Deck 11: Carbonyl Alpha-Substitution Reactions and Condensation Reactions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/38
العب
ملء الشاشة (f)
Deck 11: Carbonyl Alpha-Substitution Reactions and Condensation Reactions
1
Instructions: Consider the reaction below to answer the following question(s). 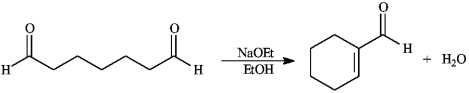
Refer to instructions. This reaction is an example of:
A) an intramolecular Claisen condensation
B) an intramolecular aldol condensation
C) a Robinson annulation
D) a Michael reaction

Refer to instructions. This reaction is an example of:
A) an intramolecular Claisen condensation
B) an intramolecular aldol condensation
C) a Robinson annulation
D) a Michael reaction
an intramolecular aldol condensation
2
Give major product(s): 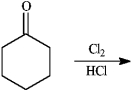


3
Which of the following would form an enolate ion on treatment with a base?
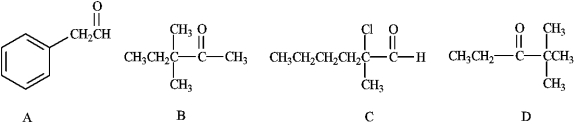
A) A
B) B
C) C
D) D
E) All of these except C
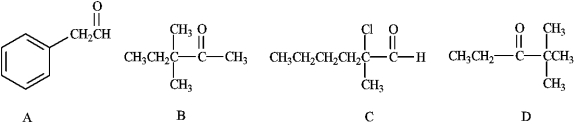
A) A
B) B
C) C
D) D
E) All of these except C
All of these except C
4
How would you prepare 3-phenylpropanoic acid using a malonic ester synthesis?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
5
Instructions: Consider the structures below to answer the following question(s). 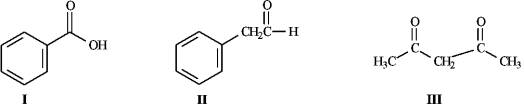
Refer to instructions. Underline the most acidic hydrogen atoms in each of the molecules.

Refer to instructions. Underline the most acidic hydrogen atoms in each of the molecules.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
6
Instructions: Draw the structure of the aldol self-condensation product of each compound for the following question(s). If a compound does not undergo aldol self-condensation, explain why it does not.
Draw and explain: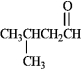
Draw and explain:
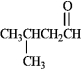

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
7
An enolate ion
A) is a resonance hybrid.
B) can be protonated to from the corresponding enol.
C) can be protonated to form the keto tautomer.
D) forms under basic conditions.
E) All of these
A) is a resonance hybrid.
B) can be protonated to from the corresponding enol.
C) can be protonated to form the keto tautomer.
D) forms under basic conditions.
E) All of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
8
Instructions: Each of the following compounds in the following question(s) can be prepared by a mixed aldol condensation reaction.
a)Give the structures of the aldehyde and/or ketone precursors for each aldol condensation product and formulate the reaction.
b)Give the structure of the intermediate aldol product.
Refer to instructions. Use the following compound: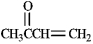
a)Give the structures of the aldehyde and/or ketone precursors for each aldol condensation product and formulate the reaction.
b)Give the structure of the intermediate aldol product.
Refer to instructions. Use the following compound:
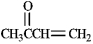

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
9
Instructions: Draw the structure of the aldol self-condensation product of each compound for the following question(s). If a compound does not undergo aldol self-condensation, explain why it does not.
-Draw and explain: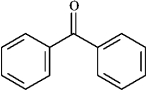
-Draw and explain:


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
10
Instructions: Consider the reaction below to answer the following question(s). 
Refer to instructions. Write the complete stepwise mechanism for the reaction above. Show all intermediate structures and all electron flow with arrows.

