Deck 34: Geometric Optics
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/89
العب
ملء الشاشة (f)
Deck 34: Geometric Optics
1
Radioactivity: If a nucleus decays by β- decay to a daughter nucleus, which of the following statements about this decay are correct? (There may be more than one correct choice.)
A) The daughter nucleus has more protons than the original nucleus.
B) The daughter nucleus has more neutrons than the original nucleus.
C) The daughter nucleus has the same number of nucleons as the original nucleus.
D) The daughter nucleus has fewer protons than the original nucleus.
E) The daughter nucleus has fewer neutrons than the original nucleus.
A) The daughter nucleus has more protons than the original nucleus.
B) The daughter nucleus has more neutrons than the original nucleus.
C) The daughter nucleus has the same number of nucleons as the original nucleus.
D) The daughter nucleus has fewer protons than the original nucleus.
E) The daughter nucleus has fewer neutrons than the original nucleus.
The daughter nucleus has more protons than the original nucleus.
The daughter nucleus has the same number of nucleons as the original nucleus.
The daughter nucleus has fewer neutrons than the original nucleus.
The daughter nucleus has the same number of nucleons as the original nucleus.
The daughter nucleus has fewer neutrons than the original nucleus.
2
Nuclear binding energy: Going from medium mass nuclei to heavy nuclei, the average binding energy per nucleon
A) decreases.
B) behaves randomly with no clear pattern.
C) does not change.
D) increases.
E) doubles.
A) decreases.
B) behaves randomly with no clear pattern.
C) does not change.
D) increases.
E) doubles.
decreases.
3
Radioactive decay: The decay rate of an isotope is initially R0, but after one half-life has gone by, the rate is R0/2. At the end of the NEXT half-life, what will the decay rate be?
A) 0
B) R0/16
C) R0/e
D) R0/4
E) R0/e2
A) 0
B) R0/16
C) R0/e
D) R0/4
E) R0/e2
R0/4
4
Nuclear fission: Which of the following descriptions best describes the process by which energy is released in a conventional nuclear reactor?
A) The radiation given off by a naturally radioactive substance, uranium, is collected and used to make steam.
B) Uranium is reacted with oxygen in a combustion process that releases large amounts of radioactivity and heat.
C) Deuterium and tritium are joined together to form helium.
D) Uranium, when bombarded by neutrons, splits into fragments and releases two or three neutrons, and these neutrons in turn strike more uranium nuclei that split, thereby setting off a chain reaction that releases energy.
E) A uranium nucleus is energized to an excited state by neutron irradiation, and it then decays by emitting beta rays and gamma rays that heat water and create steam.
A) The radiation given off by a naturally radioactive substance, uranium, is collected and used to make steam.
B) Uranium is reacted with oxygen in a combustion process that releases large amounts of radioactivity and heat.
C) Deuterium and tritium are joined together to form helium.
D) Uranium, when bombarded by neutrons, splits into fragments and releases two or three neutrons, and these neutrons in turn strike more uranium nuclei that split, thereby setting off a chain reaction that releases energy.
E) A uranium nucleus is energized to an excited state by neutron irradiation, and it then decays by emitting beta rays and gamma rays that heat water and create steam.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
5
Radioactive dating: Modern nuclear bomb tests have created an extra high level of 14C in our atmosphere. Suppose that future archaeologists date samples from our era, but do not know about this testing. Will their dates be too young, too old, or still correct? If correct they are correct, why?
A) too young
B) too old
C) correct, because 14C from bomb tests is different from that produced naturally
D) correct, because modern biological materials do not gather 14C from bomb tests
A) too young
B) too old
C) correct, because 14C from bomb tests is different from that produced naturally
D) correct, because modern biological materials do not gather 14C from bomb tests

