Deck 22: Coordination Chemistry
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/132
العب
ملء الشاشة (f)
Deck 22: Coordination Chemistry
1
What is the electron configuration of a ground-state Fe2+ ion?
A) [Ar]3d5
B) [Ar]4s13d5
C) [Ar]3d6
D) [Ar] 4s14d5
E) [Ar]4d6
A) [Ar]3d5
B) [Ar]4s13d5
C) [Ar]3d6
D) [Ar] 4s14d5
E) [Ar]4d6
[Ar]4s13d5
2
What is the electron configuration of a ground-state Ag atom?
A) [Kr]5s14d10
B) [Kr]5s15d10
C) [Kr]5s24d9
D) [Kr]5s25d9
E) [Kr]5d10
A) [Kr]5s14d10
B) [Kr]5s15d10
C) [Kr]5s24d9
D) [Kr]5s25d9
E) [Kr]5d10
[Kr]5s14d10
3
How many 4d electrons does a ground-state Ag+ ion have?
A) 8
B) 1
C) 10
D) 2
E) 9
A) 8
B) 1
C) 10
D) 2
E) 9
10
4
How many 3d electrons does a ground-state Sc3+ ion have?
A) 1
B) 3
C) 2
D) 10
E) 0
A) 1
B) 3
C) 2
D) 10
E) 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
5
How many 3d electrons does a ground-state Pd2+ ion have?
A) 8
B) 10
C) 9
D) 7
E) 6
A) 8
B) 10
C) 9
D) 7
E) 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
6
What is the electron configuration of a ground-state Cu+ ion?
A) [Ar]3d10
B) [Ar]4d10
C) [Ar]3d9
D) [Ar]4d9
E) None of these choices.
A) [Ar]3d10
B) [Ar]4d10
C) [Ar]3d9
D) [Ar]4d9
E) None of these choices.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which statement is correct about coordination compounds?
A) They consist only of nonmetals.
B) They contain ionic bonds.
C) They are formed by reactions of metal ions with groups of anions or polar molecules.
D) They contain only metals.
A) They consist only of nonmetals.
B) They contain ionic bonds.
C) They are formed by reactions of metal ions with groups of anions or polar molecules.
D) They contain only metals.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
8
What type of structure is exhibited by most of the transition metals?
A) Close-packed
B) Tetrahedral
C) Trigonal planar
D) Trigonal pyramidal
E) None of the answers is correct.
A) Close-packed
B) Tetrahedral
C) Trigonal planar
D) Trigonal pyramidal
E) None of the answers is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
9
What is the electron configuration of a ground-state Zn2+ ion?
A) 1s22s22p63s23p64s13d9
B) 1s22s22p63s23p64s14d9
C) 1s22s22p63s23p64s23d8
D) 1s22s22p63s23p64s24d8
E) 1s22s22p63s23p63d10
A) 1s22s22p63s23p64s13d9
B) 1s22s22p63s23p64s14d9
C) 1s22s22p63s23p64s23d8
D) 1s22s22p63s23p64s24d8
E) 1s22s22p63s23p63d10

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
10
How many 4d electrons does a ground-state Cd2+ ion have?
A) 8
B) 2
C) 9
D) 7
E) 10
A) 8
B) 2
C) 9
D) 7
E) 10

