Deck 1: Covalent Bonding and Shapes of Molecules
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/114
العب
ملء الشاشة (f)
Deck 1: Covalent Bonding and Shapes of Molecules
1
A neutral carbon has how many valence electrons?
A) 2
B) 4
C) 6
D) 8
A) 2
B) 4
C) 6
D) 8
4
2
A neutral nitrogen has how many valence electrons?
A) 4
B) 5
C) 6
D) 7
A) 4
B) 5
C) 6
D) 7
5
3
Which is the correct Lewis structure for acetic acid (CH3CO2H)?
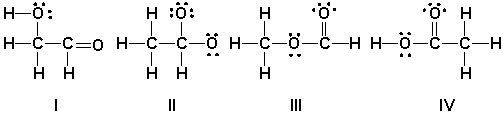
A) I
B) II
C) III
D) IV
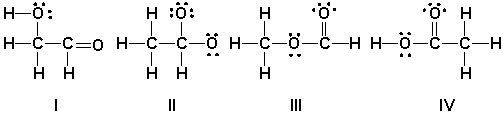
A) I
B) II
C) III
D) IV
IV
4
A neutral oxygen has how many valence electrons?
A) 4
B) 5
C) 6
D) 7
A) 4
B) 5
C) 6
D) 7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which Lewis structures are correct?
HINT: Perform a total valence count and check formal charges.
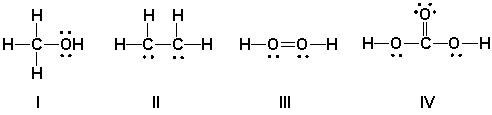
A) I
B) II
C) III
D) IV
HINT: Perform a total valence count and check formal charges.
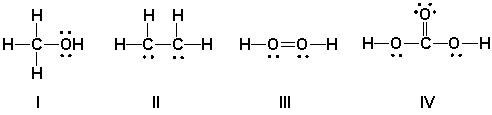
A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
6
Using the VSEPR model, predict which species have bond angles of about 109°.
HINT: Assume that the charges are correct. Add the missing lone pairs before applying the VSEPR theory!
A) I, III, IV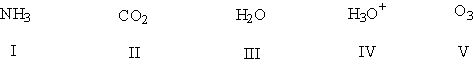
B) II, III, V
C) I, IV
D) III, IV, V
HINT: Assume that the charges are correct. Add the missing lone pairs before applying the VSEPR theory!
A) I, III, IV
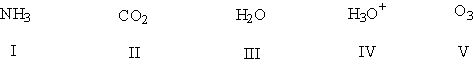
B) II, III, V
C) I, IV
D) III, IV, V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which is the electronic configuration that describes Mg2+?
A) 1s2, 2s2
B) 1s2, 2s2, 2p6
C) 1s2, 2s2, 2p6, 3s2
D) 1s2, 2s2, 2p6, 3s2, 3p6
A) 1s2, 2s2
B) 1s2, 2s2, 2p6
C) 1s2, 2s2, 2p6, 3s2
D) 1s2, 2s2, 2p6, 3s2, 3p6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
8
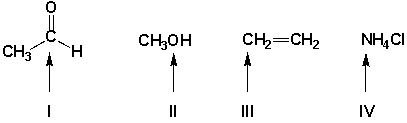
Which functional groups have correct Lewis structures?
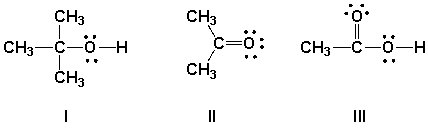
A) I, II
B) II, III
C) I, II, III
D) I, III

A) I, II
B) II, III
C) I, II, III
D) I, III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which atom is described by the electronic configuration 1s22s22p63s23p3?
A) Mg
B) Al
C) Si
D) P
A) Mg
B) Al
C) Si
D) P

