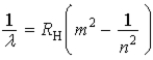
C)
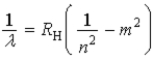
D)
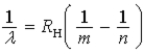
E)
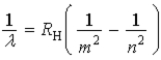

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
42
What is the difference in frequency for spectral lines emitted by hydrogen for transitions from the n = 16 level to the n = 2 level and transitions from the n = 15 level to the n = 2 level? (RH = 1.097 × 107 m−1.)
A) 5.65 × 10−13 Hz
B) 31 Hz
C) 1.77 × 1012 Hz
D) 2.55 × 1016 Hz
E) 1.02 × 1017 Hz
A) 5.65 × 10−13 Hz
B) 31 Hz
C) 1.77 × 1012 Hz
D) 2.55 × 1016 Hz
E) 1.02 × 1017 Hz

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
43
A hydrogen atom emits a photon of wavelength 657.7 nm. From what energy state to what lower energy state did the electron jump?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
44
A headwaiter at a restaurant decides to apply the exclusion principle to the seating of patrons. He will treat tables as sub-shells, and will only seat patrons if the number of the people to be seated adds up to a complete sub-shell. Of the numbers below, the number he would not be willing to seat at one table is
A) 2.
B) 4.
C) 6.
D) 10.
E) 14.
A) 2.
B) 4.
C) 6.
D) 10.
E) 14.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
45
Quantum physics agrees with the classical physics limit when
A) the total angular momentum is a small multiple of
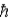 .
.
B) the total energy is a small multiple of the energy in the lowest quantized state.
C) the difference in energy between adjacent quantized levels becomes vanishingly small.
D) all electron spins are paired so that L = 0.
E) there is a vacancy in an inner level in the atom.
A) the total angular momentum is a small multiple of
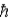 .
.B) the total energy is a small multiple of the energy in the lowest quantized state.
C) the difference in energy between adjacent quantized levels becomes vanishingly small.
D) all electron spins are paired so that L = 0.
E) there is a vacancy in an inner level in the atom.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
46
Adam and Eve are contemplating the beauty of the hydrogen atom. Adam claims that the quantum states with a given value of the principal quantum number n can have any value of the orbital quantum number 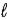 . Eve says that the Snake told her that a state with a given value of
. Eve says that the Snake told her that a state with a given value of 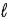 could have any value of n. Which one, if either, is correct, and why?
could have any value of n. Which one, if either, is correct, and why?
A) Adam, because the man is always right.
B) Adam because n ≤
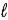 − 1.
− 1.
C) Eve, because n ≤
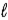 − 1.
− 1.
D) Eve, because
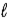 ≤ n − 1.
≤ n − 1.
E) Neither, because Adam is wrong and the Snake told a subtle lie.
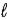 . Eve says that the Snake told her that a state with a given value of
. Eve says that the Snake told her that a state with a given value of 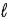 could have any value of n. Which one, if either, is correct, and why?
could have any value of n. Which one, if either, is correct, and why?A) Adam, because the man is always right.
B) Adam because n ≤
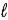 − 1.
− 1.C) Eve, because n ≤
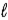 − 1.
− 1.D) Eve, because
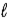 ≤ n − 1.
≤ n − 1.E) Neither, because Adam is wrong and the Snake told a subtle lie.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
47
The energy difference between the upper and lower levels in a certain laser is 1.9593 eV. What is the wavelength of the light emitted by the laser?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
48
What is the difference in wavelength for spectral lines emitted by hydrogen for transitions from the n = 16 level to the n = 2 level and transitions from the n = 15 level to the n = 2 level? (RH = 1.097 × 107 m−1.)
A) 1.0 × 10−10 m
B) 2.0 × 10−10 m
C) 4.1 × 10−10 m
D) 8.1 × 10−10 m
E) 1.6 × 10−9 m
A) 1.0 × 10−10 m
B) 2.0 × 10−10 m
C) 4.1 × 10−10 m
D) 8.1 × 10−10 m
E) 1.6 × 10−9 m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
49
Aline says that the magnetic moment of an atom originates in the orbital angular momentum of the electron. Bevis says that it comes from the electron spin. Which one, if either, is correct, and why?
A) Aline, because only atoms, not electrons, can have angular momentum.
B) Bevis, because only atoms, not electrons, can have angular momentum.