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
6
Properties of the nucleus: Consider two different isotopes of the same neutral element. Which statements about these isotopes are true? (There may be more than one correct choice.)
A) Both isotopes contain the same number of neutrons.
B) Both isotopes contain the same number of protons.
C) Both isotopes contain the same number of nucleons.
D) Both isotopes contain the same number of orbital electrons.
E) The sum of the protons and neutrons is the same for both isotopes.
A) Both isotopes contain the same number of neutrons.
B) Both isotopes contain the same number of protons.
C) Both isotopes contain the same number of nucleons.
D) Both isotopes contain the same number of orbital electrons.
E) The sum of the protons and neutrons is the same for both isotopes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
7
Radioactivity: If a nucleus decays by β+ decay to a daughter nucleus, which of the following statements about this decay are correct? (There may be more than one correct choice.)
A) The daughter nucleus has more protons than the original nucleus.
B) The daughter nucleus has more neutrons than the original nucleus.
C) The daughter nucleus has the same number of nucleons as the original nucleus.
D) The daughter nucleus has fewer protons than the original nucleus.
E) The daughter nucleus has fewer neutrons than the original nucleus.
A) The daughter nucleus has more protons than the original nucleus.
B) The daughter nucleus has more neutrons than the original nucleus.
C) The daughter nucleus has the same number of nucleons as the original nucleus.
D) The daughter nucleus has fewer protons than the original nucleus.
E) The daughter nucleus has fewer neutrons than the original nucleus.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
8
Radioactive decay: The half-life of cobalt-60 is 5.3 years, while that of strontium-90 is 28 years. Suppose that samples of cobalt-60 and strontium-90 are such that they initially have the same activity (number of decays per second). What is true about the initial numbers of cobalt-60 and strontium-90 nuclei in these samples?
A) There are more strontium-90 than cobalt-60 nuclei.
B) There are equal numbers of cobalt-60 and strontium-90 nuclei.
C) There are more cobalt-60 than strontium-90 nuclei.
D) It is not possible to compare numbers of nuclei without knowing the masses of the samples.
A) There are more strontium-90 than cobalt-60 nuclei.
B) There are equal numbers of cobalt-60 and strontium-90 nuclei.
C) There are more cobalt-60 than strontium-90 nuclei.
D) It is not possible to compare numbers of nuclei without knowing the masses of the samples.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
9
Properties of the nucleus: Which of the following statements about the atomic nucleus is correct? (There may be more than one correct choice.)
A) Large nuclei are denser than light nuclei.
B) All nuclei have nearly the same density.
C) The nucleus is held together more by the electrical force than by the gravitational force.
D) A nucleus containing 20 nucleons will have approximately twice the radius as a nucleus containing 10 nucleons.
E) As the number of nucleons increases the binding energy per nucleon always increases.
A) Large nuclei are denser than light nuclei.
B) All nuclei have nearly the same density.
C) The nucleus is held together more by the electrical force than by the gravitational force.
D) A nucleus containing 20 nucleons will have approximately twice the radius as a nucleus containing 10 nucleons.
E) As the number of nucleons increases the binding energy per nucleon always increases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
10
Radioactive decay: The half-life of cobalt-60 is 5.3 years, while that of strontium-90 is 28 years. Suppose you have a sample of each, such that they initially contain equal numbers of atoms of these nuclides. How will the activities (number of decays per second) of the samples compare?
A) The activity of the cobalt-60 sample will be greater.
B) The activities cannot be compared without more information.
C) The activities will be equal.
D) The activity of the strontium-90 sample will be greater.
A) The activity of the cobalt-60 sample will be greater.
B) The activities cannot be compared without more information.
C) The activities will be equal.
D) The activity of the strontium-90 sample will be greater.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
11
Radioactivity: If a nucleus decays by gamma decay to a daughter nucleus, which of the following statements about this decay are correct? (There may be more than one correct choice.)
A) The daughter nucleus has more protons than the original nucleus.
B) The daughter nucleus has more neutrons than the original nucleus.
C) The daughter nucleus has the same number of nucleons as the original nucleus.
D) The daughter nucleus has fewer protons than the original nucleus.
E) The daughter nucleus has fewer neutrons than the original nucleus.
A) The daughter nucleus has more protons than the original nucleus.
B) The daughter nucleus has more neutrons than the original nucleus.
C) The daughter nucleus has the same number of nucleons as the original nucleus.
D) The daughter nucleus has fewer protons than the original nucleus.
E) The daughter nucleus has fewer neutrons than the original nucleus.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
12
Properties of the nucleus: For a 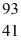 Nb atom, the number of protons, neutrons, and electrons in the atom is
Nb atom, the number of protons, neutrons, and electrons in the atom is
A) 41, 52, 93.
B) 41, 52, 52.
C) 41, 52, 41.
D) 41, 52, 0.
E) 52, 41, 0.
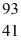 Nb atom, the number of protons, neutrons, and electrons in the atom is
Nb atom, the number of protons, neutrons, and electrons in the atom isA) 41, 52, 93.
B) 41, 52, 52.
C) 41, 52, 41.
D) 41, 52, 0.
E) 52, 41, 0.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
13
Radioactivity: Which of the following statements about β+ decay are correct? (There may be more than one correct choice.) During β+ decay
A) an orbital electron is captured by the nucleus.
B) a proton is emitted from the nucleus.
C) a neutron in the nucleus decays to a proton and an electron.
D) a proton in the nucleus decays to a positron and a neutron.
E) the atomic number Z of the isotope increases by one unit but the atomic weight A remains unchanged.
A) an orbital electron is captured by the nucleus.
B) a proton is emitted from the nucleus.
C) a neutron in the nucleus decays to a proton and an electron.
D) a proton in the nucleus decays to a positron and a neutron.
E) the atomic number Z of the isotope increases by one unit but the atomic weight A remains unchanged.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
14
Radioactive decay: A radioactive nuclide of atomic number Z emits an electron, then the daughter nuclide emits a gamma ray. What is the atomic number of the resulting nuclide after both processes?
A) Z + 1
B) Z - 1
C) Z - 2
D) Z - 3
E) Z + 2
A) Z + 1
B) Z - 1
C) Z - 2
D) Z - 3
E) Z + 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
15
Nuclear binding energy: The iron nucleus has the greatest binding energy of any nucleus.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
16
Radioactive decay: A radioisotope has a half-life of τ at a temperature of 150 K. If its temperature is increased to 300 K, what will its half-life be?
A) 4τ
B) 2 τ
C) τ
D) τ/2
E) τ/4
A) 4τ
B) 2 τ
C) τ
D) τ/2
E) τ/4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
17
Radioactivity: If a nucleus decays by alpha decay to a daughter nucleus, which of the following statements about this decay are correct? (There may be more than one correct choice.)
A) The daughter nucleus has more protons than the original nucleus.
B) The daughter nucleus has more neutrons than the original nucleus.
C) The daughter nucleus has the same number of nucleons as the original nucleus.
D) The daughter nucleus has fewer protons than the original nucleus.
E) The daughter nucleus has fewer neutrons than the original nucleus.
A) The daughter nucleus has more protons than the original nucleus.
B) The daughter nucleus has more neutrons than the original nucleus.
C) The daughter nucleus has the same number of nucleons as the original nucleus.
D) The daughter nucleus has fewer protons than the original nucleus.
E) The daughter nucleus has fewer neutrons than the original nucleus.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
18
Nuclear binding energy: Heavier stable nuclei tend to have
A) half as many protons as neutrons.
B) the same number of neutrons and protons.
C) more neutrons than protons.
D) no clear trend in the relative number of neutrons and protons.
E) more protons than neutrons.
A) half as many protons as neutrons.
B) the same number of neutrons and protons.
C) more neutrons than protons.
D) no clear trend in the relative number of neutrons and protons.
E) more protons than neutrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
19
Properties of the nucleus: Which of the following statements about the strong nuclear force are correct? (There may be more than one correct choice.)
A) It acts equally on protons and neutrons but not on electrons.
B) It acts equally on protons, neutrons, and electrons.
C) It has a much longer range than the electric force.
D) It keeps electrons in their orbits around the nucleus.
E) Because of its very short range, there is a limit to how large the nucleus can be.
A) It acts equally on protons and neutrons but not on electrons.
B) It acts equally on protons, neutrons, and electrons.
C) It has a much longer range than the electric force.
D) It keeps electrons in their orbits around the nucleus.
E) Because of its very short range, there is a limit to how large the nucleus can be.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
20
Radioactive decay: A radioactive isotope decays by β- emission with a half-life of 1.0 min. During the first 1.0 min, a particular sample emits 1000 β- particles. During the next 1.0 min, the number of β- particles this sample will emit will be closest to
A) 250.
B) 500.
C) 1000.
D) 1500.
E) 2000.
A) 250.
B) 500.
C) 1000.
D) 1500.
E) 2000.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
21
Nuclear binding energy: Uranium-238 decays into thorium-234 plus an alpha particle. How much energy is released in this process? 1 u = 931.494 MeV/c2, and the relevant mass values are 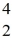 He: 4.002603 u
He: 4.002603 u 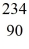 Th: 234.043583 u
Th: 234.043583 u 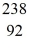 U: 238.050786 u
U: 238.050786 u
A) 4.28 MeV
B) 3.76 MeV
C) 3.18 MeV
D) 2.89 MeV
E) 5.05 MeV
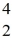 He: 4.002603 u
He: 4.002603 u 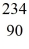 Th: 234.043583 u
Th: 234.043583 u 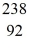 U: 238.050786 u
U: 238.050786 uA) 4.28 MeV
B) 3.76 MeV
C) 3.18 MeV
D) 2.89 MeV
E) 5.05 MeV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
22
Nuclear fusion: A fusion reaction releases energy because the binding energy of the resulting nucleus
A) is greater than the binding energy of the original nuclei.
B) is equal to the binding energy of the original nuclei.
C) is less than the binding energy of the original nuclei.
D) is released in the process.
E) is absorbed in the process.
A) is greater than the binding energy of the original nuclei.
B) is equal to the binding energy of the original nuclei.
C) is less than the binding energy of the original nuclei.
D) is released in the process.
E) is absorbed in the process.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
23
Nuclear fusion: The primary source of the energy radiated by a star, such as the sun, is
A) beta decay.
B) alpha decay.
C) fission reactions involving uranium.
D) fusion reactions in which hydrogen is fused to form helium.
E) fusion reactions in which helium is fused to form iron.
A) beta decay.
B) alpha decay.
C) fission reactions involving uranium.
D) fusion reactions in which hydrogen is fused to form helium.
E) fusion reactions in which helium is fused to form iron.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
24
Nuclear binding energy: The neutral deuterium atom,  H, has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. What is the binding energy of the
H, has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. What is the binding energy of the 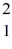 H nucleus? (1 u = 931.494 MeV/c2)
H nucleus? (1 u = 931.494 MeV/c2)
A) 1.1 MeV
B) 1.7 MeV
C) 2.2 MeV
D) 2.9 MeV
E) 3.4 MeV
 H, has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. What is the binding energy of the
H, has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. What is the binding energy of the 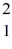 H nucleus? (1 u = 931.494 MeV/c2)
H nucleus? (1 u = 931.494 MeV/c2)A) 1.1 MeV
B) 1.7 MeV
C) 2.2 MeV
D) 2.9 MeV
E) 3.4 MeV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
25
Nuclear binding energy: The carbon in your body was formed in nuclear reactions in long-dead stars. How much energy was released when three 4He nuclei combined to make 12C? The mass of 4He is 4.002603 u, the mass of 12C is 12.0000 u, and 1 u = 931.494 MeV/c2.
A) 7.274 MeV
B) 3716 MeV
C) 8.424 MeV
D) 2.106 MeV
A) 7.274 MeV
B) 3716 MeV
C) 8.424 MeV
D) 2.106 MeV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
26
Nuclear binding energy: The set of nuclear reactions that power our sun can be summarized a 4p+ → 4He+2 + 2e+. The masses of the particles involved are 938.272 MeV/c2 (proton, p+), 3727.38 MeV/c2 (alpha particle, 4He+2), and 0.511 MeV/c2 (positron, e+). How much energy is released by each set of these reactions?
A) 24.69 MeV
B) 28.3 MeV
C) 2790 MeV
D) 279 MeV
A) 24.69 MeV
B) 28.3 MeV
C) 2790 MeV
D) 279 MeV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
27
Nuclear binding energy: A stationary plutonium-239 nucleus decays into a uranium-235 nucleus plus an alpha particle. The energy released in the process is 5.24 MeV. Given the following mass values 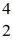 He: 4.002603 u
He: 4.002603 u 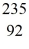 U: 235.043924 u what is the kinetic energy of the
U: 235.043924 u what is the kinetic energy of the  U nucleus? (1 u =931.494 MeV/c2)
U nucleus? (1 u =931.494 MeV/c2)
A) 0.0829 MeV
B) 0.0837 MeV
C) 0.0852 MeV
D) 0.0863 MeV
E) 0.0877 MeV
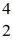 He: 4.002603 u
He: 4.002603 u 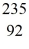 U: 235.043924 u what is the kinetic energy of the
U: 235.043924 u what is the kinetic energy of the  U nucleus? (1 u =931.494 MeV/c2)
U nucleus? (1 u =931.494 MeV/c2)A) 0.0829 MeV
B) 0.0837 MeV
C) 0.0852 MeV
D) 0.0863 MeV
E) 0.0877 MeV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
28
Nuclear fusion: How does the mass of the products of a nuclear fusion reaction compare to the mass of the original elements?
A) The mass of the products is greater than the mass of the original elements.
B) The mass of the products is less than the mass of the original elements.
C) The mass of the products is equal to the mass of the original elements.
D) The mass of the products is unrelated to the mass of the original elements.
A) The mass of the products is greater than the mass of the original elements.
B) The mass of the products is less than the mass of the original elements.
C) The mass of the products is equal to the mass of the original elements.
D) The mass of the products is unrelated to the mass of the original elements.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
29
Properties of the nucleus: A certain nucleus containing 8 protons and 7 neutrons has a density ρ. Which of the following values would be closest to the expected value of the density of a nucleus having 51 protons and 69 neutrons?
A) 1.00 ρ
B) 1.85 ρ
C) 2.00 ρ
D) 2.14 ρ
E) 8.00 ρ
A) 1.00 ρ
B) 1.85 ρ
C) 2.00 ρ
D) 2.14 ρ
E) 8.00 ρ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
30
Nuclear binding energy: The following masses are known:  n (neutron) 1.008665 u
n (neutron) 1.008665 u  H 1.007825 u
H 1.007825 u  Fe 56.935399 u What is the binding energy of
Fe 56.935399 u What is the binding energy of  Fe, in MeV? (1 u = 1.6605 × 10-27 kg = 931.5 MeV/c2)
Fe, in MeV? (1 u = 1.6605 × 10-27 kg = 931.5 MeV/c2)
A) 500 MeV
B) 550 MeV
C) 610 MeV
D) 660 MeV
E) 710 MeV
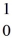 n (neutron) 1.008665 u
n (neutron) 1.008665 u 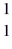 H 1.007825 u
H 1.007825 u  Fe 56.935399 u What is the binding energy of
Fe 56.935399 u What is the binding energy of  Fe, in MeV? (1 u = 1.6605 × 10-27 kg = 931.5 MeV/c2)
Fe, in MeV? (1 u = 1.6605 × 10-27 kg = 931.5 MeV/c2)A) 500 MeV
B) 550 MeV
C) 610 MeV
D) 660 MeV
E) 710 MeV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
31
Nuclear binding energy: Radium-226 decays into radon-222 plus an alpha particle. How much energy is released in this process? 1 u = 931.494 MeV/c2, and the relevant mass values are 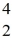 He: 4.002603 u
He: 4.002603 u 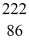 Rn: 222.017570 u
Rn: 222.017570 u  Ra: 226.025402 u
Ra: 226.025402 u
A) 4.24 MeV
B) 3.76 MeV
C) 4.87 MeV
D) 5.05 MeV
E) 5.39 MeV
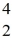 He: 4.002603 u
He: 4.002603 u  Rn: 222.017570 u
Rn: 222.017570 u  Ra: 226.025402 u
Ra: 226.025402 uA) 4.24 MeV
B) 3.76 MeV
C) 4.87 MeV
D) 5.05 MeV
E) 5.39 MeV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
32
Properties of the nucleus: What would be the expected radius of a nucleus having 82 protons and 125 neutrons?
A) 5.2 fm
B) 5.9 fm
C) 6.0 fm
D) 7.1 fm
E) 17 fm
A) 5.2 fm
B) 5.9 fm
C) 6.0 fm
D) 7.1 fm
E) 17 fm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
33
Nuclear fusion: In massive stars, three helium nuclei fuse together, forming a carbon nucleus. This reaction heats the core of the star. The net mass of the three helium nuclei must therefore be
A) higher than that of the carbon nucleus.
B) less than that of the carbon nucleus.
C) the same as that of the carbon nucleus since mass is always conserved.
D) the same as that of the carbon nucleus since energy is always conserved.
A) higher than that of the carbon nucleus.
B) less than that of the carbon nucleus.
C) the same as that of the carbon nucleus since mass is always conserved.
D) the same as that of the carbon nucleus since energy is always conserved.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
34
Nuclear binding energy: What is the binding energy per nucleon for 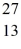 Al? The neutral
Al? The neutral 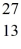 Al atom has a mass of 26.981539 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. (1 u = 931.494 MeV/c2)
Al atom has a mass of 26.981539 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. (1 u = 931.494 MeV/c2)
A) 8.3 MeV
B) 6.7 MeV
C) 5.4 MeV
D) 3.4 MeV
E) 2.8 MeV
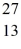 Al? The neutral
Al? The neutral 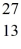 Al atom has a mass of 26.981539 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. (1 u = 931.494 MeV/c2)
Al atom has a mass of 26.981539 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. (1 u = 931.494 MeV/c2)A) 8.3 MeV
B) 6.7 MeV
C) 5.4 MeV
D) 3.4 MeV
E) 2.8 MeV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
35
Properties of the nucleus: If a nucleus had a diameter of 8.0 fm, what would be its expected mass, in atomic mass units?
A) 7 u
B) 296 u
C) 37 u
D) 64 u
E) 128 u
A) 7 u
B) 296 u
C) 37 u
D) 64 u
E) 128 u

