Deck 2: Acids and Bases
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/47
العب
ملء الشاشة (f)
Deck 2: Acids and Bases
1
What is the strongest acid in the following reaction if the equilibrium constant is much less than 0.01?
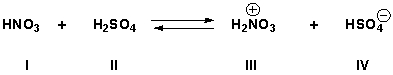
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV
III
2
What is the role of water in the following reaction?

A) acid
B) base
C) conjugate acid
D) conjugate base

A) acid
B) base
C) conjugate acid
D) conjugate base
conjugate base
3
Identify the conjugate acids in the following reactions.
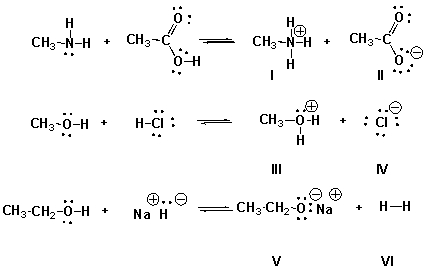
A) I, IV, VI
B) I, III, VI
C) II, IV, V
D) I, III, V

A) I, IV, VI
B) I, III, VI
C) II, IV, V
D) I, III, V
I, III, VI
4
Identify the Arrhenius acids:
I. HCl
II. NaOH
III. HNO3
IV. Ca(OH)2
A) I, II
B) I, III, IV
C) II, IV
D) I, III
I. HCl
II. NaOH
III. HNO3
IV. Ca(OH)2
A) I, II
B) I, III, IV
C) II, IV
D) I, III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
5
List the bonds in order of decreasing acidity (most to least).

A) I, III, II, IV
B) IV, II, III, I
C) II, III, IV, I
D) I, II, III, IV

A) I, III, II, IV
B) IV, II, III, I
C) II, III, IV, I
D) I, II, III, IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which equilibria have equilibrium constants greater than 1.0?
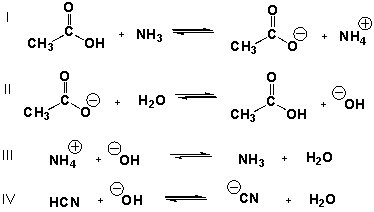
A) II, III
B) I, III, IV
C) III, IV
D) I, II, III

A) II, III
B) I, III, IV
C) III, IV
D) I, II, III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which are acid-base reactions according to Brønsted-Lowry theory?
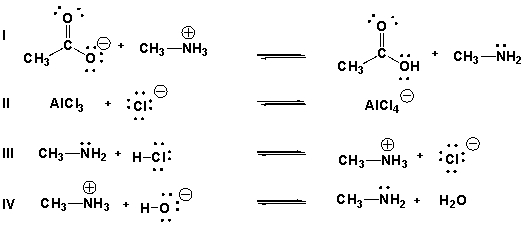
A) I, III
B) I, II, II, IV
C) I, II, III
D) I, III, IV

A) I, III
B) I, II, II, IV
C) I, II, III
D) I, III, IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which is the stronger base if the equilibrium lies to the right? (Sec. 2.4, HARD)
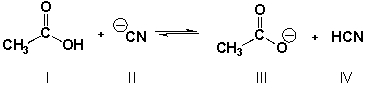
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which ion is the strongest base?
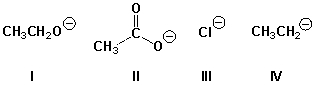
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
10
Identify the conjugate bases in the following reactions.
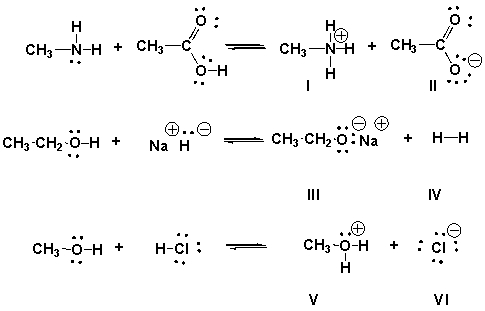
A) II, III, VI
B) I, IV, V
C) I, III, V
D) II, IV

A) II, III, VI
B) I, IV, V
C) I, III, V
D) II, IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
11
Identify the Arrhenius bases:
I. NH3
II. NaOH
III. HI
IV. Ca(OH)2
A) I, II
B) II, IV
C) I, III
D) I, II, IV
I. NH3
II. NaOH
III. HI
IV. Ca(OH)2
A) I, II
B) II, IV
C) I, III
D) I, II, IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which of these has the lowest numerical value of pKa and is therefore the strongest acid?
A) CH3COOH
B) H2O
C) NH4+
D) HCl
A) CH3COOH
B) H2O
C) NH4+
D) HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
13
Identify the Brønsted-Lowry acids in the following reactions.
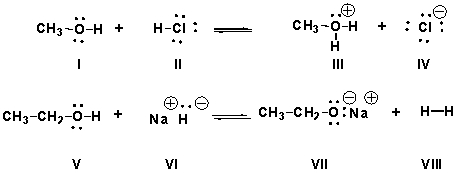
A) I, III,VI, VII
B) II, VI
C) I, IV, V, VIII
D) II, III, V, VIII