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
11
How many 3d electrons does a ground-state Nb3+ ion have?
A) 2
B) 4
C) 3
D) 10
E) 5
A) 2
B) 4
C) 3
D) 10
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
12
What is the electron configuration of a ground-state Cr atom?
A) 1s22s22p63s23p64s23d4
B) 1s22s22p63s23p63d6
C) 1s22s22p63s23p64s14d5
D) 1s22s22p63s23p64s24d4
E) 1s22s22p63s23p64s13d5
A) 1s22s22p63s23p64s23d4
B) 1s22s22p63s23p63d6
C) 1s22s22p63s23p64s14d5
D) 1s22s22p63s23p64s24d4
E) 1s22s22p63s23p64s13d5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which are the most common transition metals?
A) The transition metals in the 3rd period.
B) The transition metals in the 4th period.
C) The elements in Group 6B.
D) The transition metals in the 5th period.
E) The transition metals in the 6th period.
A) The transition metals in the 3rd period.
B) The transition metals in the 4th period.
C) The elements in Group 6B.
D) The transition metals in the 5th period.
E) The transition metals in the 6th period.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
14
In the complex ion [Fe(CN)6]4-, what is the oxidation number of Fe?
A) +1
B) +2
C) +3
D) -4
E) +6
A) +1
B) +2
C) +3
D) -4
E) +6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which statement is correct about transition metals?
A) They have incompletely filled d subshells.
B) They form ions with incompletely filled d subshells.
C) They form paramagnetic compounds.
D) They have catalytic activity.
E) All of the answers are correct.
A) They have incompletely filled d subshells.
B) They form ions with incompletely filled d subshells.
C) They form paramagnetic compounds.
D) They have catalytic activity.
E) All of the answers are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
16
What is the electron configuration of a ground-state Cu atom?
A) 1s22s22p63s23p64s13d10
B) 1s22s22p63s23p63d10
C) 1s22s22p63s23p64s14d10
D) 1s22s22p63s23p64s24d9
E) 1s22s22p63s23p64s23d9
A) 1s22s22p63s23p64s13d10
B) 1s22s22p63s23p63d10
C) 1s22s22p63s23p64s14d10
D) 1s22s22p63s23p64s24d9
E) 1s22s22p63s23p64s23d9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
17
What is the electron configuration of a ground-state Nb2+ ion?
A) 1s22s22p63s23p64s23d104p65s24d1
B) 1s22s22p63s23p64s23d104p64d3
C) 1s22s22p63s23p64s23d104p65s14d2
D) 1s22s22p63s23p64s23d104p64d5
E) 1s22s22p63s23p64s23d104p65s24d3
A) 1s22s22p63s23p64s23d104p65s24d1
B) 1s22s22p63s23p64s23d104p64d3
C) 1s22s22p63s23p64s23d104p65s14d2
D) 1s22s22p63s23p64s23d104p64d5
E) 1s22s22p63s23p64s23d104p65s24d3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
18
How many 3d electrons does a ground-state Fe3+ ion have?
A) 3
B) 5
C) 6
D) 7
E) 9
A) 3
B) 5
C) 6
D) 7
E) 9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
19
How many 4d electrons does a ground-state Mo atom have?
A) 1
B) 4
C) 3
D) 5
E) 2
A) 1
B) 4
C) 3
D) 5
E) 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
20
In K4[Fe(CN)6], how many 3d electrons does the iron atom have?
A) 3
B) 4
C) 5
D) 6
E) 7
A) 3
B) 4
C) 5
D) 6
E) 7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
21
Write the formula for diamminedichloroethylenediaminecobalt(III) bromide.
A) [CoCl2(en)(NH3)2]Br
B) [CoCl2(en)(NH3) 2]Br2
C) [CoCl2(en)2(NH3)2]Br
D) [CoCl2(en)2(NH3)2]Br2
E) (NH3)2Cl2(en)Co3Br
A) [CoCl2(en)(NH3)2]Br
B) [CoCl2(en)(NH3) 2]Br2
C) [CoCl2(en)2(NH3)2]Br
D) [CoCl2(en)2(NH3)2]Br2
E) (NH3)2Cl2(en)Co3Br

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which of the following ligands has two different types of donor atoms?
A) NH3
B) H2O
C) NH4+
D) NO2-
E) ethylenediamine
A) NH3
B) H2O
C) NH4+
D) NO2-
E) ethylenediamine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
23
What is the formula for pentaamminechlorocobalt(III) chloride?
A) [Co(NH3)5Cl]Cl
B) [Co(NH3)5Cl]Cl2
C) [Co(NH3)5Cl]Cl3
D) [Co(NH3)5Cl]Cl4
E) [CoCl3](NH3)5
A) [Co(NH3)5Cl]Cl
B) [Co(NH3)5Cl]Cl2
C) [Co(NH3)5Cl]Cl3
D) [Co(NH3)5Cl]Cl4
E) [CoCl3](NH3)5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
24
In the compound K[Co(C2O4)2(H2O)2] (where C2O42- = oxalate) the oxidation number and coordination number of cobalt are ________ and ________, respectively.
A) -1; 4
B) -1; 6
C) +3; 4
D) +3; 6
E) +1; 6
A) -1; 4
B) -1; 6
C) +3; 4
D) +3; 6
E) +1; 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
25
In the complex ion [Co(en)2Br2]+, what is the oxidation number of Co?
A) +1
B) +2
C) +3
D) -2
E) -1
A) +1
B) +2
C) +3
D) -2
E) -1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
26
What is the systematic name for [Cu(NH3)4]Cl2?
A) dichlorotetraamminecuprate(II)
B) tetraamminecopper(II) chloride
C) copper(II) ammonium chloride
D) tetraaminocopper(II) chloride
E) tetramminecopper(II) dichloride
A) dichlorotetraamminecuprate(II)
B) tetraamminecopper(II) chloride
C) copper(II) ammonium chloride
D) tetraaminocopper(II) chloride
E) tetramminecopper(II) dichloride