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
36
Properties of the nucleus: What would be the expected radius of the nucleus of 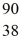 Sr?
Sr?
A) 4.0 fm
B) 1.2 fm
C) 5.4 fm
D) 0.11 pm
E) 0.54 pm
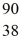 Sr?
Sr?A) 4.0 fm
B) 1.2 fm
C) 5.4 fm
D) 0.11 pm
E) 0.54 pm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
37
Properties of the nucleus: Two identical nuclei of mass 18 u are made to unite to make a single nucleus of mass 36 u. What is the radius of the result of this fusion?
A) 4.0 fm
B) 6.3 fm
C) 4.5 fm
D) 7.2 fm
A) 4.0 fm
B) 6.3 fm
C) 4.5 fm
D) 7.2 fm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
38
Nuclear binding energy: Plutonium-239 decays into uranium-235 plus an alpha particle. The energy released in the process is 5.24 MeV. Given the following mass values 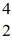 He: 4.002603 u
He: 4.002603 u 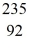 U: 235.043924 u what is the mass of
U: 235.043924 u what is the mass of  Pu in atomic mass units? (1 u = 931.494 MeV/c2)
Pu in atomic mass units? (1 u = 931.494 MeV/c2)
A) 239.05215 u
B) 239.02775 u
C) 239.00189 u
D) 238.99919 u
E) 238.98884 u
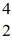 He: 4.002603 u
He: 4.002603 u 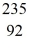 U: 235.043924 u what is the mass of
U: 235.043924 u what is the mass of  Pu in atomic mass units? (1 u = 931.494 MeV/c2)
Pu in atomic mass units? (1 u = 931.494 MeV/c2)A) 239.05215 u
B) 239.02775 u
C) 239.00189 u
D) 238.99919 u
E) 238.98884 u

