Deck 19: Temperature, Thermal Expansion, and Gas Laws
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/45
العب
ملء الشاشة (f)
Deck 19: Temperature, Thermal Expansion, and Gas Laws
1
An auditorium has dimensions 10 m × 10 m × 60 m. How many moles of air fill this volume at one atmosphere and 0°C?
A)2.7 × 102
B)2.7 × 104
C)2.7 × 103
D)2.7 × 105
E)2.7 × 106
A)2.7 × 102
B)2.7 × 104
C)2.7 × 103
D)2.7 × 105
E)2.7 × 106
2.7 × 105
2
A gallon container is filled with gasoline. How many gallons are lost if the temperature increases by 25°F? (The volume expansion of gasoline is 9.6 × 10−4 (°C)−1.) (Neglect the change in volume of the container.)
A)2.4 × 10−2
B)1.3 × 10−2
C)3.6 × 10−2
D)4.8 × 10−2
E)9.6 × 10−2
A)2.4 × 10−2
B)1.3 × 10−2
C)3.6 × 10−2
D)4.8 × 10−2
E)9.6 × 10−2
1.3 × 10−2
3
A child has a temperature of 104°F. What is the temperature in degrees kelvin?
A)40
B)406
C)401
D)313
E)349
A)40
B)406
C)401
D)313
E)349
313
4
A pressure of 10.0 mm Hg is measured at the triple-point of water using a constant-volume gas thermometer. What will the pressure be (in mm Hg) at 50.0°C?
A)68.3
B)1.80
C)31.8
D)11.8
E)8.50
A)68.3
B)1.80
C)31.8
D)11.8
E)8.50

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
5
A thermometer registers a change in temperature of 100°F. What change in temperature does this correspond to on the Kelvin Scale?
A)453
B)328
C)180
D)55.6
E)24.5
A)453
B)328
C)180
D)55.6
E)24.5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
6
An auditorium has a volume of 6 × 103 m3. How many molecules of air are needed to fill the auditorium at one atmosphere and 0°C?
A)1.6 × 1029
B)1.6 × 1027
C)1.6 × 1025
D)1.6 × 1023
E)1.6 × 1020
A)1.6 × 1029
B)1.6 × 1027
C)1.6 × 1025
D)1.6 × 1023
E)1.6 × 1020

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
7
One mole of an ideal gas has a temperature of 25°C. If the volume is held constant and the pressure is doubled, the final temperature (in °C) will be
A)174
B)596
C)50
D)323
E)25
A)174
B)596
C)50
D)323
E)25

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
8
In order to understand the concept of temperature it is necessary to understand
A)the zeroth law of thermodynamics.
B)the first law of thermodynamics.
C)the second law of thermodynamics.
D)all of the above.
E)only (b) and (c) above.
A)the zeroth law of thermodynamics.
B)the first law of thermodynamics.
C)the second law of thermodynamics.
D)all of the above.
E)only (b) and (c) above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
9
A bridge is made with segments of concrete 50 m long. If the linear expansion coefficient is 12 × 10−6 (°C)−1, how much spacing (in cm) is needed to allow for expansion during an extreme temperature change of 150°F?
A)10
B)2.5
C)7.5
D)5.0
E)9.5
A)10
B)2.5
C)7.5
D)5.0
E)9.5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
10
In order for two objects to have the same temperature, they must
A)be in thermal equilibrium.
B)be in thermal contact with each other.
C)have the same relative "hotness" or "coldness" when touched.
D)have all of the properties listed above.
E)have only properties (b) and (c) above.
A)be in thermal equilibrium.
B)be in thermal contact with each other.
C)have the same relative "hotness" or "coldness" when touched.
D)have all of the properties listed above.
E)have only properties (b) and (c) above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
11
Two thermometers are calibrated, one in degrees Celsius and the other in degrees Fahrenheit. At what temperature (in kelvins) do their readings measure the same temperature?
A)218.15
B)233.15
C)273.15
D)40.15
E)0
A)218.15
B)233.15
C)273.15
D)40.15
E)0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
12
One mole of an ideal gas is held at a constant volume of 1 liter. Find the change in pressure if the temperature increases by 50°C.
A)3 atm
B)4 atm
C)2 atm
D)1 atm
E)5 atm
A)3 atm
B)4 atm
C)2 atm
D)1 atm
E)5 atm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
13
Helium condenses into the liquid phase at approximately 4 K. What temperature, in degrees Fahrenheit, does this correspond to?
A)−182
B)−269
C)−118
D)−452
E)−484
A)−182
B)−269
C)−118
D)−452
E)−484

