Deck 11: Nuclear Chemistry
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/122
العب
ملء الشاشة (f)
Deck 11: Nuclear Chemistry
1
With the discovery of nuclear reactions, and particularly transmutation, which postulates of Dalton's atomic theory must be modified?
A) Matter is composed of atoms.
B) Atoms of the same element are the same; atoms of different elements are different.
C) Atoms combine with other atoms in fixed, whole number ratios.
D) Atoms may not be changed into other atoms.
A) Matter is composed of atoms.
B) Atoms of the same element are the same; atoms of different elements are different.
C) Atoms combine with other atoms in fixed, whole number ratios.
D) Atoms may not be changed into other atoms.
Atoms may not be changed into other atoms.
2
Which of the following particles has a mass of 1 amu and a charge of 0?
A) neutron
B) proton
C) electron
D) positron
A) neutron
B) proton
C) electron
D) positron
neutron
3
When thorium-234 undergoes beta decay, what other product is formed?
A) thorium-233
B) protactinium-234
C) radium-88
D) uranium-238
A) thorium-233
B) protactinium-234
C) radium-88
D) uranium-238
protactinium-234
4
When radium-223 undergoes alpha decay, what other product is formed?
A) radium-219
B) radium-224
C) actinium-224
D) radon-219
A) radium-219
B) radium-224
C) actinium-224
D) radon-219

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
5
Atoms that have the same atomic number but different numbers of neutrons are
A) radioactive.
B) isotopes.
C) impossible.
D) ions.
A) radioactive.
B) isotopes.
C) impossible.
D) ions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
6
Radiation with enough energy to knock electrons off atoms or molecules is known as
A) electron capture.
B) fundamental radiation
C) ionizing radiation.
D) transmuting radiation.
A) electron capture.
B) fundamental radiation
C) ionizing radiation.
D) transmuting radiation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
7
An alpha particle is the same as a
A) helium-5 nucleus.
B) helium-4 nucleus.
C) hydrogen-1 nucleus.
D) neutron.
A) helium-5 nucleus.
B) helium-4 nucleus.
C) hydrogen-1 nucleus.
D) neutron.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
8
 Rn decays by emission of an alpha particle. The product of this decay is
Rn decays by emission of an alpha particle. The product of this decay isA)
 Ra
RaB)
 Po
PoC)
 Ra
RaD)
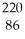 Rn
Rn
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
9
If it were possible to obtain a cubic centimeter of pure atomic nuclei, its mass would be approximately
A) 1 g.
B) 19 g.
C) 1.0 × 108 metric tons.
D) 1 kg.
A) 1 g.
B) 19 g.
C) 1.0 × 108 metric tons.
D) 1 kg.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
10
Uranium-238 decays by emission of an alpha particle. The product of this decay is
A)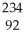 U
U
B)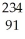 Pa
Pa
C)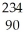 Th
Th
D)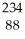 Ra
Ra
A)
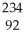 U
UB)
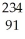 Pa
PaC)
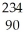 Th
ThD)
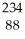 Ra
Ra
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
11
The diameter of an atom is 100,000 times as great as the diameter of its nucleus. If an atom could be expanded to the size of a classroom the nucleus would be about the size of a
A) BB.
B) marble.
C) basketball.
D) period at the end of a sentence.
A) BB.
B) marble.
C) basketball.
D) period at the end of a sentence.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
12
All of the following are known to be forms of ionizing radiation EXCEPT
A) radio waves.
B) gamma rays.
C) ultraviolet radiation.
D) X-rays.
A) radio waves.
B) gamma rays.
C) ultraviolet radiation.
D) X-rays.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
13
Thorium-234 undergoes beta decay: 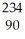 Th →
Th →  e + Q What is Q?
e + Q What is Q?
A)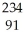 Pa
Pa
B)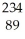 Ac
Ac
C)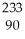 Th
Th
D)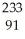 Th
Th
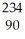 Th →
Th → 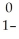 e + Q What is Q?
e + Q What is Q?A)
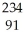 Pa
PaB)
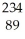 Ac
AcC)
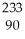 Th
ThD)
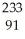 Th
Th
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
14
Cosmic rays are
A) high energy radiation produced by the sun.
B) high energy radiation produced in the ozone layer.
C) high energy radiation produced by the earth's core.
D) none of these
A) high energy radiation produced by the sun.
B) high energy radiation produced in the ozone layer.
C) high energy radiation produced by the earth's core.
D) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
15
The major part of the average exposure of humans to radiation comes from
A) nuclear power plants.
B) diagnostic X-rays.
C) other human made sources.
D) naturally occurring sources.
A) nuclear power plants.
B) diagnostic X-rays.
C) other human made sources.
D) naturally occurring sources.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
16
The following nuclear reaction is an example of  Ra →
Ra → 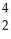 He +
He +  Rn
Rn
A) alpha decay.
B) beta decay.
C) gamma decay.
D) an impossible reaction.
 Ra →
Ra → 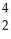 He +
He +  Rn
RnA) alpha decay.
B) beta decay.
C) gamma decay.
D) an impossible reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
17
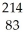 Bi decays by beta emission. The product is
Bi decays by beta emission. The product isA)
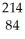 Bi
BiB)
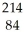 Po
PoC)
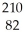 Pa
PaD)
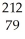 Au
Au
فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
18
The nucleon number decreases by 4 during what type of radioactive decay?
A) beta decay
B) alpha decay
C) gamma radiation
D) positron emission
A) beta decay
B) alpha decay
C) gamma radiation
D) positron emission

