Deck 2: Atoms, Molecules, and Ions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/124
العب
ملء الشاشة (f)
Deck 2: Atoms, Molecules, and Ions
1
What is the term for the total number of neutrons and protons in the nucleus of each atom of an element?
A) Isotope number
B) Mass number
C) Mass-to-charge ratio
D) Atomic number
E) Atomic mass units
A) Isotope number
B) Mass number
C) Mass-to-charge ratio
D) Atomic number
E) Atomic mass units
Mass number
2
Rutherford's experiment with alpha particle scattering by gold foil established that
A) positive charge not evenly distributed throughout an atom.
B) electrons have a negative charge.
C) electrons have a positive charge.
D) atoms are made of protons, neutrons, and electrons.
E) protons are 1840 times heavier than electrons.
A) positive charge not evenly distributed throughout an atom.
B) electrons have a negative charge.
C) electrons have a positive charge.
D) atoms are made of protons, neutrons, and electrons.
E) protons are 1840 times heavier than electrons.
positive charge not evenly distributed throughout an atom.
3
The scientist who determined the magnitude of the electric charge on the electron was
A) John Dalton.
B) Robert Millikan.
C) J. J. Thomson.
D) Henry Moseley.
E) J. Burdge.
A) John Dalton.
B) Robert Millikan.
C) J. J. Thomson.
D) Henry Moseley.
E) J. Burdge.
Robert Millikan.
4
Millikan's oil-drop experiment
A) established the charge on an electron.
B) showed that all oil drops carried the same charge.
C) provided support for the nuclear model of the atom.
D) suggested that some oil drops carried fractional numbers of electrons.
E) suggested the presence of a neutral particle in the atom.
A) established the charge on an electron.
B) showed that all oil drops carried the same charge.
C) provided support for the nuclear model of the atom.
D) suggested that some oil drops carried fractional numbers of electrons.
E) suggested the presence of a neutral particle in the atom.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which isotope is not possible?
A)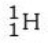
B)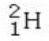
C)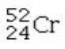
D)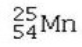
E) All of these isotopes are possible.
A)
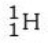
B)
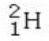
C)
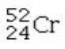
D)
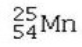
E) All of these isotopes are possible.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which of the following is a type of radioactive radiation that consists of positively charged particles and is deflected away from the positively charged plate?
A) α rays
B) β rays
C) γ rays
D) δ rays
E) ε rays
A) α rays
B) β rays
C) γ rays
D) δ rays
E) ε rays

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
7
When J. J. Thomson discovered the electron, what physical property of the electron did he measure?
A) its charge, e
B) its charge-to-mass ratio, e/m
C) its temperature, T
D) its mass, m
E) its atomic number, Z
A) its charge, e
B) its charge-to-mass ratio, e/m
C) its temperature, T
D) its mass, m
E) its atomic number, Z

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
8
Atoms of the same element with different mass numbers are called
A) ions.
B) neutrons.
C) chemical groups.
D) chemical families.
E) isotopes.
A) ions.
B) neutrons.
C) chemical groups.
D) chemical families.
E) isotopes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which one of the following statements about atoms and subatomic particles is correct?
A) Rutherford discovered the atomic nucleus by bombarding gold foil with electrons.
B) The proton and the neutron have identical masses.
C) The neutron's mass is equal to that of a proton plus an electron.
D) A neutral atom contains equal numbers of protons and electrons.
E) An atomic nucleus contains equal numbers of protons and neutrons.
A) Rutherford discovered the atomic nucleus by bombarding gold foil with electrons.
B) The proton and the neutron have identical masses.
C) The neutron's mass is equal to that of a proton plus an electron.
D) A neutral atom contains equal numbers of protons and electrons.
E) An atomic nucleus contains equal numbers of protons and neutrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
10
Who is credited with discovering the atomic nucleus?
A) Dalton
B) Gay-Lussac
C) Thomson
D) Chadwick
E) Rutherford
A) Dalton
B) Gay-Lussac
C) Thomson
D) Chadwick
E) Rutherford

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
11
What is the term for the number of protons in the nucleus of each atom of an element? It also indicates the number of electrons in the atom.
A) Isotope number
B) Mass number
C) Mass-to-charge ratio
D) Atomic number
E) Atomic mass units
A) Isotope number
B) Mass number
C) Mass-to-charge ratio
D) Atomic number
E) Atomic mass units