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
27
What is the systematic name for [CoCl3(H2O)]-?
A) cobalt(III) chloride monohydrate
B) aquatrichlorocobalt(II)
C) aquatrichlorocobaltate(II)
D) aquatrichlorocobaltite(I)
E) monoaquotris(chloro)cobalt(IV)
A) cobalt(III) chloride monohydrate
B) aquatrichlorocobalt(II)
C) aquatrichlorocobaltate(II)
D) aquatrichlorocobaltite(I)
E) monoaquotris(chloro)cobalt(IV)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
28
In the coordination compound [Pt(NH3)2Cl2], the coordination number and oxidation number of the central atom are ________ and ________, respectively.
A) 2; 0
B) 4; +4
C) 5; 0
D) 4; +2
E) 6; +2
A) 2; 0
B) 4; +4
C) 5; 0
D) 4; +2
E) 6; +2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which response gives the correct coordination number (C.N.) and oxidation number (O.N.) of the transition metal atom in [Co(NH3)2(H2O)2Cl2]+?
A) C.N. = 2; O.N. = +3
B) C.N. = 3; O.N. = +1
C) C.N. = 4; O.N. = +2
D) C.N. = 6; O.N. = +1
E) C.N. = 6; O.N. = +3
A) C.N. = 2; O.N. = +3
B) C.N. = 3; O.N. = +1
C) C.N. = 4; O.N. = +2
D) C.N. = 6; O.N. = +1
E) C.N. = 6; O.N. = +3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
30
In the coordination compound K2[Co(en)Cl4], the coordination number (C.N.) and oxidation number (O.N.) of cobalt are, respectively,
A) C.N. = 6; O.N. = +2.
B) C.N. = 6; O.N. = +3.
C) C.N. = 5; O.N. = +2.
D) C.N. = 5; O.N. = +4.
E) C.N. = 4; O.N. = +3.
A) C.N. = 6; O.N. = +2.
B) C.N. = 6; O.N. = +3.
C) C.N. = 5; O.N. = +2.
D) C.N. = 5; O.N. = +4.
E) C.N. = 4; O.N. = +3.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
31
The compound K3[Fe(CN)6] is used in calico printing and wool dyeing. What is its systematic name?
A) potassium iron(III) hexacyanate
B) tripotassium iron(III) hexacyanate
C) potassium hexacyanoferrate(III)
D) potassium hexacyanideferrate
E) tripotassium hexacyanoiron(III)
A) potassium iron(III) hexacyanate
B) tripotassium iron(III) hexacyanate
C) potassium hexacyanoferrate(III)
D) potassium hexacyanideferrate
E) tripotassium hexacyanoiron(III)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
32
What is the systematic name for Cr(CO)3(NH3)3?
A) chromiumtriaminotricarbonyl
B) triamminechromium carbonate
C) triamminetricarbonylchromate(0)
D) triamminetricarbonylchromium(0)
E) tris(amino)tris(carbonyl)chromium
A) chromiumtriaminotricarbonyl
B) triamminechromium carbonate
C) triamminetricarbonylchromate(0)
D) triamminetricarbonylchromium(0)
E) tris(amino)tris(carbonyl)chromium

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following geometries is not common in transition-metal complex ions?
A) Tetrahedral
B) Octahedral
C) Linear
D) Trigonal planar
E) Square planar
A) Tetrahedral
B) Octahedral
C) Linear
D) Trigonal planar
E) Square planar