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
19
The process of beta emission can be envisioned as the
A) conversion of a proton to a neutron and electron. The electron is emitted.
B) conversion of a neutron to a proton and electron. The electron is emitted.
C) conversion of a neutron to a proton and electron. The proton is emitted.
D) conversion of a proton to a neutron and electron. The proton is emitted.
A) conversion of a proton to a neutron and electron. The electron is emitted.
B) conversion of a neutron to a proton and electron. The electron is emitted.
C) conversion of a neutron to a proton and electron. The proton is emitted.
D) conversion of a proton to a neutron and electron. The proton is emitted.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
20
What does not describe ionizing radiation?
A) Ionizing radiation can devastate living cells by interfering with their normal chemical processes.
B) Ionizing radiation can cause changes in the molecules of DNA in reproductive cells.
C) Ionizing radiation can cause molecules to be split into free radicals, which can disrupt vital cellular processes.
D) Ionizing radiation can affect blood sugar levels and causes diabetes.
A) Ionizing radiation can devastate living cells by interfering with their normal chemical processes.
B) Ionizing radiation can cause changes in the molecules of DNA in reproductive cells.
C) Ionizing radiation can cause molecules to be split into free radicals, which can disrupt vital cellular processes.
D) Ionizing radiation can affect blood sugar levels and causes diabetes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
21
The process of beta decay results in what change in the atomic number?
A) a decrease of 2
B) a decrease of 1
C) an increase of 1
D) no change
A) a decrease of 2
B) a decrease of 1
C) an increase of 1
D) no change

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
22
A piece of cloth is dated using carbon-14. The cloth is determined to be 1400 years old. The half-life of C-14 is 5730 years. The C-14 radioactivity in the cloth will be ________ the radioactivity in a new cloth.
A) greater than
B) the same as
C) less than
D) Radioactivity cannot be predicted from the above information.
A) greater than
B) the same as
C) less than
D) Radioactivity cannot be predicted from the above information.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
23
Phosphorus-30 decays by positron emission. The product is
A) silicon-30.
B) phosphorus-29.
C) sulfur-30.
D) sulfur-29.
A) silicon-30.
B) phosphorus-29.
C) sulfur-30.
D) sulfur-29.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
24
The process of positron emission results in what change in the atomic number?
A) a decrease of 2
B) a decrease of 1
C) an increase of 1
D) no change
A) a decrease of 2
B) a decrease of 1
C) an increase of 1
D) no change