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
14
A bicycle pump contains air at one atmosphere and 0°C. As the tire is pumped up, the volume of air decreases by 50% with each stroke. What is the new pressure of air (in atm) in the chamber after the first stroke, assuming no temperature change?
A)2
B)1
C)0.5
D)0.1
E)3
A)2
B)1
C)0.5
D)0.1
E)3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
15
A building made with a steel structure is 650 m high on a winter day when the temperature is 0°F. How much taller (in cm) is the building when it is 100°F? (The linear expansion coefficient of steel is 11 × 10−6(°C)−1.)
A)71
B)36
C)40
D)46
E)65
A)71
B)36
C)40
D)46
E)65

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
16
A pressure of 10.0 mm Hg is measured using a constant-volume gas thermometer at a temperature of 50.0°C. What is the pressure (in mm Hg) at the zero-point temperature?
A)31.8
B)11.8
C)8.45
D)54.6
E)68.3
A)31.8
B)11.8
C)8.45
D)54.6
E)68.3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
17
A helium-filled balloon has a volume of 1 m3. As it rises in the earth's atmosphere, its volume expands. What will its new volume be (in m3) if its original temperature and pressure are 20°C and 1 atm, and its final temperature and pressure are −40°C and 0.1 atm?
A)4
B)6
C)8
D)10
E)1.5
A)4
B)6
C)8
D)10
E)1.5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
18
One mole of an ideal gas is held at a constant pressure of 1 atm. Find the change in volume (in liters) if the temperature changes by 50°C.
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
19
A temperature difference of 5 K is equal to
A)a difference of 9 on the Celsius scale.
B)a difference of 9 on the Fahrenheit scale.
C)a difference of 2.8 on the Rankine scale.
D)a difference of 0.5 on the Fahrenheit scale.
E)a difference of 2.8 on the Celsius scale.
A)a difference of 9 on the Celsius scale.
B)a difference of 9 on the Fahrenheit scale.
C)a difference of 2.8 on the Rankine scale.
D)a difference of 0.5 on the Fahrenheit scale.
E)a difference of 2.8 on the Celsius scale.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
20
At what temperature is the Celsius scale reading equal to twice the Fahrenheit scale reading?
A)−12.3°F
B)−24.6°F
C)−12.3°C
D)−6.1°C
E)−20°F
A)−12.3°F
B)−24.6°F
C)−12.3°C
D)−6.1°C
E)−20°F