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
34
What is the coordination number of cobalt in the complex ion [Co(en)Cl4]-? (en = ethylenediamine)
A) 1
B) 2
C) 4
D) 6
E) 8
A) 1
B) 2
C) 4
D) 6
E) 8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
35
In the coordination compound [Cr(NH3)(en)2Cl]Br2, the coordination number (C.N.) and oxidation number (O.N.) of the metal atom are, respectively,
A) C.N. = 6; O.N. = +4.
B) C.N. = 6; O.N. = +3.
C) C.N. = 5; O.N. = +2.
D) C.N. = 4; O.N. = +2.
E) C.N. = 4; O.N. = +3.
A) C.N. = 6; O.N. = +4.
B) C.N. = 6; O.N. = +3.
C) C.N. = 5; O.N. = +2.
D) C.N. = 4; O.N. = +2.
E) C.N. = 4; O.N. = +3.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
36
In the complex ion [Cr(C2O4)2(H2O)2]-, what is the oxidation number of Cr?
A) +1
B) +2
C) +3
D) -2
E) -1
A) +1
B) +2
C) +3
D) -2
E) -1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which of the following metals is capable of forming complexes spanning the smallest range of oxidation numbers?
A) Cu
B) Sc
C) Fe
D) Mn
E) Ni
A) Cu
B) Sc
C) Fe
D) Mn
E) Ni

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
38
In the coordination compound [Co(en)2Cl2]Cl, the coordination number and oxidation number of the central atom are ________ and ________, respectively.
A) 4; +3
B) 6; +2
C) 4; +2
D) 6; +3
E) 4; +1
A) 4; +3
B) 6; +2
C) 4; +2
D) 6; +3
E) 4; +1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
39
In the coordination compound [Cr(NH3)2(en)Cl2]Br2, the coordination number (C.N.) and oxidation number (O.N.) of the metal atom, respectively, are
A) C.N. = 6; O.N. = +4.
B) C.N. = 6; O.N. = +3.
C) C.N. = 5; O.N. = +2.
D) C.N. = 5; O.N. = +4.
E) C.N. = 4; O.N. = +3.
A) C.N. = 6; O.N. = +4.
B) C.N. = 6; O.N. = +3.
C) C.N. = 5; O.N. = +2.
D) C.N. = 5; O.N. = +4.
E) C.N. = 4; O.N. = +3.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which of the following metals is capable of forming complexes spanning the largest range of oxidation numbers?
A) Cu
B) Sc
C) Fe
D) Mn
E) Ni
A) Cu
B) Sc
C) Fe
D) Mn
E) Ni