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which statement about orbitals is false?
A) Orbitals are regions of space where electrons are found.
B) Orbitals may contain up to two electrons each.
C) Orbitals are filled in order of decreasing energy.
D) Orbitals of equivalent energy are half filled before adding two electrons to any one of them.
A) Orbitals are regions of space where electrons are found.
B) Orbitals may contain up to two electrons each.
C) Orbitals are filled in order of decreasing energy.
D) Orbitals of equivalent energy are half filled before adding two electrons to any one of them.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which is the electronic configuration that describes a neutral carbon atom?
A) 1s2, 2s2, 2p5
B) 1s2, 2s2, 2p6, 3s2
C) 1s2, 2s2, 2p2
D) 1s2, 2s2, 2p6
A) 1s2, 2s2, 2p5
B) 1s2, 2s2, 2p6, 3s2
C) 1s2, 2s2, 2p2
D) 1s2, 2s2, 2p6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
12
What is the formal charge of oxygen in H3O+?
A) -1
B) 0
C) +1
D) +2
A) -1
B) 0
C) +1
D) +2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which atom is described by the Lewis structure ?
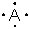
A) C
B) P
C) Se
D) I
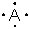
A) C
B) P
C) Se
D) I

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
14
Nitrogen has a negative formal charge in which of the following compounds?
A) NaNH2
B) N2
C) NH4Cl
D) HCN
A) NaNH2
B) N2
C) NH4Cl
D) HCN

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
15
Arrange the bonds in increasing order of ionic character (least first).
C-C Na-O C-N O-H C-O
I II III IV V
A) III, I, IV, II, V
B) V, III, I, II, IV
C) I, V, III, IV, II
D) I, III, V, IV, II
C-C Na-O C-N O-H C-O
I II III IV V
A) III, I, IV, II, V
B) V, III, I, II, IV
C) I, V, III, IV, II
D) I, III, V, IV, II

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
16
Using the VSEPR model, predict which atoms have bond angles of about 120°.
A) II, IV
B) I, IV
C) II, III
D) I, III

A) II, IV
B) I, IV
C) II, III
D) I, III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which atom is described by the electron configuration 1s22s22p63s23p5?
A) S
B) Se
C) Cl
D) Br
A) S
B) Se
C) Cl
D) Br

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
18
Which atom is described by the Lewis structure ?
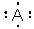
A) C
B) P
C) Se
D) I
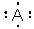
A) C
B) P
C) Se
D) I

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which Lewis structures are correct?
HINT: Perform a total valence count and check formal charges.
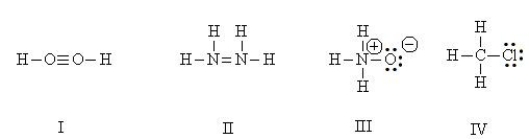
A) I, II
B) II, IV
C) III, IV
D) I, III
HINT: Perform a total valence count and check formal charges.
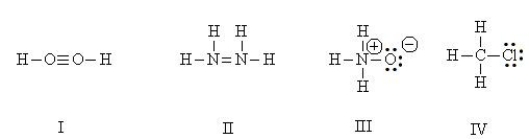
A) I, II
B) II, IV
C) III, IV
D) I, III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
20
According to VSEPR model, what is your prediction for the arrangement of electron pairs for CH3-?
A) linear
B) tetrahedral
C) bent
D) trigonal
A) linear
B) tetrahedral
C) bent
D) trigonal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which compounds are classified correctly?
HINT: Assume that the charges are correct. Add the missing lone pairs!
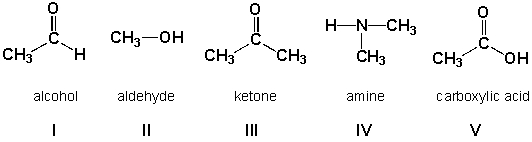
A) III, IV, V
B) II, III, IV
C) I, III, V
D) I, III, IV
HINT: Assume that the charges are correct. Add the missing lone pairs!