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
21
Death Valley in California receives many German tourists. When you convert a summer temperature reading of 130°F to the Celsius scale they use at home, you find that the Celsius temperature is about
A)26°C.
B)54°C.
C)72°C.
D)176°C
E)327°C.
A)26°C.
B)54°C.
C)72°C.
D)176°C
E)327°C.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
22
A student has written the equation below to convert a temperature in degrees Fahrenheit into Kelvins. What is wrong with this equation? 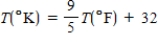
A)The factor in front of T(ºF) should be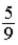 .
.
B)The numerical factor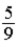 should multiply (T(ºF) − 32).
should multiply (T(ºF) − 32).
C)An additional 273.15 Kelvins must be added to the right side of the equation.
D)All the corrections above are required.
E)Only corrections (b) and (c) are required.
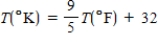
A)The factor in front of T(ºF) should be
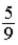 .
.B)The numerical factor
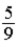 should multiply (T(ºF) − 32).
should multiply (T(ºF) − 32).C)An additional 273.15 Kelvins must be added to the right side of the equation.
D)All the corrections above are required.
E)Only corrections (b) and (c) are required.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
23
At which of the following temperatures would one liter of water weigh the most?
A)2°C
B)4°C
C)8°C
D)90°C
E)It would weigh the same at all these temperatures.
A)2°C
B)4°C
C)8°C
D)90°C
E)It would weigh the same at all these temperatures.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
24
Two identical containers, A and B, hold equal amounts of the same ideal gas at the same Po, Vo and To. The pressure of A then decreases by a half while its volume doubles; the pressure of B doubles while its volume decreases by a half. Which statement correctly describes the temperatures of the gases after the changes?
A)TA = 0.5TB = To.
B)TB = 0.5TA = To.
C)TB = TA = To.
D)TA = 2TB = To.
E)TB = 2TA = To.
A)TA = 0.5TB = To.
B)TB = 0.5TA = To.
C)TB = TA = To.
D)TA = 2TB = To.
E)TB = 2TA = To.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
25
A bubble having a diameter of 1.00 cm is released from the bottom of a swimming pool where the depth is 5.00 m. What will the diameter of the bubble be when it reaches the surface? The temperature of the water at the surface is 20.0°C, whereas it is 15.0°C at the bottom. (The density of water is 1.00 × 103 kg/m3.)
A)1.05 cm
B)1.15 cm
C)1.45 cm
D)1.65 cm
E)1.35 cm
A)1.05 cm
B)1.15 cm
C)1.45 cm
D)1.65 cm
E)1.35 cm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
26
The mass of a sulfur atom is 32.0 u, and the mass of a fluorine atom is 19.0 u. What is the mass of a mole of sulfur hexafluoride (SF6)?
A)41.0 g
B)20.5 g
C)106 g
D)146 g
E)211 g
A)41.0 g
B)20.5 g
C)106 g
D)146 g
E)211 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
27
Two bodies can be in thermal equilibrium with one another when they are at the same temperature even if they
A)absorb different quantities of thermal energy from their surroundings in equal time intervals.
B)have different masses.
C)have different volumes.
D)have any of the properties listed above.
E)have any of the properties listed above and one of them is contact with a third body at a different temperature.
A)absorb different quantities of thermal energy from their surroundings in equal time intervals.
B)have different masses.
C)have different volumes.
D)have any of the properties listed above.
E)have any of the properties listed above and one of them is contact with a third body at a different temperature.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
28
A bowling ball-size probe from a spaceship and a pebble-size object land on a large asteroid that is far from any star. After a long period of time has passed, it is highly probable that the pebble and the probe
A)have each had the same change in temperature.
B)have each had the same change in volume.
C)are in thermal equilibrium with one another.
D)are not in thermal equilibrium with one another.
E)are in thermal equilibrium with one another, but are not at the same temperature.
A)have each had the same change in temperature.
B)have each had the same change in volume.
C)are in thermal equilibrium with one another.
D)are not in thermal equilibrium with one another.
E)are in thermal equilibrium with one another, but are not at the same temperature.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
29
Equal volumes of hydrogen and helium gas are at the same pressure. The atomic mass of helium is four times that of hydrogen. If the total mass of both gases is the same, the ratio of the temperature of helium (He) to that of hydrogen (H2) is
A)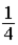 .
.
B)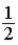 .
.
C)1.
D)2.
E)4.
A)
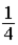 .
.B)
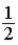 .
.C)1.
D)2.
E)4.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
30
A constant-volume gas thermometer has a pressure of 2.00 atm at 100°C. What would its pressure be at 0°C?
A)0.732 atm
B)1.46 atm
C)1.24 atm
D)1.37 atm
E)More information is needed to find the answer.
A)0.732 atm
B)1.46 atm
C)1.24 atm
D)1.37 atm
E)More information is needed to find the answer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