Refer to instructions. Write the complete stepwise mechanism for the reaction above. Show all intermediate structures and all electron flow with arrows.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
11
Instructions: Consider the reaction below to answer the following question(s). 
-Refer to instructions. The product of this reaction is:
A) a , -unsaturated aldehyde
B) an , -unsaturated ketone
C) an , -unsaturated aldehyde
D) an enol

-Refer to instructions. The product of this reaction is:
A) a , -unsaturated aldehyde
B) an , -unsaturated ketone
C) an , -unsaturated aldehyde
D) an enol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
12
Instructions: Refer to the compounds below to answer the following question(s). 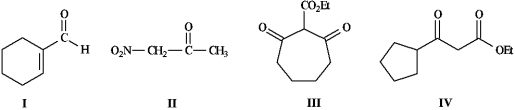
-Refer to instructions. Choose the most acidic compound from Compounds I - IV. Explain your choice.

-Refer to instructions. Choose the most acidic compound from Compounds I - IV. Explain your choice.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
13
The common feature of -substitution and condensation reactions of carbonyl groups:
A) involve two carbonyl partners
B) involve the formation of an enol or enolate ion
C) involve a nucleophile
D) produce a new carbon to carbon bond
E) all of these describe both types of reactions
A) involve two carbonyl partners
B) involve the formation of an enol or enolate ion
C) involve a nucleophile
D) produce a new carbon to carbon bond
E) all of these describe both types of reactions

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of the following is common to both tautomers and resonance forms of a compound?
A) have the same molecular formula
B) differ only in the position of electrons
C) rapidly interconvertible
D) differ in connectivity of atoms
E) all of these describe both tautomers and resonance forms
A) have the same molecular formula
B) differ only in the position of electrons
C) rapidly interconvertible
D) differ in connectivity of atoms
E) all of these describe both tautomers and resonance forms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
15
Instructions: Refer to the compounds below to answer the following question(s). 
Refer to instructions. Underline all the acidic hydrogen atoms in Compounds I through IV.

Refer to instructions. Underline all the acidic hydrogen atoms in Compounds I through IV.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
16
Instructions: Refer to the compounds below to answer the following question(s). 
Refer to instructions. Draw the structure for the enol and enolate ions corresponding to Compound I.

Refer to instructions. Draw the structure for the enol and enolate ions corresponding to Compound I.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
17
Instructions: Each of the following compounds in the following question(s) can be prepared by a mixed aldol condensation reaction.
a)Give the structures of the aldehyde and/or ketone precursors for each aldol condensation product and formulate the reaction.
b)Give the structure of the intermediate aldol product.
Refer to instructions. Use the following compound: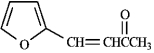
a)Give the structures of the aldehyde and/or ketone precursors for each aldol condensation product and formulate the reaction.
b)Give the structure of the intermediate aldol product.
Refer to instructions. Use the following compound:


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
18
Instructions: Consider the structures below to answer the following question(s). 
Refer to instructions. Rank the molecules above in order of increasing acidity (least acidic to most acidic).
A) III, II, I
B) II, III, I
C) I, II, III
D) II, I, III

Refer to instructions. Rank the molecules above in order of increasing acidity (least acidic to most acidic).
A) III, II, I
B) II, III, I
C) I, II, III
D) II, I, III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
19
Instructions: Draw the structure of the aldol self-condensation product of each compound for the following question(s). If a compound does not undergo aldol self-condensation, explain why it does not.
Draw and explain: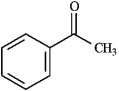
Draw and explain:


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
20
Instructions: Give the major organic product(s) of each reaction or sequences of reactions for the following question(s). Show all relevant stereochemistry.
-Refer to instructions.
a)Give major product(s):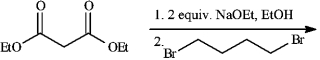
b)Why are two equivalents of Na+ OEt - required?
-Refer to instructions.
a)Give major product(s):
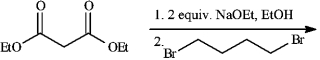
b)Why are two equivalents of Na+ OEt - required?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
21
Rank the following hydrogens in terms of decreasing acidity (most acidic > least acidic): 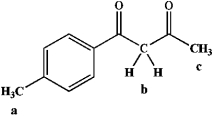