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
41
Consider the following octahedral complex structures, each involving ethylene diamine and two different, unidentate ligands X and Y. 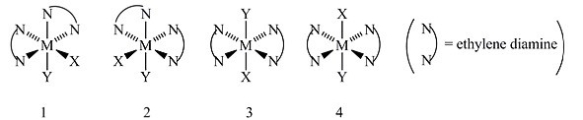 Which, if any, of the following pairs are optical isomers?
Which, if any, of the following pairs are optical isomers?
A) Structures 1 and 2
B) Structures 1 and 3
C) Structures 1 and 4
D) Structures 3 and 4
E) Structures 1, 2, 3, and 4
 Which, if any, of the following pairs are optical isomers?
Which, if any, of the following pairs are optical isomers?A) Structures 1 and 2
B) Structures 1 and 3
C) Structures 1 and 4
D) Structures 3 and 4
E) Structures 1, 2, 3, and 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
42
Consider the following structures (1 and 2 are octahedral; 3 and 4 are square planar). 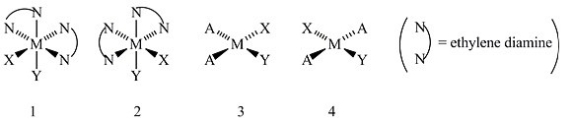 Which one of the following statements about the above structures is correct?
Which one of the following statements about the above structures is correct?
A) Structures 1 and 2 are superimposable.
B) Structures 1 and 2 are geometric isomers.
C) Structures 3 and 4 are structural isomers.
D) Structures 3 and 4 are optical isomers.
E) Structures 3 and 4 are geometric isomers.
 Which one of the following statements about the above structures is correct?
Which one of the following statements about the above structures is correct?A) Structures 1 and 2 are superimposable.
B) Structures 1 and 2 are geometric isomers.
C) Structures 3 and 4 are structural isomers.
D) Structures 3 and 4 are optical isomers.
E) Structures 3 and 4 are geometric isomers.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
43
Which of these diagrams represent the crystal field splitting between d orbitals in a tetrahedral complex?
A)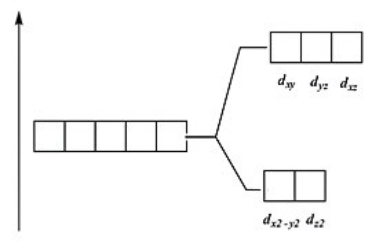
B)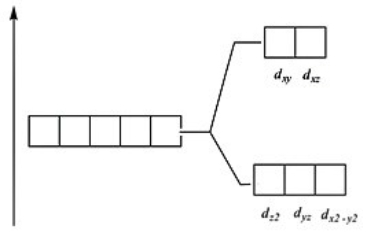
C)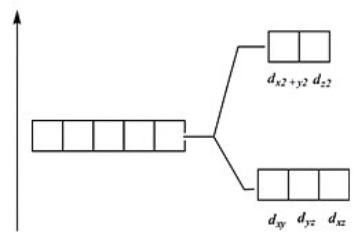
D)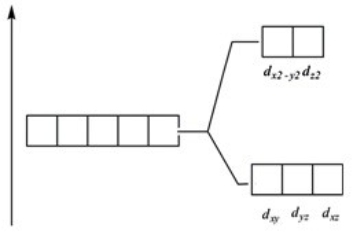
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
44
In the spectrochemical series, which one of the following ligands has the strongest field?
A) H2O
B) CN-
C) NH3
D) OH-
E) Cl-
A) H2O
B) CN-
C) NH3
D) OH-
E) Cl-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
45
Which of the following octahedral complexes should have the largest crystal field splitting energy, Δ?
A) [Cr(H2O)6]3+
B) [Cr(SCN)6]3
C) [Cr(NH3)6]3+
D) [Cr(CN)6]3-
E) [Cr(en)3]3+ (en = ethylenediamine)
A) [Cr(H2O)6]3+
B) [Cr(SCN)6]3
C) [Cr(NH3)6]3+
D) [Cr(CN)6]3-
E) [Cr(en)3]3+ (en = ethylenediamine)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
46
Which of the following ions is most likely to form colored compounds?
A) Sc3+
B) Cu+
C) Zn2+
D) Cr3+
E) Ca2+
A) Sc3+
B) Cu+
C) Zn2+
D) Cr3+