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
25
In carbon-14 dating,
A) the radioactivity of carbon is artificially induced.
B) the radioactivity of carbon occurs naturally.
C) radioactive carbon is added to the sample to be dated.
D) none of the above
A) the radioactivity of carbon is artificially induced.
B) the radioactivity of carbon occurs naturally.
C) radioactive carbon is added to the sample to be dated.
D) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
26
The process of alpha decay results in what change in the atomic number?
A) a decrease of 2
B) a decrease of 1
C) an increase of 1
D) no change
A) a decrease of 2
B) a decrease of 1
C) an increase of 1
D) no change

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
27
After five half-lives, what percentage of the original radioactive isotope remains in a sample?
A) 0%
B) 50%
C) 3.13%
D) 6.25%
A) 0%
B) 50%
C) 3.13%
D) 6.25%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
28
Plants incorporate carbon as long they live. Once a plant dies, it takes how many years for 75% of the carbon-14 to decay (half-life of C-14 is 5730 years)?
A) 573 yrs
B) 2865 yrs
C) 5730 yrs
D) 11460 yrs
A) 573 yrs
B) 2865 yrs
C) 5730 yrs
D) 11460 yrs

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
29
The process of electron capture can be considered as the
A) conversion of an electron and a proton to a neutron.
B) conversion of an electron and a neutron to a proton.
C) conversion of a proton to a neutron and an electron. The electron is emitted.
D) conversion of a neutron to a proton and an electron. The electron is emitted.
A) conversion of an electron and a proton to a neutron.
B) conversion of an electron and a neutron to a proton.
C) conversion of a proton to a neutron and an electron. The electron is emitted.
D) conversion of a neutron to a proton and an electron. The electron is emitted.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
30
Nitrogen-13 has a half-life of 10 minutes. How much of a 128 mg sample would remain after 20 minutes?
A) 0 mg
B) 4 mg
C) 16 mg
D) 32 mg
A) 0 mg
B) 4 mg
C) 16 mg
D) 32 mg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
31
Exposure to radioactive material is considered safe after 10 half-lives because
A) less than a tenth of 1% of the material remains.
B) less than 12.5% of the material remains.
C) ten is an even number.
D) all of the material will have decayed at that time.
A) less than a tenth of 1% of the material remains.
B) less than 12.5% of the material remains.
C) ten is an even number.
D) all of the material will have decayed at that time.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
32
Sodium-24 has a half-life of 15 hours. How much of a 20 mg sample would remain after two half-lives?
A) 0 mg
B) 2 mg
C) 5 mg
D) 10 mg
A) 0 mg
B) 2 mg
C) 5 mg
D) 10 mg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
33
The isotope that is used for the dating of brandy is
A) uranium-238.
B) lead-210.
C) carbon-14.
D) hydrogen-3.
A) uranium-238.
B) lead-210.
C) carbon-14.
D) hydrogen-3.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
34
Nitrogen-13 has a half-life of 10 minutes. How much of a 128 mg sample would remain after 40 minutes?
A) 4 mg
B) 8 mg
C) 16 mg
D) 32 mg
A) 4 mg
B) 8 mg
C) 16 mg
D) 32 mg