A) III, IV, V
B) II, III, IV
C) I, III, V
D) I, III, IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
22
______ is the number of valence electrons for a neutral sulfur atom.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
23
What is the correct structure for the aldehyde, which has the formula C4H8O?
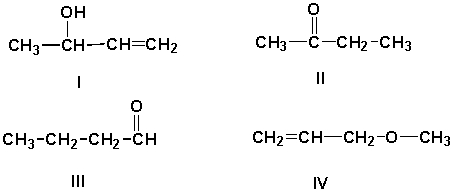
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
24
Using the VSEPR model, predict which molecules have bond angles of about 120°.
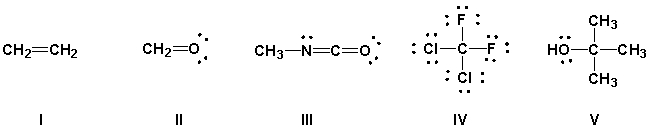
A) I, II, IV
B) I, II, III
C) III, IV, V
D) II, III, IV

A) I, II, IV
B) I, II, III
C) III, IV, V
D) II, III, IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
25
Outer shell electrons are called _________ electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
26
The carbon has the correct orbital hybridization in which structures?
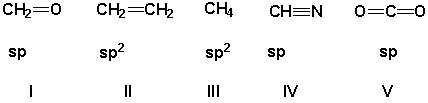
A) II, IV, V
B) II, III, IV
C) I, II, III
D) I, IV, V
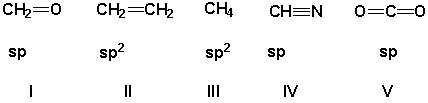
A) II, IV, V
B) II, III, IV
C) I, II, III
D) I, IV, V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which of the three molecules aspirin, paracetamol and ibuprofen contains a carboxyl group?
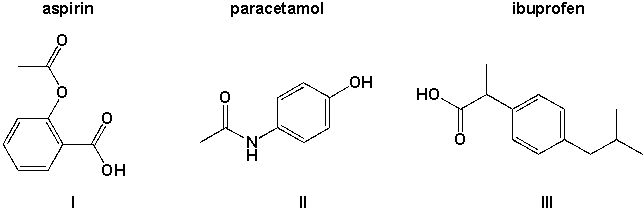
A) I
B) I, III
C) II,III
D) I, II, III

A) I
B) I, III
C) II,III
D) I, II, III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
28
What is the formal charge of indicated carbon in the following molecule?
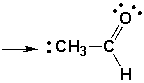
A) -2
B) -1
C) 0
D) +1
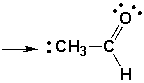
A) -2
B) -1
C) 0
D) +1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which compounds contain both covalent and ionic bonds?
CH3OH Na2CO3 NH4Cl NaCl
I II III IV
A) I, II
B) II, IV
C) I, II, IV
D) II, III
CH3OH Na2CO3 NH4Cl NaCl
I II III IV
A) I, II
B) II, IV
C) I, II, IV
D) II, III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which of these molecules are polar?
HINT: look for the presence of at least one polar covalent bond in these molecules!
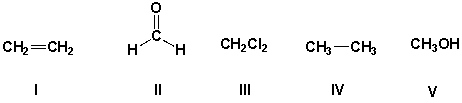
A) III, IV, V
B) I, IV
C) II, III, V
D) I, III
HINT: look for the presence of at least one polar covalent bond in these molecules!
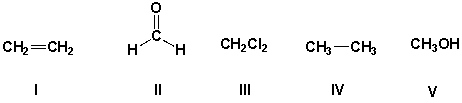
A) III, IV, V
B) I, IV
C) II, III, V
D) I, III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which of these molecules are polar?
HINT: look for the presence of at least one polar covalent bond in these molecules!
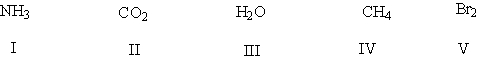
A) I,IV
B) I, III, III
C) II, III, IV
D) III, IV, V
HINT: look for the presence of at least one polar covalent bond in these molecules!
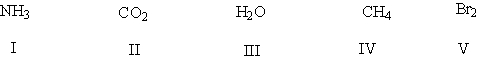
A) I,IV
B) I, III, III
C) II, III, IV
D) III, IV, V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
32
The spins of two electrons in the same orbital must be _______ .