E) Ca2+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
47
The crystal field splitting energy, Δ,
A) is larger for tetrahedral complexes than for octahedral complexes.
B) depends on the metal but not on the ligand.
C) determines the color of a complex.
D) is larger for ionic ligands like chloride than for molecular ligands like carbon monoxide, CO.
E) determines the charge of a complex.
A) is larger for tetrahedral complexes than for octahedral complexes.
B) depends on the metal but not on the ligand.
C) determines the color of a complex.
D) is larger for ionic ligands like chloride than for molecular ligands like carbon monoxide, CO.
E) determines the charge of a complex.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
48
What is the systematic name for [Ru(NH3)2(en)](NO3)2?
A) (ethylenediamine)diammineruthenium(II) nitrate
B) diamminebis(ethylenediamine)ruthenium(III) nitrate
C) diammine(ethylenediamine)ruthenium(II) nitrate
D) diammine(ethylenediamine)nitratoruthenium(III)
E) bis(ethylene)diamminenitratoruthenate(II)
A) (ethylenediamine)diammineruthenium(II) nitrate
B) diamminebis(ethylenediamine)ruthenium(III) nitrate
C) diammine(ethylenediamine)ruthenium(II) nitrate
D) diammine(ethylenediamine)nitratoruthenium(III)
E) bis(ethylene)diamminenitratoruthenate(II)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
49
Which of these diagrams represent the crystal field splitting between d orbitals in an octahedral complex?
A)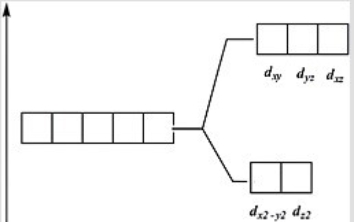
B)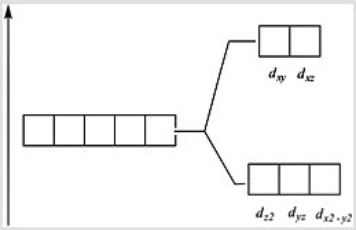
C)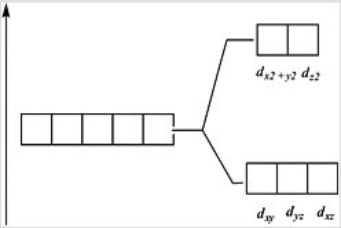
D)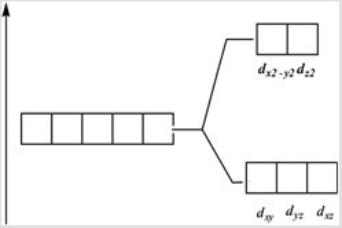
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
50
Which of these electron energy level patterns would absorb light with the shortest wavelength?
A)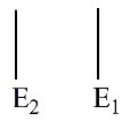
B)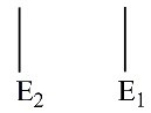
C)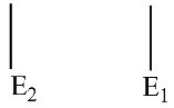
D)
A)
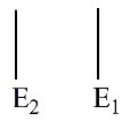
B)
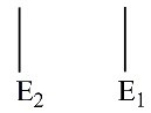
C)
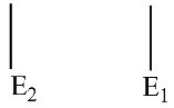
D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
51
Which of the following ions is least likely to form colored compounds?
A) Mn2+
B) Cr5+
C) Sc3+
D) Fe3+
E) Co2+
A) Mn2+
B) Cr5+
C) Sc3+
D) Fe3+
E) Co2+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
52
Which of these square planar complex ions can have cis-trans isomers?
A) [Pt(NH3)4]2+
B) [Ni(NH3)4]2+
C) [Pt(NH3)2Cl2]
D) [Pt(NH3)Cl3]-
E) [Ni(NH3)3Cl]+
A) [Pt(NH3)4]2+
B) [Ni(NH3)4]2+
C) [Pt(NH3)2Cl2]
D) [Pt(NH3)Cl3]-
E) [Ni(NH3)3Cl]+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
53
Which of the following ligands is most likely to form a low-spin octahedral complex with iron(III)?
A) Cl-
B) H2O
C) NH3
D) OH-
E) CO
A) Cl-
B) H2O
C) NH3
D) OH-
E) CO