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
39
Properties of the nucleus: A certain nucleus containing 8 protons and 7 neutrons has a radius R. Which of the following values would be closest to the expected value of the radius of a nucleus having 51 protons and 69 neutrons?
A) 1.85R
B) 2.00R
C) 2.14R
D) 6.38R
E) 8.00R
A) 1.85R
B) 2.00R
C) 2.14R
D) 6.38R
E) 8.00R

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
40
Nuclear binding energy: How much energy is released when 1.40 μg of 3H have decayed to 3He? The mass of 3He is 3.016029 u, the mass of 3H is 3.016049 u, and 1 u = 931.494 MeV/c2.
A) 830 J
B) 11,900 J
C) 7970 J
D) 71,700 J
E) 23,900 J
A) 830 J
B) 11,900 J
C) 7970 J
D) 71,700 J
E) 23,900 J

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
41
Radioactivity: The stability of 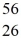 Fe with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known:
Fe with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known: 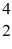 He: 4.002603 u
He: 4.002603 u 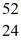 Cr: 51.944768 u
Cr: 51.944768 u 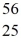 Mn: 55.938907 u
Mn: 55.938907 u 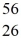 Fe: 55.934939 u
Fe: 55.934939 u 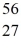 Co: 55.939841 u The
Co: 55.939841 u The 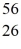 Fe nuclide is
Fe nuclide is
A) not subject to alpha, β+, or β- decay.
B) subject to alpha decay only.
C) subject to β+decay only.
D) subject to β- decay only.
E) subject to β+ or β- decay, but not to alpha decay.
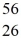 Fe with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known:
Fe with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known: 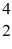 He: 4.002603 u
He: 4.002603 u 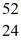 Cr: 51.944768 u
Cr: 51.944768 u 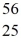 Mn: 55.938907 u
Mn: 55.938907 u 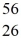 Fe: 55.934939 u
Fe: 55.934939 u 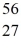 Co: 55.939841 u The
Co: 55.939841 u The 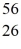 Fe nuclide is
Fe nuclide isA) not subject to alpha, β+, or β- decay.
B) subject to alpha decay only.
C) subject to β+decay only.
D) subject to β- decay only.
E) subject to β+ or β- decay, but not to alpha decay.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
42
Radioactivity: The stability of 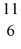 C with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known:
C with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known: 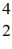 He: 4.002603 u
He: 4.002603 u 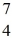 Be: 7.016928 u
Be: 7.016928 u 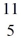 B: 11.009305 u
B: 11.009305 u 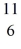 C: 11.011433 u
C: 11.011433 u 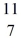 N: 11.026742 u The
N: 11.026742 u The 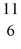 C nuclide is
C nuclide is
A) not subject to alpha, β+, or β- decay.
B) subject to alpha decay only.
C) subject to β+ decay only.
D) subject to β- decay only.
E) subject to β+ or β- decay, but not to alpha decay.
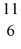 C with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known:
C with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known: 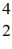 He: 4.002603 u
He: 4.002603 u 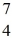 Be: 7.016928 u
Be: 7.016928 u 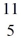 B: 11.009305 u
B: 11.009305 u 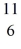 C: 11.011433 u
C: 11.011433 u 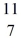 N: 11.026742 u The
N: 11.026742 u The 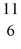 C nuclide is
C nuclide isA) not subject to alpha, β+, or β- decay.
B) subject to alpha decay only.
C) subject to β+ decay only.
D) subject to β- decay only.
E) subject to β+ or β- decay, but not to alpha decay.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
43
Radioactive decay: Fermium-253 has a half-life of 3.00 d. A sample of fermium contains 7.37 × 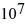 nuclei. How long will it take for there to be only 3.36 ×
nuclei. How long will it take for there to be only 3.36 × 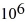 fermium nuclei in this sample?
fermium nuclei in this sample?
A) 2.75 d
B) 9.80 d
C) 13.4 d
D) 15.7 d
E) 58.6 d
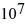 nuclei. How long will it take for there to be only 3.36 ×
nuclei. How long will it take for there to be only 3.36 × 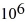 fermium nuclei in this sample?
fermium nuclei in this sample?A) 2.75 d
B) 9.80 d
C) 13.4 d
D) 15.7 d
E) 58.6 d