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
35
One difference between a chemical reaction and a nuclear reaction is that in a nuclear reaction
A) atoms retain their identity.
B) atoms often change from one element to another.
C) only the valence electrons are involved.
D) only small amounts of energy are absorbed or emitted.
A) atoms retain their identity.
B) atoms often change from one element to another.
C) only the valence electrons are involved.
D) only small amounts of energy are absorbed or emitted.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
36
The process of positron emission can be considered as the
A) conversion of a neutron to a proton and a positron. The positron is emitted.
B) conversion of a neutron to a proton and an electron. The electron is emitted.
C) conversion of a proton to a neutron and a positron. The neutron is emitted.
D) conversion of a proton to an neutron and a positron. The positron is emitted.
A) conversion of a neutron to a proton and a positron. The positron is emitted.
B) conversion of a neutron to a proton and an electron. The electron is emitted.
C) conversion of a proton to a neutron and a positron. The neutron is emitted.
D) conversion of a proton to an neutron and a positron. The positron is emitted.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
37
Carbon-14 dating of ancient objects is particularly effective because C-14
A) has the shortest half-life.
B) is continuously produced in the upper atmosphere and its ratio to C-12 in living systems and the environment is constant.
C) is a stable isotope and is therefore particularly easy to work with.
D) decays by a simple fission reaction.
A) has the shortest half-life.
B) is continuously produced in the upper atmosphere and its ratio to C-12 in living systems and the environment is constant.
C) is a stable isotope and is therefore particularly easy to work with.
D) decays by a simple fission reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
38
The amount of a radioactive isotope that remains after two half-lives have passed is
A) 98%.
B) 75%.
C) 50%.
D) 25%.
A) 98%.
B) 75%.
C) 50%.
D) 25%.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
39
The process of electron capture results in what change in the atomic number?
A) a decrease of 2
B) a decrease of 1
C) an increase of 1
D) no change
A) a decrease of 2
B) a decrease of 1
C) an increase of 1
D) no change

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
40
A piece of fossilized wood has a carbon-14 radioactivity that is 1/4 that of new wood. The half-life of carbon-14 is 5730 years. How old is the cloth?
A) 1 × 5730 = 5730 years
B) 2 × 5730 = 11,460 years
C) 3 × 5730 = 17,190 years
D) 0.25 × 5730 = 1432 years
A) 1 × 5730 = 5730 years
B) 2 × 5730 = 11,460 years
C) 3 × 5730 = 17,190 years
D) 0.25 × 5730 = 1432 years