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
12
Atoms X, Y, Z, and R have the following nuclear compositions: 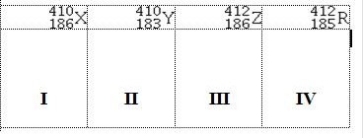 Which of the following are isotopes of the same element?
Which of the following are isotopes of the same element?
A) I & II
B) I & IV
C) II & IV
D) III & IV
E) I & III
 Which of the following are isotopes of the same element?
Which of the following are isotopes of the same element?A) I & II
B) I & IV
C) II & IV
D) III & IV
E) I & III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
13
Bromine is the only nonmetal that is a liquid at room temperature. Consider the isotope bromine-81,  . Select the combination which lists the correct atomic number, number of neutrons, and mass number, respectively.
. Select the combination which lists the correct atomic number, number of neutrons, and mass number, respectively.
A) 35, 46, 81
B) 35, 81, 46
C) 81, 46, 35
D) 46, 81, 35
E) 35, 81, 116
 . Select the combination which lists the correct atomic number, number of neutrons, and mass number, respectively.
. Select the combination which lists the correct atomic number, number of neutrons, and mass number, respectively.A) 35, 46, 81
B) 35, 81, 46
C) 81, 46, 35
D) 46, 81, 35
E) 35, 81, 116

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
14
Who discovered the neutron, the subatomic particle having a neutral charge?
A) Millikan
B) Dalton
C) Chadwick
D) Rutherford
E) Thomson
A) Millikan
B) Dalton
C) Chadwick
D) Rutherford
E) Thomson

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which of the following is a type of radioactive radiation that has no charge and is unaffected by external electric or magnetic fields?
A) α rays
B) β rays
C) γ rays
D) δ rays
E) ε rays
A) α rays
B) β rays
C) γ rays
D) δ rays
E) ε rays

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which of the following is a type of radioactive radiation that consists of electrons and is deflected away from the negatively charged plate?
A) α rays
B) β rays
C) γ rays
D) δ rays
E) ε rays
A) α rays
B) β rays
C) γ rays
D) δ rays
E) ε rays

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which field of study made a big contribution toward understanding the composition of the atom?
A) Electricity
B) Radiation
C) Solution chemistry
D) Electrochemistry
E) Quantum mechanics
A) Electricity
B) Radiation
C) Solution chemistry
D) Electrochemistry
E) Quantum mechanics

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
18
Who is credited with first measuring the charge of the electron?
A) Dalton
B) Gay-Lussac
C) Thomson
D) Millikan
E) Rutherford
A) Dalton
B) Gay-Lussac
C) Thomson
D) Millikan
E) Rutherford

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
19
Which of these scientists developed the nuclear model of the atom?
A) John Dalton
B) Robert Millikan
C) J. J. Thomson
D) Henry Moseley
E) Ernest Rutherford
A) John Dalton
B) Robert Millikan
C) J. J. Thomson
D) Henry Moseley
E) Ernest Rutherford

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
20
Who is credited with measuring the mass/charge ratio of the electron?
A) Dalton
B) Chadwick
C) Thomson
D) Millikan
E) Rutherford
A) Dalton
B) Chadwick
C) Thomson
D) Millikan
E) Rutherford

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
21
The elements in Group 2A are known by what name?
A) Transition metals
B) Halogens
C) Alkali metals
D) Alkaline earth metals
E) Noble gases
A) Transition metals
B) Halogens
C) Alkali metals
D) Alkaline earth metals
E) Noble gases

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
22
Two isotopes of an element differ only in their
A) symbol.
B) atomic number.
C) atomic mass.
D) number of protons.
E) number of electrons.
A) symbol.
B) atomic number.
C) atomic mass.
D) number of protons.
E) number of electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which of these elements is chemically similar to oxygen?
A) Sulfur
B) Calcium
C) Iron
D) Nickel
E) Potassium
A) Sulfur
B) Calcium
C) Iron
D) Nickel
E) Potassium

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
24
Which of these materials are typically poor conductors of heat and electricity?
A) Metals
B) Metalloids
C) Nonmetals
D) Alkaline earth metals
E) Alkali metals
A) Metals
B) Metalloids
C) Nonmetals
D) Alkaline earth metals
E) Alkali metals

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
25
What term defines a mass which is exactly equal to 1/12 the mass of one carbon-12 atom?
A) Isotope number
B) Mass number
C) Mass-to-charge ratio
D) Atomic number
E) Atomic mass unit
A) Isotope number
B) Mass number
C) Mass-to-charge ratio
D) Atomic number
E) Atomic mass unit