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
44
Radioactive decay: How many days are required for a radioactive sample, with a half-life of 5.7 d and an initial activity of 1.07 × 105 Bq, to decay to an activity of 100 Bq?
A) 57 d
B) 46 d
C) 68 d
D) 39 d
A) 57 d
B) 46 d
C) 68 d
D) 39 d

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
45
Radioactivity: A radioactive atom has 98 protons and 249 nucleons. If it undergoes alpha decay, what are the number of protons and nucleons, respectively, in the daughter nucleus?
A) 100, 245
B) 94, 247
C) 96, 245
D) 96, 247
E) 100, 249
A) 100, 245
B) 94, 247
C) 96, 245
D) 96, 247
E) 100, 249

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
46
Radioactive decay: Rutherfordium-261 has a half-life of 1.08 min. How long will it take for a sample of rutherfordium to lose one-third of its nuclei?
A) 1.02 min
B) 1.62 min
C) 0.632 min
D) 2.70 min
E) 3.24 min
A) 1.02 min
B) 1.62 min
C) 0.632 min
D) 2.70 min
E) 3.24 min

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
47
Radioactive decay: The material used in certain nuclear bombs is 239Pu, which has a half-life of about 20,000 years. How long must we wait for a buried stockpile of this substance to decay to 4.0% of its original 239Pu mass?
A) 93,000 y
B) 64,000 y
C) 45,000 y
D) 800 y
A) 93,000 y
B) 64,000 y
C) 45,000 y
D) 800 y

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
48
Radioactive decay: An air sample is contaminated with 15O, which has a half-life of 2.03 min. One possible way to minimize its hazard is to pass it through a long pipe to allow it to decay inside the pipe until it can be safely released into the atmosphere. If the oxygen moves at a speed of 1.1 m/s in the pipe, how long must the pipe be for the sample to have decayed to 3.0% of its original activity just as it leaves the pipe?
A) 680 m
B) 8.0 m
C) 7.0 m
D) 2.0 m
A) 680 m
B) 8.0 m
C) 7.0 m
D) 2.0 m

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
49
Radioactivity: A sphere made of a radioactive isotope initially has a mass of 6.88 kg. The half-life of this isotope is 1.34 h, and it decays by β- emission. At the end of 2.68 h, what is the mass of this sphere?
A) 6.88 kg
B) 3.44 kg
C) 1.72 kg
D) 2.53 kg
A) 6.88 kg
B) 3.44 kg
C) 1.72 kg
D) 2.53 kg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
50
Radioactive decay: In a laboratory accident a work area is contaminated with radioactive material. Health physicists monitor the area during a 30-day period and, after correcting for the background rate, obtain the data shown in the table.  The accident occurred at t = 0. They determine that it will not be safe for workers to enter the area until the radioactivity level has dropped to 133 counts per minute. Of the choices listed below, which one is the earliest time that workers could safely return
The accident occurred at t = 0. They determine that it will not be safe for workers to enter the area until the radioactivity level has dropped to 133 counts per minute. Of the choices listed below, which one is the earliest time that workers could safely return
A) 38 days
B) 44 days
C) 50 days
D) 32 days
E) 24 days
 The accident occurred at t = 0. They determine that it will not be safe for workers to enter the area until the radioactivity level has dropped to 133 counts per minute. Of the choices listed below, which one is the earliest time that workers could safely return
The accident occurred at t = 0. They determine that it will not be safe for workers to enter the area until the radioactivity level has dropped to 133 counts per minute. Of the choices listed below, which one is the earliest time that workers could safely returnA) 38 days
B) 44 days
C) 50 days
D) 32 days
E) 24 days

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
51
Radioactive decay: A radioactive sample has a half-life of 10 min. What fraction of the sample is left after 40 min?
A) 1/2
B) 1/4
C) 1/8
D) 1/16
E) 1/32
A) 1/2
B) 1/4
C) 1/8
D) 1/16
E) 1/32

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
52
Radioactive decay: A certain substance has a half-life of 5.0 hours. How many nuclei of the substance are required to give an initial activity of 6.0 μCi? 1 Ci = 3.7 × 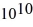 Bq.
Bq.
A) 5.8 ×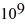
B) 8.5 ×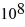
C) 6.3 ×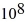
D) 3.2 ×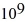
E) 2.4 ×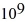
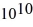 Bq.
Bq.A) 5.8 ×
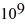
B) 8.5 ×
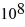
C) 6.3 ×
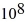
D) 3.2 ×
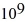
E) 2.4 ×
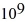

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
53
Radioactive decay: What mass of 14C (having a half-life of 5730 years) do you need to provide a decay rate of 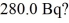 (1 u = 1.6605 × 10-27 kg)
(1 u = 1.6605 × 10-27 kg)
A) 1.70 × 10-12 kg
B) 5.38 × 10-19 kg
C) 3.84 × 10-20 kg
D) 8.68 × 10-13 kg
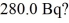 (1 u = 1.6605 × 10-27 kg)
(1 u = 1.6605 × 10-27 kg)A) 1.70 × 10-12 kg
B) 5.38 × 10-19 kg
C) 3.84 × 10-20 kg
D) 8.68 × 10-13 kg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
54
Radioactive decay: An isotope of Tc having a half-life of 6.0 h is used in bone scans. If a certain amount of this Tc is injected into the body, how long does it take for its initial decay rate to decrease BY 99%?
A) (0.060 h)
B) (3.3 h)
C) (33 h)
D) (40 h)
E) (slightly more than a month)
A) (0.060 h)
B) (3.3 h)
C) (33 h)
D) (40 h)
E) (slightly more than a month)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
55
Radioactive dating: Carbon-14 has a half-life of 5730 y. A sample of wood has been recovered by an archaeologist. The sample is sent to a laboratory, where it is determined that the activity of the sample is 0.144 Bq/g. By comparing this activity with the activity of living organic matter, 0.230 Bq/g, the scientist determines how old the wood sample is, or more precisely, when the tree that the sample came from died. How old is the sample of wood?
A) 3870 y
B) 4250 y
C) 4590 y
D) 2630 y
E) 2940 y
A) 3870 y
B) 4250 y
C) 4590 y
D) 2630 y
E) 2940 y