C) Neither, because electron spin and orbital angular momentum always cancel exactly.
D) Neither, because the magnetic moment of an atom comes only from the spin of the nucleus.
E) Both, because both the orbital angular momentum and the spins of the electrons contribute to the magnetic moment of an atom.
A) Aline, because only atoms, not electrons, can have angular momentum.
B) Bevis, because only atoms, not electrons, can have angular momentum.
C) Neither, because electron spin and orbital angular momentum always cancel exactly.
D) Neither, because the magnetic moment of an atom comes only from the spin of the nucleus.
E) Both, because both the orbital angular momentum and the spins of the electrons contribute to the magnetic moment of an atom.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
50
The number of electrons in the n = 4, 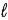 = 2 subshell in strontium (Z = 38) is ____ the number of electrons in the n =4,
= 2 subshell in strontium (Z = 38) is ____ the number of electrons in the n =4, 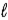 =2 subshell in barium (Z = 56).
=2 subshell in barium (Z = 56).
A)
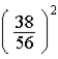 times
times
B)
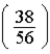 times
times
C) equal to
D)
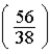 times
times
E)
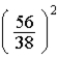 times
times
 = 2 subshell in strontium (Z = 38) is ____ the number of electrons in the n =4,
= 2 subshell in strontium (Z = 38) is ____ the number of electrons in the n =4,  =2 subshell in barium (Z = 56).
=2 subshell in barium (Z = 56).A)
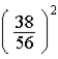 times
timesB)
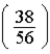 times
timesC) equal to
D)
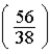 times
timesE)
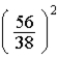 times
times
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
51
In the Bohr model of the hydrogen atom, the total energy of the electron-proton system is
A)
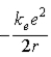 .
.
B)
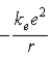 .
.
C) 0.
D)
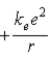 .
.
E)
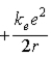 .
.
A)
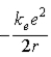 .
.B)
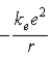 .
.C) 0.
D)
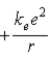 .
.E)
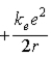 .
.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
52
Suppose a beam of electrons is incident on a collection of hydrogen atoms, all of which are in the lowest energy state (n = 1). What is the minimum energy the electrons can have if they are to excite the hydrogen atoms into the n = 2 state?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
53
In an atom that has an electron in a sub-shell for which 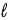 = 4, with respect to the magnetic field vector
= 4, with respect to the magnetic field vector  the magnetic moment vector
the magnetic moment vector 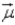 of the electron is allowed to be oriented in
of the electron is allowed to be oriented in
A) any direction.
B)
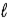 discrete directions
discrete directions
C)
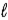 − 1 discrete directions.
− 1 discrete directions.
D)
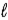 + 1 discrete directions.
+ 1 discrete directions.
E) 2
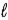 + 1 discrete directions.
+ 1 discrete directions.
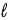 = 4, with respect to the magnetic field vector
= 4, with respect to the magnetic field vector  the magnetic moment vector
the magnetic moment vector 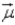 of the electron is allowed to be oriented in
of the electron is allowed to be oriented inA) any direction.
B)
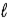 discrete directions
discrete directionsC)
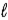 − 1 discrete directions.
− 1 discrete directions.D)
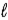 + 1 discrete directions.
+ 1 discrete directions.E) 2
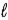 + 1 discrete directions.
+ 1 discrete directions.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
54
In an allowed electron transition in a hydrogen atom,
A) Δ
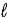 = 0;
= 0;
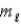 = 0, ±1.
= 0, ±1.
B) Δ
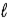 = 0, ±1;
= 0, ±1;
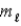 = ±1.
= ±1.
C) Δ
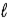 = 0, ±1;
= 0, ±1;
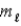 = 0, ±1.
= 0, ±1.
D) Δ
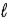 = ±1;
= ±1;
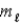 = 0, ±1.
= 0, ±1.
E) Δ
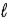 = ±1;
= ±1;
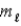 = ±1.
= ±1.
A) Δ
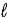 = 0;
= 0;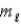 = 0, ±1.
= 0, ±1.B) Δ
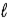 = 0, ±1;
= 0, ±1;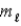 = ±1.
= ±1.C) Δ
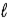 = 0, ±1;
= 0, ±1;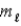 = 0, ±1.