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
41
The bombardment of nitrogen-14 with alpha particles produces oxygen-17 and a proton. This process is called
A) alpha emission.
B) transmutation.
C) isotopic enrichment.
D) fission.
A) alpha emission.
B) transmutation.
C) isotopic enrichment.
D) fission.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
42
Radioisotopes have been used as tracers in a variety of applications because
A) radioactive isotopes of an element have the same chemical properties as nonradioactive isotopes.
B) it is easy to follow the movement of a radioactive isotope.
C) the decay products are easily detected.
D) All of the above are true.
A) radioactive isotopes of an element have the same chemical properties as nonradioactive isotopes.
B) it is easy to follow the movement of a radioactive isotope.
C) the decay products are easily detected.
D) All of the above are true.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
43
Radioisotopes have been used to determine the age of all of the following EXCEPT
A) brandy.
B) cloth.
C) living plants.
D) wood.
A) brandy.
B) cloth.
C) living plants.
D) wood.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
44
In 1932, James Chadwick bombarded beryllium-9 with alpha particles. One product was a neutron. This led to the direct experimental verification of the existence of neutrons. The other product of Chadwick's nuclear reaction was
A) nitrogen-14.
B) carbon-12.
C) boron-12.
D) helium-4.
A) nitrogen-14.
B) carbon-12.
C) boron-12.
D) helium-4.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
45
Transmutation
A) can be carried out by chemical means, but not nuclear processes.
B) can be carried out by chemical means and nuclear processes.
C) cannot be carried out by chemical means but can be accomplished by nuclear processes.
D) cannot be carried out by chemical means or nuclear processes.
A) can be carried out by chemical means, but not nuclear processes.
B) can be carried out by chemical means and nuclear processes.
C) cannot be carried out by chemical means but can be accomplished by nuclear processes.
D) cannot be carried out by chemical means or nuclear processes.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
46
The radioactive decay of 99mTc to 99Tc MUST occur with the emission of
A) an alpha particle alone.
B) two beta particles.
C) a gamma ray alone.
D) the combination of a beta particle and a gamma ray.
A) an alpha particle alone.
B) two beta particles.
C) a gamma ray alone.
D) the combination of a beta particle and a gamma ray.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
47
Which of the following statements about the use of radioisotopes to irradiate foods is NOT true?
A) Radioisotopes kill microorganisms that cause food spoilage.
B) The taste and appearance of irradiated food undergoes a major change.
C) There is no residual radiation left in the food after irradiation.
D) Irradiation has been used for years in some countries.
A) Radioisotopes kill microorganisms that cause food spoilage.
B) The taste and appearance of irradiated food undergoes a major change.
C) There is no residual radiation left in the food after irradiation.
D) Irradiation has been used for years in some countries.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
48
Ernest Rutherford bombarded nitrogen-14 with alpha particles and produced oxygen-17 and a proton. What was the significance of this experiment?
A) It showed that most of the mass of an atom is in its nucleus.
B) It was the first time that protons were produced.
C) It showed that protons were part of the nucleus for an atom other than hydrogen.
D) It showed that the nucleus contains protons and neutrons.
A) It showed that most of the mass of an atom is in its nucleus.
B) It was the first time that protons were produced.
C) It showed that protons were part of the nucleus for an atom other than hydrogen.
D) It showed that the nucleus contains protons and neutrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
49
Which of the following statements about transmutation is true? In transmutation,
A) an atom of gold will be produced.
B) the original element changes into a different element.
C) the particles in the nucleus of the product are the same as the particles in the nucleus of the starting atom.
D) you need to balance electrons.
A) an atom of gold will be produced.
B) the original element changes into a different element.
C) the particles in the nucleus of the product are the same as the particles in the nucleus of the starting atom.
D) you need to balance electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
50
In positron emission tomography (PET), a positron collides with an electron and the two particles disappear in a burst of
A) an alpha particle and a gamma ray.
B) two beta particles.
C) a neutron and an alpha particle.
D) two gamma rays.
A) an alpha particle and a gamma ray.
B) two beta particles.
C) a neutron and an alpha particle.
D) two gamma rays.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
51
The radioisotope used to detect anemia is
A) gadolinium-153.
B) iron-59.
C) plutonium-238.
D) iodine-131.
A) gadolinium-153.
B) iron-59.
C) plutonium-238.
D) iodine-131.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
52
Gadolinium-153 is the most widely used radioisotope in medicine. It is used for the detection of
A) lung tumor.
B) skin cancer.
C) osteoporosis.
D) heart problems.
A) lung tumor.
B) skin cancer.
C) osteoporosis.
D) heart problems.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
53
The use of radioisotopes as tracers in medical and environmental research takes advantage of the fact that isotopes
A) generally behave identically in chemical and physical processes.
B) generally behave differently in chemical and physical processes.
C) have different masses.
D) none of the above
A) generally behave identically in chemical and physical processes.
B) generally behave differently in chemical and physical processes.
C) have different masses.
D) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
54
Positron emission tomography (PET) is a
A) therapy for cancer using positrons.
B) diagnostic technique for monitoring dynamic processes in the body, such as brain activity.
C) device for containing a nuclear fusion reaction.
D) mechanism for transmutation of elements.
A) therapy for cancer using positrons.
B) diagnostic technique for monitoring dynamic processes in the body, such as brain activity.
C) device for containing a nuclear fusion reaction.
D) mechanism for transmutation of elements.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
55
Which of the following resulted in the first demonstration of artificial transmutation?
A) Rutherford's bombardment of nitrogen-14 with alpha particles
B) Fermi and Segre's bombardment of uranium atoms with neutrons
C) the change of lead into gold by alchemists in the 14th century
D) Goldstein's production of protons in a gas discharge tube
A) Rutherford's bombardment of nitrogen-14 with alpha particles
B) Fermi and Segre's bombardment of uranium atoms with neutrons
C) the change of lead into gold by alchemists in the 14th century
D) Goldstein's production of protons in a gas discharge tube

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
56
Which one of the following radioactive isotopes is useful in the investigation of thyroid problems?
A) Uranium-235
B) Barium-120
C) Iodine-131
D) Cesium-145
A) Uranium-235
B) Barium-120
C) Iodine-131
D) Cesium-145