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
56
Radioactive decay: A hospital patient has been given some 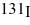 (half-life = 8.04 d) which decays at 4.2 times the acceptable level for exposure to the general public. How long must the patient wait for the decay rate to reach the acceptable level? Assume that the material merely decays and is not excreted by the body.
(half-life = 8.04 d) which decays at 4.2 times the acceptable level for exposure to the general public. How long must the patient wait for the decay rate to reach the acceptable level? Assume that the material merely decays and is not excreted by the body.
A) 17 d
B) 12 d
C) 8.0 d
D) 7.2 d
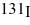 (half-life = 8.04 d) which decays at 4.2 times the acceptable level for exposure to the general public. How long must the patient wait for the decay rate to reach the acceptable level? Assume that the material merely decays and is not excreted by the body.
(half-life = 8.04 d) which decays at 4.2 times the acceptable level for exposure to the general public. How long must the patient wait for the decay rate to reach the acceptable level? Assume that the material merely decays and is not excreted by the body.A) 17 d
B) 12 d
C) 8.0 d
D) 7.2 d

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
57
Radioactivity: The stability of 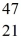 Sc with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known:
Sc with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known: 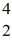 He: 4.002603 u
He: 4.002603 u 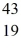 K: 42.960717 u
K: 42.960717 u 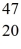 Ca: 46.954543 u
Ca: 46.954543 u 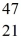 Sc 46.952409 u
Sc 46.952409 u 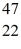 Ti: 46.951764 u The
Ti: 46.951764 u The 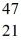 Sc nuclide is
Sc nuclide is
A) not subject to alpha, β+, or β- decay.
B) subject to alpha decay only.
C) subject to β+ decay only.
D) subject to β- decay only.
E) subject to β+ or β- decay, but not to alpha decay.
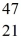 Sc with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known:
Sc with respect to alpha, β+, and β- decay is to be determined. Do not consider the possibility of decay by electron capture. The following atomic masses are known: 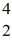 He: 4.002603 u
He: 4.002603 u 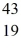 K: 42.960717 u
K: 42.960717 u 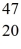 Ca: 46.954543 u
Ca: 46.954543 u 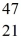 Sc 46.952409 u
Sc 46.952409 u 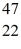 Ti: 46.951764 u The
Ti: 46.951764 u The 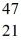 Sc nuclide is
Sc nuclide isA) not subject to alpha, β+, or β- decay.
B) subject to alpha decay only.
C) subject to β+ decay only.
D) subject to β- decay only.
E) subject to β+ or β- decay, but not to alpha decay.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
58
Radioactive decay: The unstable isotope 234Th decays by β emission with a half-life of 24.5 days. The initial decay rate of the sample was 9.9 × 1013 Bq. (1 u = 1.6605 × 10-27 kg)
(a) What mass of 234Th was initially present?
(b) What is the decay rate after 68 days?
(a) What mass of 234Th was initially present?
(b) What is the decay rate after 68 days?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
59
Radioactivity: Scandium, 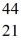 Sc, decays by emitting a positron. What is the nuclide that is the product of the decay?
Sc, decays by emitting a positron. What is the nuclide that is the product of the decay?
A)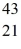 Sc
Sc
B)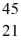 Sc
Sc
C)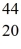 Ca
Ca
D)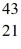 Ca
Ca
E)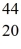 Sc
Sc
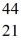 Sc, decays by emitting a positron. What is the nuclide that is the product of the decay?
Sc, decays by emitting a positron. What is the nuclide that is the product of the decay?A)
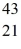 Sc
ScB)
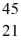 Sc
ScC)
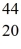 Ca
CaD)
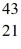 Ca
CaE)
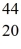 Sc
Sc
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
60
Radioactive dating: The radioactivity due to carbon-14 measured in a piece of a wood from an ancient site was found to produce 20 counts per minute from a given sample, whereas the same amount of carbon from a piece of living wood produced 160 counts per minute. The half-life of carbon-14, a beta emitter, is 5730 y. The age of the artifact is closest to
A) 5700 y.
B) 12,000 y.
C) 15,000 y.
D) 17,000 y.
E) 23,000 y.
A) 5700 y.
B) 12,000 y.
C) 15,000 y.
D) 17,000 y.
E) 23,000 y.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
61
Nuclear reactions: In the nuclear reaction
n +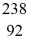 U → X + 2e-
U → X + 2e-
n is a neutron and e- is an electron, and the neutrinos have not been shown. Determine the atomic mass and atomic number of the missing nuclear product X, and write X in the standard form. It is NOT necessary to identify which atom X is.
n +
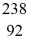 U → X + 2e-
U → X + 2e-n is a neutron and e- is an electron, and the neutrinos have not been shown. Determine the atomic mass and atomic number of the missing nuclear product X, and write X in the standard form. It is NOT necessary to identify which atom X is.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
62
Radioactive dating: An ancient rock is found to contain 40Ar gas, indicating that 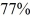 of the 40K in the rock has decayed since the rock solidified. Any argon would have boiled out of liquid rock. The half-life of 40K is 1.25 billion years. How long ago did the rock solidify?
of the 40K in the rock has decayed since the rock solidified. Any argon would have boiled out of liquid rock. The half-life of 40K is 1.25 billion years. How long ago did the rock solidify?
A) 2.6 billion years
B) 0.50 billion years
C) 1.8 billion years
D) 0.30 billion years
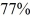 of the 40K in the rock has decayed since the rock solidified. Any argon would have boiled out of liquid rock. The half-life of 40K is 1.25 billion years. How long ago did the rock solidify?
of the 40K in the rock has decayed since the rock solidified. Any argon would have boiled out of liquid rock. The half-life of 40K is 1.25 billion years. How long ago did the rock solidify?A) 2.6 billion years
B) 0.50 billion years
C) 1.8 billion years
D) 0.30 billion years

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
63
Nuclear fission: How much energy is released in the total fission of 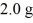 of
of 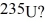 The average energy per fission is 200.0 MeV. (1 u = 931.5 MeV/c2 = 1.6605 × 10-27 kg, 1 eV = 1.60 × 10-19 J)
The average energy per fission is 200.0 MeV. (1 u = 931.5 MeV/c2 = 1.6605 × 10-27 kg, 1 eV = 1.60 × 10-19 J)
A) 1.6 × 1011 J
B) 3.9 × 1013 J
C) 1.6 × 105 J
D) 3.9 × 1010 J
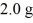 of
of 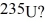 The average energy per fission is 200.0 MeV. (1 u = 931.5 MeV/c2 = 1.6605 × 10-27 kg, 1 eV = 1.60 × 10-19 J)
The average energy per fission is 200.0 MeV. (1 u = 931.5 MeV/c2 = 1.6605 × 10-27 kg, 1 eV = 1.60 × 10-19 J)A) 1.6 × 1011 J
B) 3.9 × 1013 J
C) 1.6 × 105 J
D) 3.9 × 1010 J