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
26
In the periodic table, atoms are arranged in order of
A) increasing atomic mass.
B) increasing atomic number.
C) physical properties.
D) periodicity.
E) chemical reactivities.
A) increasing atomic mass.
B) increasing atomic number.
C) physical properties.
D) periodicity.
E) chemical reactivities.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
27
How many neutrons are there in an atom of lead whose mass number is 208?
A) 82
B) 126
C) 208
D) 290
E) none of them
A) 82
B) 126
C) 208
D) 290
E) none of them

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
28
Silicon, which makes up about 25% of Earth's crust by mass, is used widely in the modern electronics industry. It has three naturally occurring isotopes, 28Si, 29Si, and 30Si. Calculate the atomic mass of silicon. 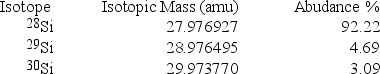
A) 29.2252 amu
B) 28.9757 amu
C) 28.7260 amu
D) 28.0855 amu
E) 27.9801 amu
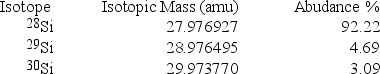
A) 29.2252 amu
B) 28.9757 amu
C) 28.7260 amu
D) 28.0855 amu
E) 27.9801 amu

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which of these elements is most likely to be a good conductor of electricity?
A) N
B) S
C) He
D) Cl
E) Fe
A) N
B) S
C) He
D) Cl
E) Fe

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which of the following is a metalloid?
A) Carbon, C, Z = 6
B) Sulfur, S, Z = 16
C) Germanium, Ge, Z = 32
D) Iridium, Ir, Z = 77
E) Bromine, Br, Z = 35
A) Carbon, C, Z = 6
B) Sulfur, S, Z = 16
C) Germanium, Ge, Z = 32
D) Iridium, Ir, Z = 77
E) Bromine, Br, Z = 35

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
31
The elements in Group 7A are known by what name?
A) Transition metals
B) Halogens
C) Alkali metals
D) Alkaline earth metals
E) Noble gases
A) Transition metals
B) Halogens
C) Alkali metals
D) Alkaline earth metals
E) Noble gases

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
32
Lithium forms compounds which are used in dry cells, storage batteries, and in high-temperature lubricants. It has two naturally occurring isotopes, 6Li (isotopic mass = 6.015123 amu) and 7Li (isotopic mass = 7.016005 amu). Lithium has an atomic mass of 6.9412 amu. What is the percent abundance of lithium-6?
A) 92.53%
B) 86.65%
C) 49.47%
D) 7.47%
E) 6.015%
A) 92.53%
B) 86.65%
C) 49.47%
D) 7.47%
E) 6.015%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
33
The elements in a column of the periodic table are known as
A) metalloids.
B) a period.
C) noble gases.
D) a group.
E) nonmetals.
A) metalloids.
B) a period.
C) noble gases.
D) a group.
E) nonmetals.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
34
The alkali metal elements are found in ________ of the periodic table.
A) Group 1A
B) Group 2A
C) Group 3A
D) Period 7
E) Period 1
A) Group 1A
B) Group 2A
C) Group 3A
D) Period 7
E) Period 1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
35
Select the correct number of protons (p), electrons (e), and neutrons (n) in one atom of chlorine-37.
A) 37 p, 37 e, 17 n
B) 17 p, 17 e, 37 n
C) 17 p, 17 e, 20 n
D) 37 p, 17 e, 20 n
E) 17 p, 37 e, 17 n
A) 37 p, 37 e, 17 n
B) 17 p, 17 e, 37 n
C) 17 p, 17 e, 20 n
D) 37 p, 17 e, 20 n
E) 17 p, 37 e, 17 n

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
36
An atom of the isotope sulfur-31 consists of how many protons, neutrons, and electrons? (p = proton, n = neutron, e = electron)
A) 15 p, 16 n, 15 e
B) 16 p, 15 n, 16 e
C) 16 p, 31 n, 16 e
D) 32 p, 31 n, 32 e
E) 16 p, 16 n, 15 e
A) 15 p, 16 n, 15 e
B) 16 p, 15 n, 16 e
C) 16 p, 31 n, 16 e
D) 32 p, 31 n, 32 e
E) 16 p, 16 n, 15 e