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
57
Technetium-99m is a radioisotope used in a variety of diagnostic tests. Technetium-99m has a short half-life (6 hr). The advantage of a short half-life for diagnostic purposes is
A) the radioactivity is easier to monitor.
B) the radioactivity does not linger in the body.
C) the radioactivity lasts for a long time.
D) the chemical reactions induced by the technetium are more rapid.
A) the radioactivity is easier to monitor.
B) the radioactivity does not linger in the body.
C) the radioactivity lasts for a long time.
D) the chemical reactions induced by the technetium are more rapid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
58
Which one of the following is most penetrating?
A) He nucleus
B) beta particle
C) gamma ray
D) visible light
A) He nucleus
B) beta particle
C) gamma ray
D) visible light

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
59
When nitrogen-14 is bombarded by alpha particles , a proton is produced. What other particle is emitted?
A) a neutron
B) oxygen-16
C) a positron
D) oxygen-17
A) a neutron
B) oxygen-16
C) a positron
D) oxygen-17

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
60
The medical application of cobalt-60 is
A) brain scans.
B) bone density determination
C) eye tumor detection.
D) radiation cancer therapy.
A) brain scans.
B) bone density determination
C) eye tumor detection.
D) radiation cancer therapy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
61
The most stable elements have
A) a high binding energy per nucleon.
B) a low binding energy per nucleon.
C) a high proton to neutron ratio.
D) an atomic number above 92.
A) a high binding energy per nucleon.
B) a low binding energy per nucleon.
C) a high proton to neutron ratio.
D) an atomic number above 92.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
62
Naturally occurring uranium contains approximately what percentage of uranium-235, the isotope that undergoes fission?
A) 0.72%
B) 10%
C) 50%
D) 100%
A) 0.72%
B) 10%
C) 50%
D) 100%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
63
The U.S. president who ordered the dropping of atomic bombs on the Japanese cities of Hiroshima and Nagasaki was
A) Bill Clinton.
B) Harry Truman.
C) Dwight Eisenhower.
D) George Bush.
A) Bill Clinton.
B) Harry Truman.
C) Dwight Eisenhower.
D) George Bush.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
64
Critical mass is
A) the minimum amount of fissionable material that will sustain a chain reaction.
B) the maximum amount of fissionable material that will sustain a chain reaction.
C) the amount of fissionable material that produces the most energy when split.
D) the largest amount of fissionable material that can be obtained from isotopic enrichment.
A) the minimum amount of fissionable material that will sustain a chain reaction.
B) the maximum amount of fissionable material that will sustain a chain reaction.
C) the amount of fissionable material that produces the most energy when split.
D) the largest amount of fissionable material that can be obtained from isotopic enrichment.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
65
Gamma rays can be stopped by
A) a sheet of paper.
B) a thin aluminum sheet.
C) a block of wood.
D) several centimeters of lead.
A) a sheet of paper.
B) a thin aluminum sheet.
C) a block of wood.
D) several centimeters of lead.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
66
The often tedious process by which a naturally occurring mixture of isotopes is separated and concentrated is called
A) isotopic transmutation.
B) isotopic ionization.
C) isotopic synthesis.
D) isotopic enrichment.
A) isotopic transmutation.
B) isotopic ionization.
C) isotopic synthesis.
D) isotopic enrichment.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
67
The source of the energy produced by the sun is
A) the burning of fossil fuels.
B) the fission of uranium-235.
C) the fusion of primarily hydrogen.
D) unknown.
A) the burning of fossil fuels.
B) the fission of uranium-235.
C) the fusion of primarily hydrogen.
D) unknown.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
68
Radioactive alpha emitters pose the greatest potential health risk when they are
A) unshielded.
B) on the skin.
C) ingested.
D) all of the above
A) unshielded.
B) on the skin.
C) ingested.
D) all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
69
In the famous relationship: E = mc2, the symbol m represents
A) mass.
B) molecules.
C) momentum.
D) metastable.
A) mass.
B) molecules.
C) momentum.
D) metastable.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
70
Which of the following was NOT a goal of the Manhattan project?
A) to isolate enriched uranium-235
B) to produce plutonium-239
C) constructing a bomb based on nuclear fission
D) to determine the amount of uranium-235 needed for an atomic power plant
A) to isolate enriched uranium-235
B) to produce plutonium-239
C) constructing a bomb based on nuclear fission
D) to determine the amount of uranium-235 needed for an atomic power plant