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
64
Radioactive dating: Living matter has 1.3 × 10-10 % of its carbon in the form of 14C which has a half-life of 5730 y. A mammoth bone has a 300-g sample of carbon separated from it, and the sample is found to have an activity of 20 decays per second. How old is the bone?
A) 15,000 y
B) 10,900 y
C) 11,500 y
D) 7600 y
E) 6400 y
A) 15,000 y
B) 10,900 y
C) 11,500 y
D) 7600 y
E) 6400 y

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
65
Nuclear reactions: For the missing product X in the reaction
neutron +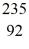 U →
U → 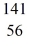 Ba + X + 3 neutrons
Ba + X + 3 neutrons
determine the atomic mass and atomic number of X, and write X in the standard form. It is NOT necessary to identify which atom X is.
neutron +
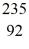 U →
U → 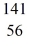 Ba + X + 3 neutrons
Ba + X + 3 neutronsdetermine the atomic mass and atomic number of X, and write X in the standard form. It is NOT necessary to identify which atom X is.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
66
Nuclear fission: In the fission reaction 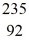 U + neutron →
U + neutron → 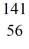 Ba +
Ba + 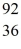 Kr + x neutrons, what is the number x of neutrons produced?
Kr + x neutrons, what is the number x of neutrons produced?
A) 0
B) 4
C) 1
D) 3
E) 2
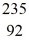 U + neutron →
U + neutron → 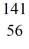 Ba +
Ba + 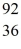 Kr + x neutrons, what is the number x of neutrons produced?
Kr + x neutrons, what is the number x of neutrons produced?A) 0
B) 4
C) 1
D) 3
E) 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
67
Nuclear fission: If a 2.0-MeV neutron released in a fission reaction loses half of its energy in each moderator collision, how many collisions are needed to reduce its energy to (1/25) eV?
A) 6
B) 18
C) 26
D) 30
E) 4
A) 6
B) 18
C) 26
D) 30
E) 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
68
Nuclear fission: An excited 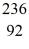 U* nucleus undergoes fission into two fragments, as shown:
U* nucleus undergoes fission into two fragments, as shown: 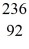 U* →
U* → 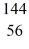 Ba +
Ba + 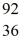 Kr The following atomic masses are known:
Kr The following atomic masses are known: 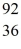 Kr: 91.926270 u
Kr: 91.926270 u 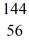 Ba: 143.922845 u
Ba: 143.922845 u 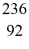 U*: 236.045563 u
U*: 236.045563 u
What is the reaction energy, in MeV, for this process? (1 u = 1.6605 × 10-27 kg = 931.5 MeV/c2)
A) 150 MeV
B) 160 MeV
C) 170 MeV
D) 180 MeV
E) 190 MeV
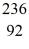 U* nucleus undergoes fission into two fragments, as shown:
U* nucleus undergoes fission into two fragments, as shown: 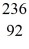 U* →
U* → 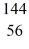 Ba +
Ba + 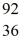 Kr The following atomic masses are known:
Kr The following atomic masses are known: 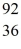 Kr: 91.926270 u
Kr: 91.926270 u 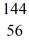 Ba: 143.922845 u
Ba: 143.922845 u 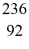 U*: 236.045563 u
U*: 236.045563 uWhat is the reaction energy, in MeV, for this process? (1 u = 1.6605 × 10-27 kg = 931.5 MeV/c2)
A) 150 MeV
B) 160 MeV
C) 170 MeV
D) 180 MeV
E) 190 MeV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
69
Reaction energy: Find the reaction energy (Q value) of the reaction 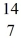 N +
N + 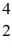 He →
He → 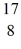 O +
O + 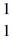 H, given the following masses:
H, given the following masses: 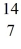 N: 14.003074 u
N: 14.003074 u 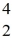 He: 4.002603 u
He: 4.002603 u 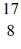 O : 16.999131 u
O : 16.999131 u 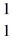 H: 1.007825 u (1 u = 931.5 MeV/c2)
H: 1.007825 u (1 u = 931.5 MeV/c2)
A) -1.191 MeV
B) -2.030 MeV
C) -3.241 MeV
D) -6.724 MeV
E) -9.055 MeV
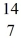 N +
N + 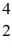 He →
He → 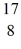 O +
O + 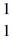 H, given the following masses:
H, given the following masses: 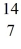 N: 14.003074 u
N: 14.003074 u 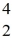 He: 4.002603 u
He: 4.002603 u 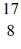 O : 16.999131 u
O : 16.999131 u 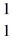 H: 1.007825 u (1 u = 931.5 MeV/c2)
H: 1.007825 u (1 u = 931.5 MeV/c2)A) -1.191 MeV
B) -2.030 MeV
C) -3.241 MeV
D) -6.724 MeV
E) -9.055 MeV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
70
Nuclear reactions: For the missing product X in the nuclear reaction
neutron +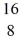 O → X + alpha particle
O → X + alpha particle
determine the atomic mass and atomic number of X, and write X in the standard form. It is NOT necessary to identify which atom X is.
neutron +
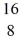 O → X + alpha particle
O → X + alpha particledetermine the atomic mass and atomic number of X, and write X in the standard form. It is NOT necessary to identify which atom X is.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
71
Reaction energy: The reaction energy (Q value) for a particular reaction is -2.4 MeV, and the reaction's threshold energy is 9.60 MeV. What is the ratio of the mass of the incident particle to the mass of the stationary target nucleus?
A) 0.75
B) 0.25
C) 3.0
D) 4.0
E) 5.0
A) 0.75
B) 0.25
C) 3.0
D) 4.0
E) 5.0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
72
Nuclear reactions: In the nuclear reaction 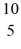 B +
B + 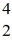 He →
He → 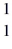 H + X, which of the following is the missing nuclear product X?
H + X, which of the following is the missing nuclear product X?
A)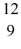 F
F
B)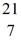 N
N
C) C
C
D)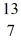 N
N
E)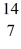 N
N
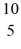 B +
B + 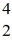 He →
He → 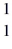 H + X, which of the following is the missing nuclear product X?
H + X, which of the following is the missing nuclear product X?A)
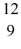 F
FB)
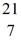 N
NC)
 C
CD)
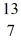 N
NE)
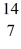 N
N
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
73
Reaction energy: The detonation of a certain nuclear device results in a mass decrease of 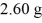 between the initial and the final ingredients. How much energy is released by this detonation? (c = 3.00 × 108 m/s)
between the initial and the final ingredients. How much energy is released by this detonation? (c = 3.00 × 108 m/s)
A) 2.34 × 1014 J
B) 7.80 × 105 J
C) 2.34 × 1013 J
D) 2.78 × 1012 J
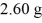 between the initial and the final ingredients. How much energy is released by this detonation? (c = 3.00 × 108 m/s)
between the initial and the final ingredients. How much energy is released by this detonation? (c = 3.00 × 108 m/s)A) 2.34 × 1014 J
B) 7.80 × 105 J
C) 2.34 × 1013 J
D) 2.78 × 1012 J