= 0, ±1.D) Δ
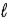 = ±1;
= ±1;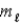 = 0, ±1.
= 0, ±1.E) Δ
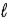 = ±1;
= ±1;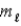 = ±1.
= ±1.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
55
In the operation of a laser
A) stimulated emission occurs.
B) there is a population inversion.
C) the excited state will tend to be metastable.
D) the photons emitted will have transitioned to the ground state.
E) For the answers (a), (b), (c), and (d), three are correct and one is incorrect.
A) stimulated emission occurs.
B) there is a population inversion.
C) the excited state will tend to be metastable.
D) the photons emitted will have transitioned to the ground state.
E) For the answers (a), (b), (c), and (d), three are correct and one is incorrect.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
56
All quantum states forming a shell have the same
A) principal quantum number n.
B) orbital quantum number
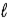 .
.
C) orbital magnetic quantum number
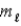 .
.
D) n,
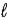 and
and
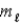
E) n and
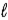 only.
only.
A) principal quantum number n.
B) orbital quantum number
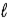 .
.C) orbital magnetic quantum number
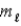 .
.D) n,
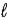 and
and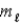
E) n and
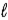 only.
only.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
57
In terms of a0, where a0 = 0.0529 nm, the radii of the allowed orbits in the Bohr model of the hydrogen atom are given by rn =
A)
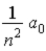 .
.
B)
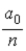 .
.
C)
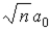 .
.
D) na0.
E) n2a0.
A)
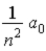 .
.B)
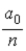 .
.C)
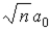 .
.D) na0.
E) n2a0.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
58
Zeke says that the magnitude of the orbital angular momentum in the hydrogen atom has the value L = 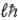 . Ruth says that the maximum magnitude of the projection of the angular momentum along the direction of a constant magnetic field vector
. Ruth says that the maximum magnitude of the projection of the angular momentum along the direction of a constant magnetic field vector  is
is 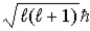 . Which one, if either, is correct, and why?
. Which one, if either, is correct, and why?
A) Ruth, because the maximum value of L is
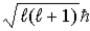 .
.
B) Ruth, because the orbital angular momentum always lines up with a magnetic field so that
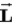 has its maximum value along the field.
has its maximum value along the field.
C) Zeke, because the maximum magnitude of
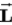 is L =
is L =
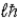 .
.
D) Zeke, because the orbital angular momentum always lines up with a magnetic field so that
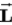 has its maximum value along the field.
has its maximum value along the field.
E) Neither, because they have interchanged the maximum magnitude of
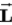 ,
,
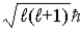 , and
, and
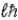 , its maximum projection along a magnetic field direction.
, its maximum projection along a magnetic field direction.
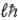 . Ruth says that the maximum magnitude of the projection of the angular momentum along the direction of a constant magnetic field vector
. Ruth says that the maximum magnitude of the projection of the angular momentum along the direction of a constant magnetic field vector  is
is 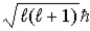 . Which one, if either, is correct, and why?
. Which one, if either, is correct, and why?A) Ruth, because the maximum value of L is
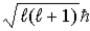 .
.B) Ruth, because the orbital angular momentum always lines up with a magnetic field so that
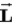 has its maximum value along the field.
has its maximum value along the field.C) Zeke, because the maximum magnitude of
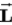 is L =
is L =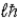 .
.D) Zeke, because the orbital angular momentum always lines up with a magnetic field so that
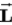 has its maximum value along the field.
has its maximum value along the field.E) Neither, because they have interchanged the maximum magnitude of
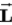 ,
,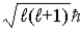 , and
, and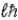 , its maximum projection along a magnetic field direction.
, its maximum projection along a magnetic field direction.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck
59
All quantum states forming a sub-shell have the same
A) principal quantum number n.
B) orbital quantum number
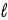 .
.
C) orbital magnetic quantum number
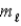 .
.
D) n,
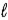 and
and
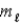
E) n and
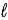 only.
only.
A) principal quantum number n.
B) orbital quantum number
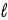 .
.C) orbital magnetic quantum number
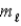 .
.D) n,
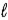 and
and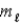
E) n and
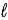 only.
only.
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 59 في هذه المجموعة.
فتح الحزمة
k this deck