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
71
In nuclear fusion,
A) large unstable nuclei are fused together, and then split with the release of binding energy.
B) large, relatively unstable, nuclei are split releasing binding energy.
C) small, relatively stable, nuclei are split releasing binding energy.
D) smaller, relatively stable, nuclei are forced together to create a larger nucleus with the release of binding energy.
A) large unstable nuclei are fused together, and then split with the release of binding energy.
B) large, relatively unstable, nuclei are split releasing binding energy.
C) small, relatively stable, nuclei are split releasing binding energy.
D) smaller, relatively stable, nuclei are forced together to create a larger nucleus with the release of binding energy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
72
UF6 containing uranium-235 can be separated from UF6 containing uranium-238 because
A) compounds containing uranium-235 react differently than compounds containing uranium-238.
B) UF6 containing uranium-235 is lighter and will travel faster than UF6 containing uranium-238.
C) UF6 containing uranium-235 is lighter and will travel more slowly than UF6 containing uranium-238.
D) They cannot be separated.
A) compounds containing uranium-235 react differently than compounds containing uranium-238.
B) UF6 containing uranium-235 is lighter and will travel faster than UF6 containing uranium-238.
C) UF6 containing uranium-235 is lighter and will travel more slowly than UF6 containing uranium-238.
D) They cannot be separated.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
73
Alpha particles can be stopped by a
A) sheet of paper.
B) block of wood.
C) thin sheet of aluminum.
D) all of these
A) sheet of paper.
B) block of wood.
C) thin sheet of aluminum.
D) all of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
74
Although Albert Einstein is considered one of the greatest scientists of all time, his work generally did not involve much of an aspect that is traditionally associated with science. That aspect is
A) creativity.
B) mathematics.
C) theories.
D) experiments.
A) creativity.
B) mathematics.
C) theories.
D) experiments.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
75
Which process will result in an increase in the binding energy per nucleon?
A) fission of large radioactive nuclei
B) fusion of small nuclei
C) both A and B
D) neither A nor B
A) fission of large radioactive nuclei
B) fusion of small nuclei
C) both A and B
D) neither A nor B

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
76
Which type of radioactive emission is very similar to X-rays?
A) positron
B) electron
C) alpha
D) gamma ray
A) positron
B) electron
C) alpha
D) gamma ray

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
77
Stagg Field, at the University of Chicago, is where
A) nuclear fission was discovered.
B) radioactivity was discovered.
C) nuclear fusion was discovered.
D) the first nuclear pile was built.
A) nuclear fission was discovered.
B) radioactivity was discovered.
C) nuclear fusion was discovered.
D) the first nuclear pile was built.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
78
Nuclear fission is a process by which the nucleus of an atom
A) splits into two or more fragments spontaneously.
B) is induced to split into two or more fragments by some external source.
C) combines with another nucleus to produce a larger nucleus.
D) loses a proton with the release of a large amount of energy.
A) splits into two or more fragments spontaneously.
B) is induced to split into two or more fragments by some external source.
C) combines with another nucleus to produce a larger nucleus.
D) loses a proton with the release of a large amount of energy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
79
In the fission of uranium-235, which particle causes and propagates the chain reaction?
A) alpha particle
B) beta particle
C) neutron
D) electron
A) alpha particle
B) beta particle
C) neutron
D) electron

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck
80
The mass of a helium nucleus is slightly less than the sum of its parts (2 protons and 2 neutrons) because
A) some of the mass is converted to binding energy.
B) some of the mass is given to electrons.
C) the mass of protons and neutrons are not precisely known.
D) the mass of a proton is larger than the mass of a neutron.
A) some of the mass is converted to binding energy.
B) some of the mass is given to electrons.
C) the mass of protons and neutrons are not precisely known.
D) the mass of a proton is larger than the mass of a neutron.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 122 في هذه المجموعة.
فتح الحزمة
k this deck