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
54
The numbers of geometrical isomers and optical isomers of the complex ion [Co(en)3]3+ are, respectively,
A) 2 and 2
B) 1 and 1
C) 3 and 2
D) 1 and 2
E) 2 and 4
A) 2 and 2
B) 1 and 1
C) 3 and 2
D) 1 and 2
E) 2 and 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
55
Which of the following species could exist as isomers?
A) [Co(H2O)4Cl2]+
B) [Pt(NH3)Br3]-
C) [Pt(en)Cl2]
D) [Pt(NH3)3Cl]+
E) [CuBr4]2+
A) [Co(H2O)4Cl2]+
B) [Pt(NH3)Br3]-
C) [Pt(en)Cl2]
D) [Pt(NH3)3Cl]+
E) [CuBr4]2+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
56
Write the formula for sodium tetracyanonickelate(II).
A) Na[Ni(CN)4]
B) Na[Ni(CN)4]2
C) Na2[Ni(CN)4]
D) Na4[Ni(CN)4]
E) Na[(NiCN)4]
A) Na[Ni(CN)4]
B) Na[Ni(CN)4]2
C) Na2[Ni(CN)4]
D) Na4[Ni(CN)4]
E) Na[(NiCN)4]

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
57
Two well-known complex ions containing Ni are [Ni(H2O)6]2+, which is green, and [Ni(en)3]2+, which is purple. Which one of these statements is true?
A) The crystal field splitting energy (Δ) is greater for [Ni(en)3]2+ than for [Ni(H2O)6]2+.
B) [Ni(en)3]2+ absorbs energy in the red region of the spectrum.
C) Both complex ions are diamagnetic.
D) [Ni(H2O)6]2+ transmits light with wavelengths of approximately 650-700 nm.
E) The green complex absorbs green light.
A) The crystal field splitting energy (Δ) is greater for [Ni(en)3]2+ than for [Ni(H2O)6]2+.
B) [Ni(en)3]2+ absorbs energy in the red region of the spectrum.
C) Both complex ions are diamagnetic.
D) [Ni(H2O)6]2+ transmits light with wavelengths of approximately 650-700 nm.
E) The green complex absorbs green light.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
58
Consider the following octahedral complex structures, each involving ethylene diamine and two different, unidentate ligands X and Y. 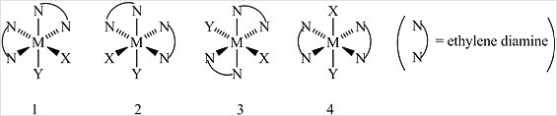 Which one of the following statements about these structures is incorrect?
Which one of the following statements about these structures is incorrect?
A) Structures 1 and 2 are optical isomers.
B) Structures 1 and 3 are optical isomers.
C) Structures 1 and 3 are different complexes.
D) Structures 1 and 4 are geometrical isomers.
E) Structures 3 and 4 are the same complex.
 Which one of the following statements about these structures is incorrect?
Which one of the following statements about these structures is incorrect?A) Structures 1 and 2 are optical isomers.
B) Structures 1 and 3 are optical isomers.
C) Structures 1 and 3 are different complexes.
D) Structures 1 and 4 are geometrical isomers.
E) Structures 3 and 4 are the same complex.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
59
Cis-trans isomerism is exhibited by which one of these ions?
A) [Pd(NH3)3Cl]+
B) [Co(NH3)5Cl]2+
C) [Fe(CN)6]3-
D) All of the choices are correct.
E) None of these choices.
A) [Pd(NH3)3Cl]+
B) [Co(NH3)5Cl]2+
C) [Fe(CN)6]3-
D) All of the choices are correct.
E) None of these choices.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
60
In a coordination compound involving a complex ion of square planar geometry, which of the following types of isomerism is/are never possible?
A) Geometric
B) Optical
C) Linkage
D) Coordination
E) More than one of these choices is correct.
A) Geometric
B) Optical
C) Linkage
D) Coordination
E) More than one of these choices is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
61
Which one of these species is a bidentate ligand?
A) CN-
B) NH3
C) CO
D) H2NCH2CH2NH2
E) EDTA
A) CN-
B) NH3
C) CO
D) H2NCH2CH2NH2
E) EDTA

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
62
How many unpaired electrons are there in the complex ion [Mn(CN)6]3-?
A) 0
B) 1
C) 2
D) 3
E) 5
A) 0
B) 1
C) 2
D) 3
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
63
The ion [Co(NH3)6]2+ is octahedral and high spin. This complex is
A) paramagnetic, with 1 unpaired electron.
B) paramagnetic, with 3 unpaired electrons.
C) paramagnetic, with 4 unpaired electrons.
D) paramagnetic, with 5 unpaired electrons.
E) diamagnetic.
A) paramagnetic, with 1 unpaired electron.
B) paramagnetic, with 3 unpaired electrons.
C) paramagnetic, with 4 unpaired electrons.
D) paramagnetic, with 5 unpaired electrons.
E) diamagnetic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
64
The net rotation of plane-polarized light by a racemic mixture is ________.
A) to the right
B) to the left
C) zero
A) to the right
B) to the left
C) zero

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
65
What is the name of the atom in a ligand that is bound directly to the metal atom?
A) Acceptor atom
B) Donor atom
C) Chelating atom
D) Coordinator
E) Chelating agent
A) Acceptor atom
B) Donor atom
C) Chelating atom
D) Coordinator
E) Chelating agent