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
22
Instructions: Consider the reaction below to answer the following question(s).
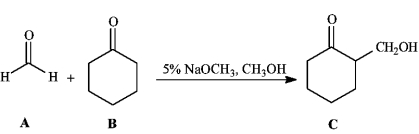
Refer to instructions. Draw the structure of the enolate ion that is generated during the course of this reaction.
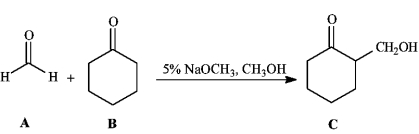
Refer to instructions. Draw the structure of the enolate ion that is generated during the course of this reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which of the following does not possess an enol form? Explain your choice.
A)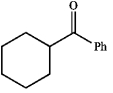
B)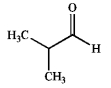
C)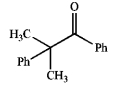
D)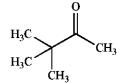
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
24
Identify products a and b.
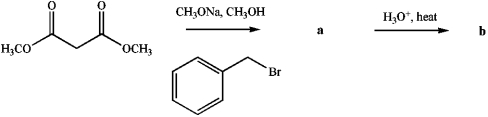


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
25
Consider the reaction below to answer the following questions. 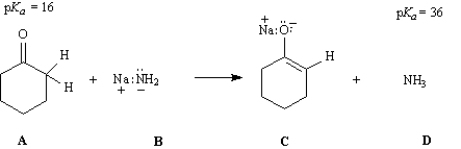
Refer to Instructions. On the structures provided above, draw arrows indicating elctron flow in the generation of the intermediate C.

Refer to Instructions. On the structures provided above, draw arrows indicating elctron flow in the generation of the intermediate C.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
26
Instructions: Consider the reaction below to answer the following question(s).
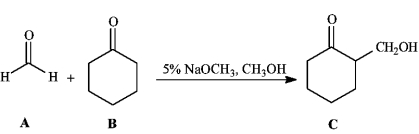
Refer to instructions. Which carbonyl compound functions as the electrophile in this reaction?
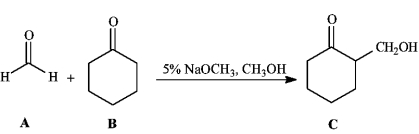
Refer to instructions. Which carbonyl compound functions as the electrophile in this reaction?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
27
Consider the reaction below to answer the following questions. 
Refer to Instructions. The strongest base in the reaction is:

Refer to Instructions. The strongest base in the reaction is:

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
28
Predict the major aldol product of the following reaction. 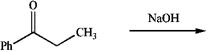

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
29
Consider the reaction below to answer the following questions. 
Refer to Instructions. The weakest acid in the reaction is:

Refer to Instructions. The weakest acid in the reaction is:

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
30
Write a resonance structure for the anion below. 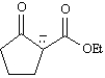


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
31
How would you prepare each of the following compounds using a malonic ester synthesis? Show all intermediate structures and all reagents.
Prepare: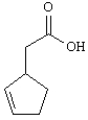
Prepare:


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
32
Write the complete stepwise mechanism for the reaction of cyclopentanone with bromine in acetic acid to give 2-bromocyclopentanone. Show all intermediate structures and all electron flow with arrows.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
33
Consider the reaction below to answer the following questions. 
Refer to Instructions. The enolate ion in the reaction is:

Refer to Instructions. The enolate ion in the reaction is:

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
34
Write a resonance structure for the anion below. 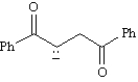


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
35
Instructions: Draw the structure of the product you would expect to obtain by Claisen condensation of the esters shown in the question(s) below. If an ester does not undergo Claisen condensation, explain why it does not.
Draw and explain: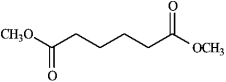
Draw and explain:


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
36
Instructions: Draw the structure of the product you would expect to obtain by Claisen condensation of the esters shown in the question(s) below. If an ester does not undergo Claisen condensation, explain why it does not.
-Draw and explain: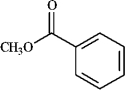
-Draw and explain:


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
37
How would you prepare each of the following compounds using a malonic ester synthesis? Show all intermediate structures and all reagents.
Prepare: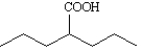
Prepare:


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck
38
When treated with base and heat, the following diketone undergoes an intramolecular aldol reaction followed by dehydration to produce cis-jasmone, a perfume component. Draw the structure of the product. 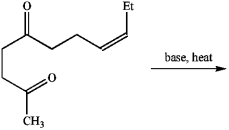


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 38 في هذه المجموعة.
فتح الحزمة
k this deck