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
74
Nuclear reactions: A proton strikes an 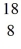 O nucleus producing
O nucleus producing 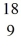 F and another particle. What is the other particle?
F and another particle. What is the other particle?
A) a neutron
B) an alpha particle
C) a β- particle
D) a β+ particle
E) a gamma ray
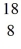 O nucleus producing
O nucleus producing 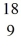 F and another particle. What is the other particle?
F and another particle. What is the other particle?A) a neutron
B) an alpha particle
C) a β- particle
D) a β+ particle
E) a gamma ray

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
75
Nuclear fission: When a neutron (n) collides with a uranium-235 nucleus it can induce a variety of fission reactions. One such reaction is 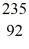 U + n →
U + n → 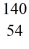 Xe +
Xe + 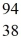 Sr + 2n. How much energy is released in this reaction, given the following mass values:
Sr + 2n. How much energy is released in this reaction, given the following mass values: 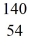 Xe: 139.921620 u
Xe: 139.921620 u 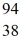 Sr : 93.915367 u
Sr : 93.915367 u 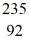 U: 235.043924 u n: 1.008665 u
U: 235.043924 u n: 1.008665 u
(1 u = 931.494 MeV/c2)
A) 185 MeV
B) 202 MeV
C) 32.6 MeV
D) 65.7 MeV
E) 98.6 MeV
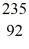 U + n →
U + n → 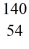 Xe +
Xe + 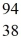 Sr + 2n. How much energy is released in this reaction, given the following mass values:
Sr + 2n. How much energy is released in this reaction, given the following mass values: 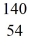 Xe: 139.921620 u
Xe: 139.921620 u 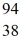 Sr : 93.915367 u
Sr : 93.915367 u 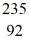 U: 235.043924 u n: 1.008665 u
U: 235.043924 u n: 1.008665 u(1 u = 931.494 MeV/c2)
A) 185 MeV
B) 202 MeV
C) 32.6 MeV
D) 65.7 MeV
E) 98.6 MeV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
76
Radioactive dating: Today, the uranium found on Earth contains 0.720% 235U (with a half-life of 0.700 billion years) and 99.28% 238U (with a half-life of 4.50 billion years). At a time 2.20 billion years ago, what percent of the uranium on Earth was 238U (assuming that no other uranium isotopes were present)?
A) 95.6%
B) 2.18%
C) 6.29%
D) 8.68%
E) 4.53%
A) 95.6%
B) 2.18%
C) 6.29%
D) 8.68%
E) 4.53%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
77
Radioactive dating: An archaeologist finds the 14C in a sample of 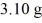 of material to be decaying at 107 counts per second. A modern 1.00-g sample of the same material decays at 151 counts per second. The half-life of 14C is 5730 y. How old is the sample?
of material to be decaying at 107 counts per second. A modern 1.00-g sample of the same material decays at 151 counts per second. The half-life of 14C is 5730 y. How old is the sample?
A) 12,200 y
B) 8460 y
C) 25,100 y
D) 12,600 y
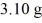 of material to be decaying at 107 counts per second. A modern 1.00-g sample of the same material decays at 151 counts per second. The half-life of 14C is 5730 y. How old is the sample?
of material to be decaying at 107 counts per second. A modern 1.00-g sample of the same material decays at 151 counts per second. The half-life of 14C is 5730 y. How old is the sample?A) 12,200 y
B) 8460 y
C) 25,100 y
D) 12,600 y

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
78
Nuclear fission: An excited 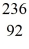 U* nucleus undergoes fission into two fragments, as shown:
U* nucleus undergoes fission into two fragments, as shown: 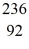 U* →
U* → 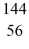 Ba +
Ba + 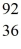 Kr The following atomic masses are known:
Kr The following atomic masses are known: 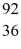 Kr: 91.926270 u
Kr: 91.926270 u 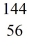 Ba: 143.922845 u
Ba: 143.922845 u 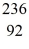 U*: 236.045563 u
U*: 236.045563 u
Assume, at a given instant, that the two fission fragments are spherical, just barely in contact, and carry spherically symmetric charge distributions. At that instant, what is the electrostatic interaction energy of the two fragments, in MeV? (1 u = 1.6605 × 10-27 kg = 931.5 MeV/c2, 1/4πε0 = 9.0 × 109 N * m2/C2)
A) 230 MeV
B) 240 MeV
C) 250 MeV
D) 260 MeV
E) 270 MeV
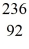 U* nucleus undergoes fission into two fragments, as shown:
U* nucleus undergoes fission into two fragments, as shown: 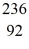 U* →
U* → 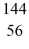 Ba +
Ba + 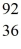 Kr The following atomic masses are known:
Kr The following atomic masses are known: 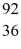 Kr: 91.926270 u
Kr: 91.926270 u 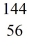 Ba: 143.922845 u
Ba: 143.922845 u 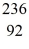 U*: 236.045563 u
U*: 236.045563 uAssume, at a given instant, that the two fission fragments are spherical, just barely in contact, and carry spherically symmetric charge distributions. At that instant, what is the electrostatic interaction energy of the two fragments, in MeV? (1 u = 1.6605 × 10-27 kg = 931.5 MeV/c2, 1/4πε0 = 9.0 × 109 N * m2/C2)
A) 230 MeV
B) 240 MeV
C) 250 MeV
D) 260 MeV
E) 270 MeV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
79
Nuclear fusion: Calculate the amount of energy that is released in the fusion reaction 2H + 2H → 4He, given the masses: 2H: 2.014102 u
4He: 4.002603 u
(1 u = 931.5 MeV/c2)
A) 24 MeV
B) 18 MeV
C) 13 MeV
D) 12 MeV
E) 36 MeV
4He: 4.002603 u
(1 u = 931.5 MeV/c2)
A) 24 MeV
B) 18 MeV
C) 13 MeV
D) 12 MeV
E) 36 MeV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck
80
Reaction energy: Calculate the reaction energy (Q value) for the reaction 7Li + 1H → 4He + 4He, given the following masses: 7Li: 7.016005 u
1H: 1.007825 u
4He: 4.002603 u
(1 u = 931.5 MeV/c2)
A) 13.35 MeV
B) 13.57 MeV
C) 15.37 MeV
D) 17.35 MeV
E) 17.53 MeV
1H: 1.007825 u
4He: 4.002603 u
(1 u = 931.5 MeV/c2)
A) 13.35 MeV
B) 13.57 MeV
C) 15.37 MeV
D) 17.35 MeV
E) 17.53 MeV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 89 في هذه المجموعة.
فتح الحزمة
k this deck








