Deck 9: Chemistry of Fire and Heat
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/19
العب
ملء الشاشة (f)
Deck 9: Chemistry of Fire and Heat
1
Explain the differences between a bomb calorimeter and a cone calorimeter.
The answer should include the following information: Bomb calorimeters convert fuel to gaseous products that are confined within a sealed container at high pressures. Cone calorimeters measure the heat of combustion of ignitable materials by monitoring the decrease in oxygen in the air collected above a burning sample. Bomb calorimeters do not provide information about time, while cone calorimeters do.
2
Determine how much heat is required to increase the temperature of a 495.0 g sample of acetone from 58°C to its autoignition temperature of 465°C, given that the specific heat capacity of acetone is 1.20 J/g·°C.
2.4 × 105 J
3
What physical process is happening between points E and F? 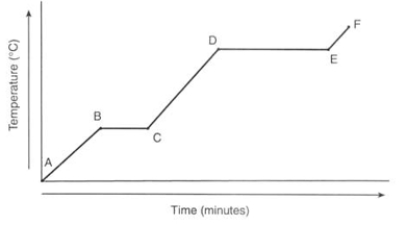
A) Heat energy converts the solid water into liquid water.
B) Solid water is being heated, resulting in the temperature of the solid increasing.
C) Liquid water is being heated, resulting in the temperature of the liquid increasing.
D) Gaseous water is being heated, resulting in the temperature of the gas increasing.

A) Heat energy converts the solid water into liquid water.
B) Solid water is being heated, resulting in the temperature of the solid increasing.
C) Liquid water is being heated, resulting in the temperature of the liquid increasing.
D) Gaseous water is being heated, resulting in the temperature of the gas increasing.
Gaseous water is being heated, resulting in the temperature of the gas increasing.
4
Calculate the specific heat capacity in J/kg·°C of an unknown metal in a 3.76 kg block that requires 1.83 × 104 J of heat to raise the temperature from 23.8°C to 61.2°C.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
5
Compounds with which type of intermolecular force will tend to have large heats of vaporization?
A) dipole-dipole
B) dipole-induced dipole
C) dispersion
D) hydrogen bonding
A) dipole-dipole
B) dipole-induced dipole
C) dispersion
D) hydrogen bonding

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
6
A 0.500 g sample of TNT (C7H5N2O6) is ignited in a bomb calorimeter and the temperature of 610 mL of water increases from 20.0°C to 23.0°C. What is the heat of combustion (J/g) of the TNT?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
7
Convert 76.2 Calories to kilojoules.
A) 0.0000182 kJ
B) 0.000319 kJ
C) 18.2 kJ
D) 319 kJ
A) 0.0000182 kJ
B) 0.000319 kJ
C) 18.2 kJ
D) 319 kJ

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
8
A 28.4 g sample of aluminum, initially at 39.4°C, heated 50.0 g of water from 22.0°C to 24.0°C upon being submerged in the water. Determine the specific heat of aluminum, given that the specific heat of water is 4.184 J/g·°C.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which element is oxidized in the following reaction? 10I- + 16H+ + 2MnO4- → 5I2 + 2Mn2+ + 8H2O
A) H
B) I
C) Mn
D) O
A) H
B) I
C) Mn
D) O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
10
What physical process is happening between points B and C? 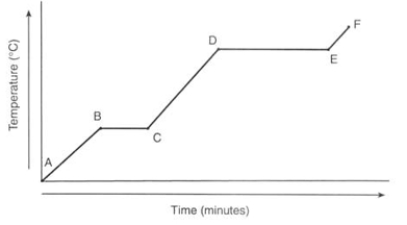
A) Heat energy converts the solid water into liquid water.
B) Heat energy converts the liquid water into gaseous water.
C) Solid water is being heated, resulting in the temperature of the solid increasing.
D) Liquid water is being heated, resulting in the temperature of the liquid increasing.
E) Gaseous water is being heated, resulting in the temperature of the gas increasing.

A) Heat energy converts the solid water into liquid water.
B) Heat energy converts the liquid water into gaseous water.
C) Solid water is being heated, resulting in the temperature of the solid increasing.
D) Liquid water is being heated, resulting in the temperature of the liquid increasing.
E) Gaseous water is being heated, resulting in the temperature of the gas increasing.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
11
Write and balance the equation for the complete combustion of pentane.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
12
Which element is reduced in the following reaction? 4Ag(s) + 2H2S(g) + O2(g) → 2Ag2S(s) + 2H2O(g)
A) Ag
B) H
C) O
D) S
Enter the appropriate word(s) to complete the statement.
A) Ag
B) H
C) O
D) S
Enter the appropriate word(s) to complete the statement.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
13
Indicate whether the following statement is true or false
Temperature is a measure of the heat content of a body.
Temperature is a measure of the heat content of a body.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
14
In which type of reaction do the products have more energy than the reactants?
A) endothermic
B) exothermic
A) endothermic
B) exothermic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
15
Write and balance the equation for the complete combustion of C6H14.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
16
What is the oxidation number of Mn in the compound Mn2S3?
A) +3/2
B) +2
C) +3
D) +6
A) +3/2
B) +2
C) +3
D) +6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
17
What is the initial temperature of a 13.5 g sample of gold if it raises the temperature of 60.0 g of water from 19.5°C to 20.5°C upon submersion? The specific heat of gold is 0.13 J/g·°C and of water is 4.184 J/g·°C.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
18
What is the oxidation number of Hg in Hg2Cl2?
A) -2
B) -1
C) +1
D) +2
A) -2
B) -1
C) +1
D) +2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck
19
Convert the heat of combustion of ethanol, 7.1 Cal/g, to J/g.
A) 1.7 × 10-3 J/g
B) 3.0 × 10-2 J/g
C) 1.7 × 103 J/g
D) 3.0 × 104 J/g
A) 1.7 × 10-3 J/g
B) 3.0 × 10-2 J/g
C) 1.7 × 103 J/g
D) 3.0 × 104 J/g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 19 في هذه المجموعة.
فتح الحزمة
k this deck