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
37
A row of the periodic table is called a(n)
A) group.
B) period.
C) isotopic mixture.
D) family.
E) subshell.
A) group.
B) period.
C) isotopic mixture.
D) family.
E) subshell.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
38
Which of the following is a metal?
A) Nitrogen, N, Z = 7
B) Phosphorus, P, Z = 15
C) Arsenic, As, Z = 33
D) Thallium, Tl, Z = 81
E) Silicon, Si, Z = 14
A) Nitrogen, N, Z = 7
B) Phosphorus, P, Z = 15
C) Arsenic, As, Z = 33
D) Thallium, Tl, Z = 81
E) Silicon, Si, Z = 14

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
39
Which of the following is a nonmetal?
A) Lithium, Li, Z = 3
B) Bromine, Br, Z = 35
C) Mercury, Hg, Z = 80
D) Bismuth, Bi, Z = 83
E) Sodium, Na, Z = 11
A) Lithium, Li, Z = 3
B) Bromine, Br, Z = 35
C) Mercury, Hg, Z = 80
D) Bismuth, Bi, Z = 83
E) Sodium, Na, Z = 11

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which of these elements is chemically similar to magnesium?
A) Sulfur
B) Calcium
C) Iron
D) Nickel
E) Potassium
A) Sulfur
B) Calcium
C) Iron
D) Nickel
E) Potassium

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
41
Which of the following contains ionic bonding?
A) CO
B) SrF2
C) Al
D) OCl2
E) HCl
A) CO
B) SrF2
C) Al
D) OCl2
E) HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
42
What element is represented by X in the atomic symbol notation 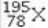 ?
?
A) Iridium
B) Platinum
C) Palladium
D) Selenium
E) Magnesium
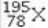 ?
?A) Iridium
B) Platinum
C) Palladium
D) Selenium
E) Magnesium

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
43
Which of these compounds is most likely to be ionic?
A) GaAs
B) SrBr2
C) NO2
D) CBr4
E) H2O
A) GaAs
B) SrBr2
C) NO2
D) CBr4
E) H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
44
The chemical name for ClO3- is "chlorate ion". What is the common name for HClO3?
A) hydrochloric acid
B) chloroform
C) hydrogen trioxychloride
D) chlorous acid
E) chloric acid
A) hydrochloric acid
B) chloroform
C) hydrogen trioxychloride
D) chlorous acid
E) chloric acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
45
Which of these pairs of elements would be most likely to form an ionic compound?
A) Cl and I
B) Al and K
C) Cl and Mg
D) C and S
E) Al and Mg
A) Cl and I
B) Al and K
C) Cl and Mg
D) C and S
E) Al and Mg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
46
Which of the following is an ionic compound?
A) H2S
B) NH3
C) I2
D) KI
E) CCl4
A) H2S
B) NH3
C) I2
D) KI
E) CCl4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
47
Determine the number of protons, electrons, and neutrons for the isotope gold-118. The symbol for gold is Au.
A) 118 protons, 118 electrons, 79 neutrons
B) 79 protons, 79 electrons, 118 neutrons
C) 79 protons, 79 electrons, 39 neutrons
D) 118 protons, 118 electrons, 39 neutrons
E) 79 protons, 39 electrons, 118 neutrons
A) 118 protons, 118 electrons, 79 neutrons
B) 79 protons, 79 electrons, 118 neutrons
C) 79 protons, 79 electrons, 39 neutrons
D) 118 protons, 118 electrons, 39 neutrons
E) 79 protons, 39 electrons, 118 neutrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which of these pairs of elements would be most likely to form an ionic compound?
A) P and Br
B) Cu and K
C) C and O
D) O and Zn
E) Al and Rb
A) P and Br
B) Cu and K
C) C and O
D) O and Zn
E) Al and Rb

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
49
Which is the correct formula for copper(II) phosphate?
A) Cu2PO4
B) Cu3(PO4)2
C) Cu2PO3
D) Cu(PO4)2
E) Cu(PO3)2
A) Cu2PO4
B) Cu3(PO4)2
C) Cu2PO3
D) Cu(PO4)2
E) Cu(PO3)2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
50
Which one of these species is an ion?
A) B3+
B) NaCl
C) He
D) (14C)
E) None of these species is an ion.
A) B3+
B) NaCl
C) He
D) (14C)
E) None of these species is an ion.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
51
Which pair of elements would be most likely to form an ionic compound?
A) P and Br
B) Zn and K
C) F and Al
D) C and S
E) Al and Rb
A) P and Br
B) Zn and K
C) F and Al
D) C and S
E) Al and Rb