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
66
Which of the following ions could exist in either the high-spin or low-spin state in an octahedral complex?
A) Sc3+
B) Ni2+
C) Mn2+
D) Ti4+
E) Zn2+
A) Sc3+
B) Ni2+
C) Mn2+
D) Ti4+
E) Zn2+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
67
A bidentate ligand always
A) forms bonds to two metal ions.
B) has a charge of 2+ or 2-.
C) forms complex ions with a charge of 2+ or 2-.
D) has two donor atoms.
E) has medical uses.
A) forms bonds to two metal ions.
B) has a charge of 2+ or 2-.
C) forms complex ions with a charge of 2+ or 2-.
D) has two donor atoms.
E) has medical uses.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
68
Which of the following ions could exist in only the high-spin state in an octahedral complex?
A) Cr2+
B) Mn4+
C) Fe3+
D) Co3+
E) Ni2+
A) Cr2+
B) Mn4+
C) Fe3+
D) Co3+
E) Ni2+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
69
Cisplatin complexes are
A) used in the extraction of silver and gold.
B) effective antidotes for heavy metal poisoning (e.g., Pb2+ and Hg2+).
C) used to provide nutrients for plants.
D) used to prevent eutrophication of lakes.
E) effective antitumor agents.
A) used in the extraction of silver and gold.
B) effective antidotes for heavy metal poisoning (e.g., Pb2+ and Hg2+).
C) used to provide nutrients for plants.
D) used to prevent eutrophication of lakes.
E) effective antitumor agents.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
70
Ethylenediaminetetraacetic acid (EDTA) is
A) not useful as a chelating agent.
B) an effective antidote for heavy metal poisoning (e.g., Pb2+ and Hg2+).
C) a monodentate ligand.
D) known to form unstable complex ions with Fe3+, Hg2+, and Zn2+.
E) known to form complexes with platinum that inhibit the growth of cancerous cells.
A) not useful as a chelating agent.
B) an effective antidote for heavy metal poisoning (e.g., Pb2+ and Hg2+).
C) a monodentate ligand.
D) known to form unstable complex ions with Fe3+, Hg2+, and Zn2+.
E) known to form complexes with platinum that inhibit the growth of cancerous cells.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
71
The complex ion [Cr(CN)6]4- has magnetic properties that correspond to how many unpaired electrons?
A) 0
B) 1
C) 2
D) 3
E) 5
A) 0
B) 1
C) 2
D) 3
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
72
How many unpaired electrons will there be in a high-spin octahedral complex of Fe(II)?
A) 0
B) 2
C) 4
D) 6
E) 8
A) 0
B) 2
C) 4
D) 6
E) 8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
73
Which of the following will be paramagnetic?
A) V5+
B) Ni2+
C) Mn7+
D) Ti4+
E) Zn2+
A) V5+
B) Ni2+
C) Mn7+
D) Ti4+
E) Zn2+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
74
What is the name of the molecules or ions that surround the metal in a complex ion?
A) Chelaters
B) Donors
C) Acceptors
D) Coordinators
E) Ligands
A) Chelaters
B) Donors
C) Acceptors
D) Coordinators
E) Ligands

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
75
Iron(III) forms an octahedral complex with the ligand CN-. How many unpaired electrons are in the d orbitals of iron?
A) 1
B) 3
C) 5
D) 7
E) 9
A) 1
B) 3
C) 5
D) 7
E) 9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
76
Which of the following will be diamagnetic?
A) Ni2+
B) Cr2+
C) Mn2+
D) Co3+
E) Ti4+
A) Ni2+
B) Cr2+
C) Mn2+
D) Co3+
E) Ti4+

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
77
How many geometric isomers can the following square-planar complex have? 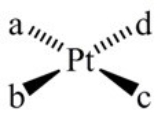
A) 1
B) 2
C) 3
D) 4
E) 6

A) 1
B) 2
C) 3
D) 4
E) 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
78
Which of the following ligands is most likely to form a high-spin octahedral complex with cobalt(II)?
A) CN-
B) en (ethylenediamine)
C) NH3
D) CO
E) I-
A) CN-
B) en (ethylenediamine)
C) NH3
D) CO
E) I-

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
79
If a solution absorbs green light, what is its likely color?
A) Red
B) Violet
C) Orange
D) Yellow
E) Blue
A) Red
B) Violet
C) Orange
D) Yellow
E) Blue

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
80
Which one of these species is a monodentate ligand?
A) CN-
B) EDTA
C) C2O4-
D) H2NCH2CH2NH2
A) CN-
B) EDTA
C) C2O4-
D) H2NCH2CH2NH2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck