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following are pairs of contributing structures?
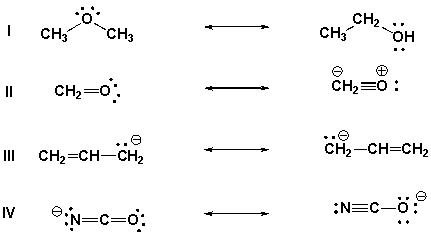
A) II, IV
B) I, II, III
C) III, IV
D) II, III, ,IV

A) II, IV
B) I, II, III
C) III, IV
D) II, III, ,IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
34
What are the correct orbital hybridizations for carbon in the following species?
HINT: Add a lone pair if the charge of the molecule suggests it!
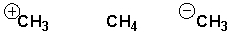
A) CH3+ is sp-hybridized
B) CH3- is sp2-hybridized
C) CH4 is sp2-hybridized
D) CH3- is sp3-hybridized
HINT: Add a lone pair if the charge of the molecule suggests it!
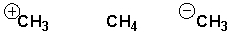
A) CH3+ is sp-hybridized
B) CH3- is sp2-hybridized
C) CH4 is sp2-hybridized
D) CH3- is sp3-hybridized

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
35
Which statement about contributing structures is false?
A) All contributing structures must have the same number of valence electrons.
B) All contributing structures must obey the rules of covalent bonding.
C) The position of nuclei may change.
D) Third period atoms may have up to 18 electrons around them.
A) All contributing structures must have the same number of valence electrons.
B) All contributing structures must obey the rules of covalent bonding.
C) The position of nuclei may change.
D) Third period atoms may have up to 18 electrons around them.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which of the three molecules testosterone, methadone and hydrocodone contains an amine?
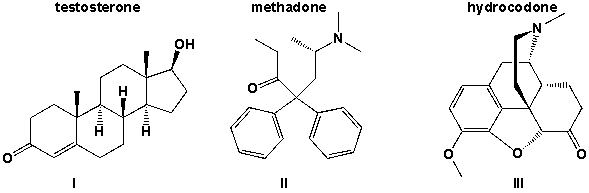
A) I,II
B) III
C) II,III
D) I, II, III

A) I,II
B) III
C) II,III
D) I, II, III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which of the three molecules (testosterone, methadone and hydrocodone) contains a ketone?
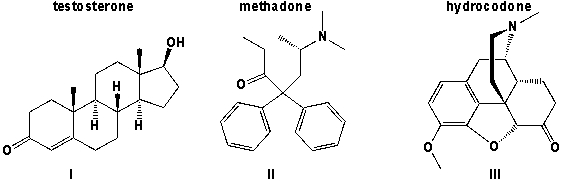
A) I,II
B) III
C) II,III
D) I, II, III

A) I,II
B) III
C) II,III
D) I, II, III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which of the following compounds is a tertiary (3°) alcohol?
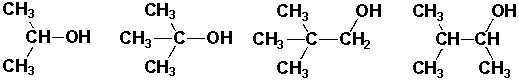
I II III IV
A) I
B) II
C) III
D) IV
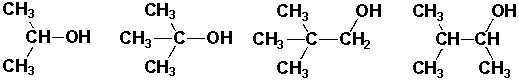
I II III IV
A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
39
Which of the three molecules testosterone, methadone and hydrocodone contains a hydroxyl group.
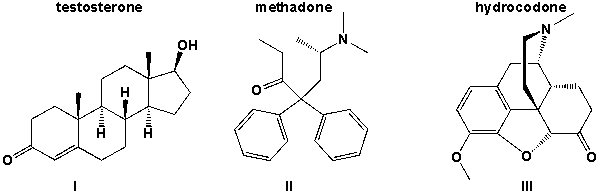
A) I
B) I, III
C) II,III
D) I, II, III

A) I
B) I, III
C) II,III
D) I, II, III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
40
Using the VSEPR model, predict which molecules have bond angles of about 109°.
A) II, IV
B) I, II, III
C) III, IV, V
D) II, III, IV

A) II, IV
B) I, II, III
C) III, IV, V
D) II, III, IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
41
Ionic bonds are characterized by the unequal sharing of electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
42
Orbitals make up the majority of the mass of an atom.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
43
Atoms of the group 2A elements react by losing two electrons to achieve a noble gas configuration.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
44
______ is the number of valence electrons for a neutral bromine atom.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
45
A __________ bond is characterized by the unequal sharing of electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
46
The following molecule is an example of a secondary amine.
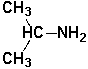
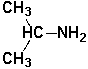

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
47
Carbon atoms usually react by gaining 4 electrons to achieve a noble gas configuration.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
48
The following molecule contains the _________ and __________ functional groups.
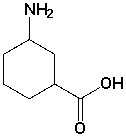


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
49
The tendency of an element to react such that it achieves a noble gas configuration is called
the ______ ______. (Sec. 1.1, EASY)
the ______ ______. (Sec. 1.1, EASY)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
50
An neutral atom that has gained electrons is called an anion.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
51
______________ are the basis for compound nomenclature.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which compound has the least exothermic (least negative) heat of hydrogenation? 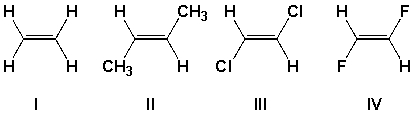
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
53
The following molecule contains the _________ and __________ functional groups.
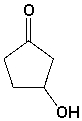


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
54
The most polar bond in the following molecule is __________.
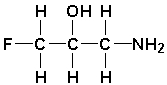


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
55
Atoms of the group 7A elements react by losing an electron to achieve a noble gas configuration.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
56
is a polar molecule.
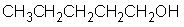
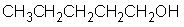

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
57
Functional groups undergo the same type of __________ in whatever compound they are found.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
58
contains only polar covalent bonds.
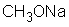
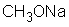

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
59
Each shell can hold two electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
60
Which is the major product from the reaction of propene with BH3 followed by NaOH/H2O2?
A) 1-propanol
B) 2-propanol
C) 1,2-propanediol
D) 1,3-propandiol
A) 1-propanol
B) 2-propanol
C) 1,2-propanediol
D) 1,3-propandiol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
61
Arrange these carbocations in order of increasing stability (least to most). 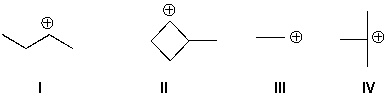
A) I, II, III, IV
B) III, II, I, IV
C) II, IV, I, III
D) III, I, II, IV

A) I, II, III, IV
B) III, II, I, IV
C) II, IV, I, III
D) III, I, II, IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
62
Which reagents react with an alkene by anti addition?
i. Cl2
II. Br2
III. H2/Pt
IV. BH3, followed by H2O2/NaOH/H2O
A) I, II
B) III, IV
C) II, III
D) I, IV
i. Cl2
II. Br2
III. H2/Pt
IV. BH3, followed by H2O2/NaOH/H2O
A) I, II
B) III, IV
C) II, III
D) I, IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
63
Which are the major products from the reaction of 1,2-dimethylcyclohexene with H2/Pt? 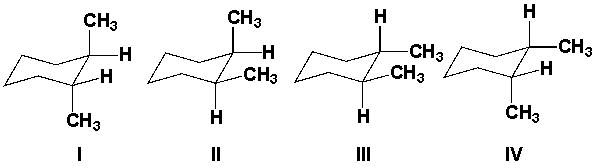
A) I and III
B) II and IV
C) II and III
D) III and IV

A) I and III
B) II and IV
C) II and III
D) III and IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
64
Compound X has a molecular formula C8H14 and reacts with H2/Pt to give compound Y, C8H16. Which is compound X? 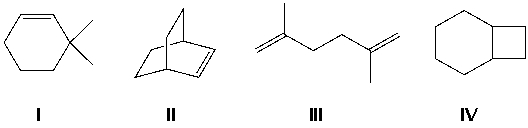
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
65
Which compound has the most exothermic (most negative) heat of hydrogenation? 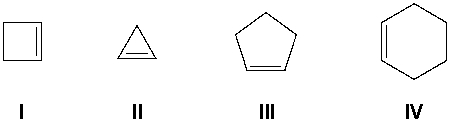
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
66
Which reagents react with 2-methyl-1-hexene in a Markovnikov orientation?
i. HBr
II. H2O/H2SO4
III. Br2
IV. BH3, followed by H2O2/NaOH/H2O
A) I, II
B) III, IV
C) II, IV
D) II, III, IV
i. HBr
II. H2O/H2SO4
III. Br2
IV. BH3, followed by H2O2/NaOH/H2O
A) I, II
B) III, IV
C) II, IV
D) II, III, IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
67
Which energy diagram represents the reaction of HBr with 2-butene? 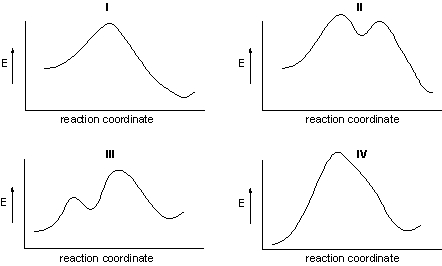
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
68
Which is the major product from the reaction of 1,2-dimethylcyclohexene with Br2? 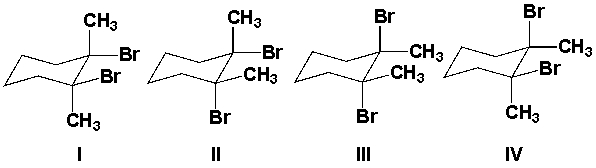
A) I and III
B) II and IV
C) II and III
D) III and IV

A) I and III
B) II and IV
C) II and III
D) III and IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
69
Which reagents react with an alkene by syn addition?
i. Cl2
II. Br2
III. H2/Pt
IV. BH3, followed by H2O2/NaOH/H2O
A) I, II
B) III, IV
C) II, III
D) I, IV
i. Cl2
II. Br2
III. H2/Pt
IV. BH3, followed by H2O2/NaOH/H2O
A) I, II
B) III, IV
C) II, III
D) I, IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
70
The hydroboration and subsequent oxidation of 1-heptene leads to which of the following products? 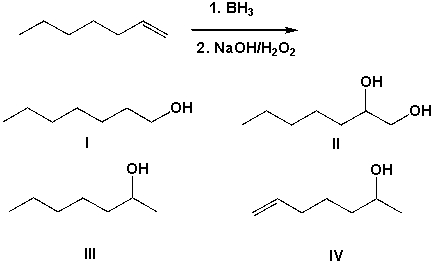
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
71
Ethynylcyclohexane reacts with sodium amide. The product of that reaction then reacts with bromocyclohexane. What is the main product that is formed? 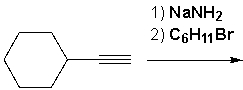
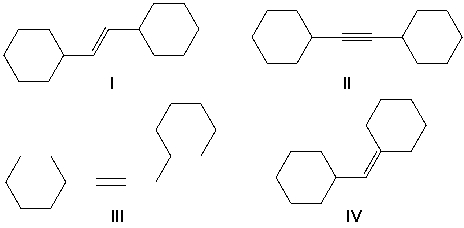
A) I
B) II
C) III
D) IV
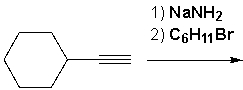