A) I, III,VI, VII
B) II, VI
C) I, IV, V, VIII
D) II, III, V, VIII

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which equilibria have equilibrium constants smaller than 1.0?
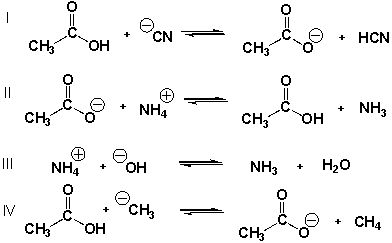
A) II
B) I, IV
C) III, IV
D) I

A) II
B) I, IV
C) III, IV
D) I

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
15
What is the stronger acid in the following reaction if the equilibrium constant is approximately 108.
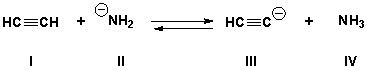
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
16
Arrange the following species in the order of increasing acidity (weakest to strongest).
I. H2O
II. H3O+
III. NH4+
A) II, III, I
B) I, II, III
C) III, II, I
D) I, III, II
I. H2O
II. H3O+
III. NH4+
A) II, III, I
B) I, II, III
C) III, II, I
D) I, III, II

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
17
Arrange the following species in order of increasing basicity (weakest to strongest).
I. OH-
II. Cl-
III. H2O
IV. NH3
A) II, III, IV, I
B) III, I, IV, II
C) IV, I, II, III
D) III, IV, I, II
I. OH-
II. Cl-
III. H2O
IV. NH3
A) II, III, IV, I
B) III, I, IV, II
C) IV, I, II, III
D) III, IV, I, II

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which statements about acid-base equilibria are true?
I. The pKa is the negative log10 of the acid equilibrium constant.
II. A stronger acid has a pKa with a smaller value than a weaker acid.
III. The stronger the base, the smaller the pKa of its conjugate acid.
IV. The Ka = K [HA].
A) I, III
B) I, II
C) I, II, III
D) II, III, IV
I. The pKa is the negative log10 of the acid equilibrium constant.
II. A stronger acid has a pKa with a smaller value than a weaker acid.
III. The stronger the base, the smaller the pKa of its conjugate acid.
IV. The Ka = K [HA].
A) I, III
B) I, II
C) I, II, III
D) II, III, IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which are acid-base reactions according to the Brønsted-Lowry theory?
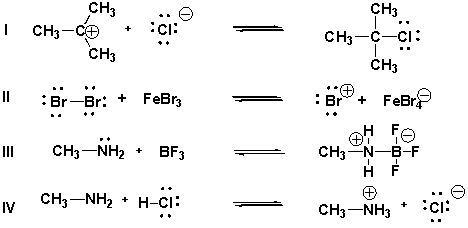
A) IV
B) I, III, IV
C) II, III
D) I, IV

A) IV
B) I, III, IV
C) II, III
D) I, IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
20
List the bonds in order of increasing acidity (least to most).
A) II, III, IV, I
B) III, I, II, IV
C) I, IV, II, III
D) IV, III, II, I
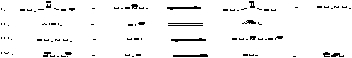
A) II, III, IV, I
B) III, I, II, IV
C) I, IV, II, III
D) IV, III, II, I

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
21
Identify the acid, base, conjugate acid, conjugate base in the following reaction.
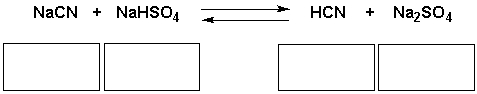


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
22
Complete the following reaction.
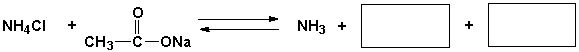
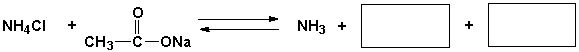

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
23
Complete the following reaction.
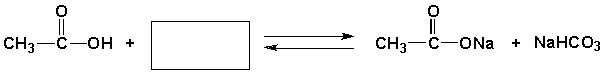


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
24
What is the role of diethyl ether in the following reaction?

A) Lewis acid
B) Lewis base
C) Brønsted acid
D) Brønsted base

A) Lewis acid
B) Lewis base
C) Brønsted acid
D) Brønsted base

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
25
The weaker the acid, the ________________ the conjugate base.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
26
Complete the following reaction scheme with the appropriate equilibrium arrow (indicating the higher concentrations at equilibrium).



فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
27
The stronger acid has the larger (more positive) pKa.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
28
What is the role of methanol (CH3OH) in the following reaction?
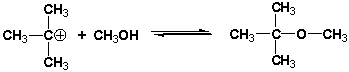
A) Lewis acid
B) Lewis base
C) Brønsted acid
D) Brønsted base
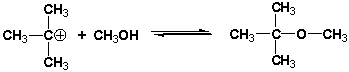
A) Lewis acid
B) Lewis base
C) Brønsted acid
D) Brønsted base

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
29
Brønsted-Lowry acids accept protons when reacting.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which are acid-base reactions according to Lewis theory but not according to the Brønsted-Lowry theory?
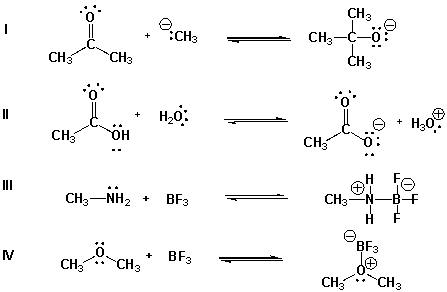
A) I, II
B) III, IV
C) I, III, IV
D) I, II, III, IV

A) I, II
B) III, IV
C) I, III, IV
D) I, II, III, IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
31
Complete the following reaction.
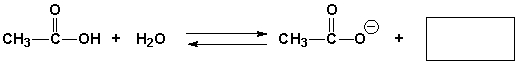
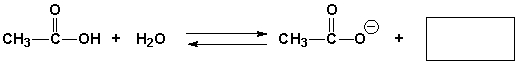

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
32
Which is the proper reaction mechanism for the reaction of boron trifluoride and diethyl ether?
Assume that the charges are correct and add electron pairs, if needed. Also consider how many electrons should be in the outer shell of boron trifluoride to make it neutral.
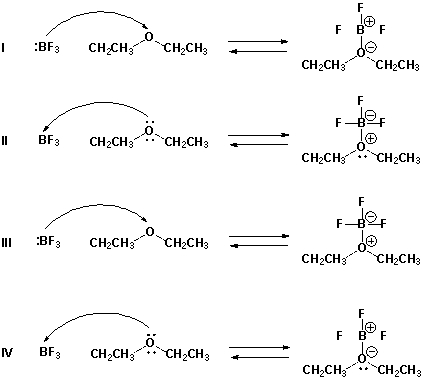
A) I
B) II
C) III
D) IV
Assume that the charges are correct and add electron pairs, if needed. Also consider how many electrons should be in the outer shell of boron trifluoride to make it neutral.

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
33
Strong acids have weak conjugate bases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
34
Identify the acid, base, conjugate acid, conjugate base in the following reaction.
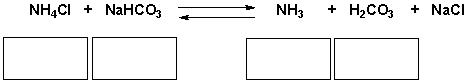


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
35
Complete the following reaction scheme with the appropriate equilibrium arrow (indicating the higher concentrations at equilibrium).



فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which is the order of increasing acid strength of the following compounds (least first)?
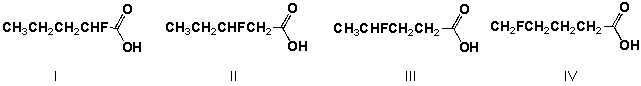
A) I, III, II, IV
B) IV, III, II, I
C) II, I, III, IV
D) IV, III, I, II

A) I, III, II, IV
B) IV, III, II, I
C) II, I, III, IV
D) IV, III, I, II

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
37
The higher concentration (reactants or products) at equilibrium will lie on the side of the ___________ acid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which substances are Lewis bases?
)
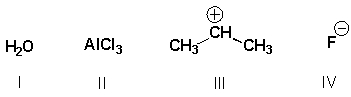
A) I, II
B) I, III
C) III, IV
D) I, IV
)

A) I, II
B) I, III
C) III, IV
D) I, IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
39
Complete the following reaction.
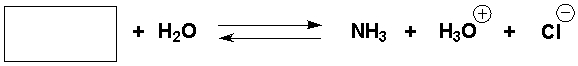


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which is the order of decreasing acid strength of the following compounds (greatest first)?
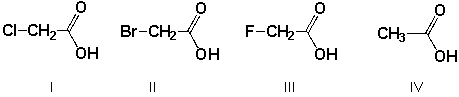
A) II, I, III, IV
B) III, IV, I, II
C) III, I, II, IV
D) IV, II, I, III

A) II, I, III, IV
B) III, IV, I, II
C) III, I, II, IV
D) IV, II, I, III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
41
The strongest acid in the following list is sodium bicarbonate.
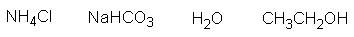
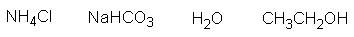

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
42
The equilibrium constant will be greater than 1.0 for the following reaction
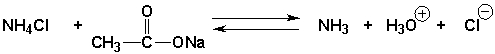


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
43
Water acts as a Brønsted-Lowry base in the following reaction.



فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
44
Lewis bases donate electrons when reacting.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
45
The weakest acid in the following list is acetic acid.
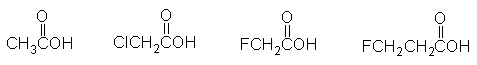


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
46
Ammonia acts as a Brønsted-Lowry base in the following reaction.



فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck
47
The equilibrium constant will be greater than 1.0 for the following reaction



فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 47 في هذه المجموعة.
فتح الحزمة
k this deck