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
52
What is the formula for the ionic compound formed by calcium and selenium?
A) CaSe
B) Ca2Se
C) CaSe2
D) Ca3Se
E) CaSe3
A) CaSe
B) Ca2Se
C) CaSe2
D) Ca3Se
E) CaSe3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
53
Which of these compounds is most likely to be ionic?
A) KF
B) CCl4
C) CS2
D) CO2
E) ICl
A) KF
B) CCl4
C) CS2
D) CO2
E) ICl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
54
Which of these compounds is most likely to be ionic?
A) NCl3
B) BaCl2
C) CO
D) SO2
E) SF4
A) NCl3
B) BaCl2
C) CO
D) SO2
E) SF4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
55
An anion is defined as
A) an atom or group of atoms with a net negative charge.
B) a stable atom.
C) a group of stable atoms.
D) an atom or group of atoms with a net positive charge.
E) neutral.
A) an atom or group of atoms with a net negative charge.
B) a stable atom.
C) a group of stable atoms.
D) an atom or group of atoms with a net positive charge.
E) neutral.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
56
The formula for magnesium sulfate is
A) MnS.
B) MgS.
C) MnSO3.
D) MgSO4.
E) MnSO4.
A) MnS.
B) MgS.
C) MnSO3.
D) MgSO4.
E) MnSO4.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
57
What is the formula for the ionic compound formed by calcium ions and nitrate ions?
A) Ca3N2
B) Ca(NO3)2
C) Ca2NO3
D) Ca2NO2
E) CaNO3
A) Ca3N2
B) Ca(NO3)2
C) Ca2NO3
D) Ca2NO2
E) CaNO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
58
Which of these elements is chemically similar to potassium?
A) calcium
B) arsenic
C) phosphorus
D) cerium
E) cesium
A) calcium
B) arsenic
C) phosphorus
D) cerium
E) cesium

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
59
Determine the number of protons and identify the correct symbol for an atom with 20 neutrons and 20 electrons.
A) 20 protons,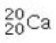
B) 20 protons,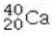
C) 20 protons,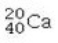
D) 40 protons,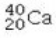
E) 40 protons,
A) 20 protons,
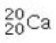
B) 20 protons,
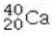
C) 20 protons,
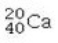
D) 40 protons,
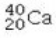
E) 40 protons,


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
60
Determine the number of electrons and identify the correct symbol for an atom with 17 protons and 18 neutrons.
A) 17 electrons,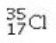
B) 18 electrons,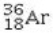
C) 17 electrons,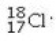
D) 17 electrons,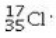
E) 18 electrons,
A) 17 electrons,
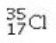
B) 18 electrons,
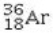
C) 17 electrons,
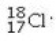
D) 17 electrons,
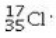
E) 18 electrons,


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
61
What is the name of PCl3?
A) phosphorus chloride
B) phosphoric chloride
C) phosphorus trichlorate
D) trichlorophosphide
E) phosphorus trichloride
A) phosphorus chloride
B) phosphoric chloride
C) phosphorus trichlorate
D) trichlorophosphide
E) phosphorus trichloride

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
62
What is the name of Mn(CO3)2?
A) manganese carbide
B) magnesium(IV) carbonate
C) manganese(II) carbonate
D) magnesium(II) carbonate
E) manganese(IV) carbonate
A) manganese carbide
B) magnesium(IV) carbonate
C) manganese(II) carbonate
D) magnesium(II) carbonate
E) manganese(IV) carbonate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
63
Iron(III) chloride hexahydrate is used as a coagulant for sewage and industrial wastes. What is its formula?
A) Fe(Cl·6H2O)3
B) Fe3Cl·6H2O
C) FeCl3(H2O)6
D) Fe3Cl(H2O)6
E) FeCl3·6H2O
A) Fe(Cl·6H2O)3
B) Fe3Cl·6H2O
C) FeCl3(H2O)6
D) Fe3Cl(H2O)6
E) FeCl3·6H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
64
The compound, P4S10, is used in the manufacture of safety matches. What is its name?
A) phosphorus sulfide
B) phosphoric sulfide
C) phosphorus decasulfide
D) tetraphosphorus decasulfide
E) phosphorus sulfite
A) phosphorus sulfide
B) phosphoric sulfide
C) phosphorus decasulfide
D) tetraphosphorus decasulfide
E) phosphorus sulfite