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
72
Which is the major product from the reaction of cyclopentene with Br2/CCl4? 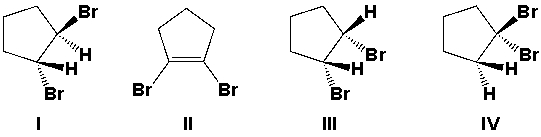
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
73
Which compound does not give two stereoisomers when reacted with Cl2/CCl4? 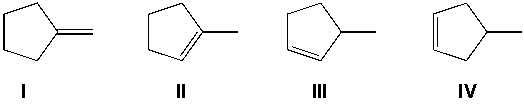
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
74
Select the stereoselective reagents from the following list.
HCl H+/H2O Br2 BH3 H2
HCl H+/H2O Br2 BH3 H2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
75
Which is the product of the reaction of 1-methylcyclohexene with H2O/H2SO4? 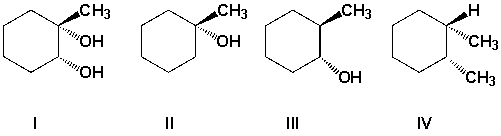
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
76
Which set of reagents will produce a electrophilic addition to the pi bond of an alkene??
i. H2O/H2SO4
II. Br2/CCl4
III. HBr
IV. H2/Pt
A) I, II, III
B) I, II, IV
C) II, IV
D) III, IV
i. H2O/H2SO4
II. Br2/CCl4
III. HBr
IV. H2/Pt
A) I, II, III
B) I, II, IV
C) II, IV
D) III, IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
77
Which point on the energy diagram represents the intermediate? 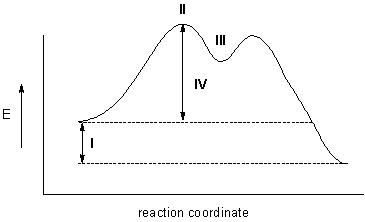
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
78
Which statement does not describe a transition state?
A) possesses a definite geometry
B) maximum on the energy diagram
C) structure can be determined experimentally
D) cannot be isolated
A) possesses a definite geometry
B) maximum on the energy diagram
C) structure can be determined experimentally
D) cannot be isolated

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
79
But-1-yn-1-ylcyclohexane reacts with molecular hydrogen under pressure in the presence of a Lindlar catalyst. What reaction product is formed? 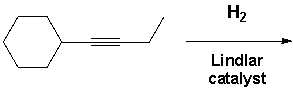
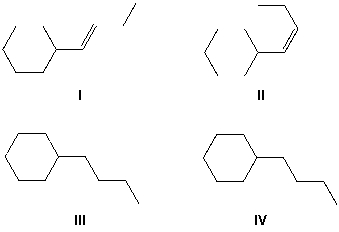
A) I
B) II
C) III
D) IV
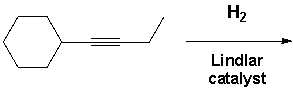

A) I
B) II
C) III
D) IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck
80
Which is the major product from acid catalyzed hydration of 2-methyl-2-pentene?
A) 2-methyl-3-pentanol
B) 2-methyl-2-pentanol
C) 4-methyl-2-pentanol
D) 3-methyl-3-pentanol
A) 2-methyl-3-pentanol
B) 2-methyl-2-pentanol
C) 4-methyl-2-pentanol
D) 3-methyl-3-pentanol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 114 في هذه المجموعة.
فتح الحزمة
k this deck