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
65
What is the name of P4Se3?
A) phosphorus selenide
B) phosphorus triselenide
C) tetraphosphorus selenide
D) phosphoric selenide
E) tetraphosphorus triselenide
A) phosphorus selenide
B) phosphorus triselenide
C) tetraphosphorus selenide
D) phosphoric selenide
E) tetraphosphorus triselenide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
66
Potassium permanganate is a strong oxidizer that reacts explosively with easily oxidized materials. What is its formula?
A) KMnO3
B) KMnO4
C) K2MnO4
D) K(MnO4)2
E) K2Mn2O7
A) KMnO3
B) KMnO4
C) K2MnO4
D) K(MnO4)2
E) K2Mn2O7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
67
What is the formula for the permanganate ion?
A) MnO2-
B) MnO4-
C) MgO42-
D) Mn2O7-
E) MgO22-
A) MnO2-
B) MnO4-
C) MgO42-
D) Mn2O7-
E) MgO22-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
68
Diiodine pentaoxide is used as an oxidizing agent that converts carbon monoxide to carbon dioxide. What is its chemical formula?
A) I2O5
B) IO5
C) 2IO5
D) I5O2
E) (IO5)2
A) I2O5
B) IO5
C) 2IO5
D) I5O2
E) (IO5)2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
69
What is the formula of iodous acid?
A) HI
B) HIO3
C) HIO
D) HIO4
E) HIO2
A) HI
B) HIO3
C) HIO
D) HIO4
E) HIO2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
70
What is the formula for lead(II) oxide?
A) PbO
B) PbO2
C) Pb2O
D) PbO4
E) Pb2O3
A) PbO
B) PbO2
C) Pb2O
D) PbO4
E) Pb2O3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
71
Tetrasulfur dinitride decomposes explosively when heated. What is its formula?
A) S2N4
B) S4N2
C) 4SN2
D) S4N
E) S2N
A) S2N4
B) S4N2
C) 4SN2
D) S4N
E) S2N

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
72
Which of the following is the oxoanion of bromine called the bromate ion?
A) BrO3-
B) BrO32-
C) BrO42-
D) BrO2-
E) BrO-
A) BrO3-
B) BrO32-
C) BrO42-
D) BrO2-
E) BrO-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
73
Which one of the following formulas of ionic compounds is the least likely to be correct?
A) NH4Cl
B) Ba(OH)2
C) Na2SO4
D) Ca2NO3
E) Cu(CN)2
A) NH4Cl
B) Ba(OH)2
C) Na2SO4
D) Ca2NO3
E) Cu(CN)2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
74
What is the name of ClO - ion?
A) hypochlorite
B) chlorate
C) chlorite
D) perchlorate
E) perchlorite
A) hypochlorite
B) chlorate
C) chlorite
D) perchlorate
E) perchlorite

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
75
What is the name of Ba(NO2)2·3H2O?
A) barium nitrite
B) trihydrobarium(II) nitrite
C) barium nitrite trihydrate
D) barium(II) nitrite trihydrate
E) barium nitrate trihydrate
A) barium nitrite
B) trihydrobarium(II) nitrite
C) barium nitrite trihydrate
D) barium(II) nitrite trihydrate
E) barium nitrate trihydrate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
76
The formula for sodium sulfide is
A) NaS.
B) K2S.
C) NaS2.
D) Na2S.
E) SeS.
A) NaS.
B) K2S.
C) NaS2.
D) Na2S.
E) SeS.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
77
What combination of element types will typically result in ionic bonding?
A) two metals
B) a nonmetal and a metal
C) two nonmetals
D) two Group 1A elements
E) two noble gases
A) two metals
B) a nonmetal and a metal
C) two nonmetals
D) two Group 1A elements
E) two noble gases

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
78
Ferric oxide is used as a pigment in metal polishing. Which of the following is its formula?
A) FeO
B) Fe2O
C) FeO3
D) Fe2O5
E) Fe2O3
A) FeO
B) Fe2O
C) FeO3
D) Fe2O5
E) Fe2O3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
79
What is the formula of hydrobromic acid?
A) H2OBr
B) HBrO3
C) HBrO
D) HBr
E) HBr·2H2O
A) H2OBr
B) HBrO3
C) HBrO
D) HBr
E) HBr·2H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck
80
The chemical formula for iron(II) nitrate is
A) Fe2(NO3)3.
B) Ir(NO2)2.
C) Fe2N3.
D) Fe(NO3)2.
E) Fe(NO2)2.
A) Fe2(NO3)3.
B) Ir(NO2)2.
C) Fe2N3.
D) Fe(NO3)2.
E) Fe(NO2)2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 124 في هذه المجموعة.
فتح الحزمة
k this deck