31
A temperature difference of 9.0 Celsius degrees is equal to a Fahrenheit temperature difference of
A)5.0 Fahrenheit degrees.
B)9.0 Fahrenheit degrees.
C)16 Fahrenheit degrees.
D)37 Fahrenheit degrees.
E)41 Fahrenheit degrees.
A)5.0 Fahrenheit degrees.
B)9.0 Fahrenheit degrees.
C)16 Fahrenheit degrees.
D)37 Fahrenheit degrees.
E)41 Fahrenheit degrees.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
32
Equal masses of hydrogen and helium gas are at the same temperature in vessels of equal volume. The atomic mass of helium is four times that of hydrogen. If the total mass of both gases is the same, the ratio of the pressure of helium (He) to that of hydrogen (H2) is
A)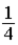 .
.
B)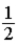 .
.
C)1.
D)2.
E)4.
A)
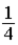 .
.B)
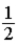 .
.C)1.
D)2.
E)4.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
33
A square plate has an area of 29.00 cm2 at 20.0°C. It will be used in a low temperature experiment at T = 10.0K where it must have an area of 28.00 cm2. What area must be removed from the plate at 20.0°C for it to have the correct area at 10.0 K? (The coefficient of linear expansion is 10 × 10−6 (°C)−1.)
A)0.079 3 cm2
B)0.159 cm2
C)0.238 cm2
D)0.836 cm2
E)0.921 cm2
A)0.079 3 cm2
B)0.159 cm2
C)0.238 cm2
D)0.836 cm2
E)0.921 cm2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
34
Angela claims that she wears a cylindrical-shaped hollow gold bracelet because it expands less than a solid one with a change in temperature. Clarissa claims that a cylindrical-shaped solid gold bracelet expands less than a hollow one. Which one, if either, is correct?
A)Angela, because the bracelet expands outward on its outer surface and inward on its inner surface.
B)Clarissa, because the bracelet expands outward on its outer surface and inward on its inner surface.
C)Angela, because the inner circumference does not change, but the outer circumference expands.
D)Clarissa, because the inner circumference does not change, but the outer circumference expands.
E)Neither, because both the inner and outer circumferences increase in length.
A)Angela, because the bracelet expands outward on its outer surface and inward on its inner surface.
B)Clarissa, because the bracelet expands outward on its outer surface and inward on its inner surface.
C)Angela, because the inner circumference does not change, but the outer circumference expands.
D)Clarissa, because the inner circumference does not change, but the outer circumference expands.
E)Neither, because both the inner and outer circumferences increase in length.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
35
A beaker is filled to the 500-ml mark with alcohol. What increase in volume (in ml) does the beaker contain when the temperature changes from 5°C to 30°C? (Neglect the expansion of the beaker, evaporation of alcohol and absorption of water vapor by alcohol.) βalcohol = 1.12 × 10−4/°C
A)0.47
B)0.93
C)1.4
D)1.7
E)2.5
A)0.47
B)0.93
C)1.4
D)1.7
E)2.5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
36
A container with a one-liter capacity at 27°C is filled with helium to a pressure of 2.0 atm. (1 atm = 1.0 × 105 N/m2.) How many moles of helium does it hold?
A)0.041
B)0.081
C)0.45
D)0.90
E)1.0
A)0.041
B)0.081
C)0.45
D)0.90
E)1.0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
37
A scuba diver has his lungs filled to half capacity (3 liters) when 10 m below the surface. If the diver holds his breath while quietly rising to the surface, what will the volume of the lungs be (in liters) at the surface? Assume the temperature is the same at all depths. (The density of water is 1.0 × 103kg/m3.)
A)5.9
B)4.5
C)6.4
D)3.9
E)3.1
A)5.9
B)4.5
C)6.4
D)3.9
E)3.1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
38
Two moles of an ideal gas are placed in a container of adjustable volume. When measurements are made
A)the pressure is inversely proportional to the volume at constant temperature.
B)the temperature is directly proportional to the volume at constant pressure.
C)the temperature is directly proportional to the pressure at constant volume.
D)all the statements above are found to be correct.
E)only statements (a) and (b) are found to be correct.
A)the pressure is inversely proportional to the volume at constant temperature.
B)the temperature is directly proportional to the volume at constant pressure.
C)the temperature is directly proportional to the pressure at constant volume.
D)all the statements above are found to be correct.
E)only statements (a) and (b) are found to be correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
39
What is the change in area (in cm2) of a 60.0-cm by 150-cm automobile windshield when the temperature changes from 0°C to 36.0°C. The coefficient of linear expansion of this glass is 9.0 × 10−6/°C.
A)1.6
B)2.9
C)3.2
D)4.9
E)5.8
A)1.6
B)2.9
C)3.2
D)4.9
E)5.8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
40
48 grams of oxygen at room temperature consists of how many moles?
A)3.0
B)1.5
C)0.75
D)more than 3.0
E)less than 0.75
A)3.0
B)1.5
C)0.75
D)more than 3.0
E)less than 0.75

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
41
The pressure of a substance is directly proportional to its volume when the temperature is held constant and inversely proportional to its temperature when the volume is held constant. Is this substance an ideal gas? Explain why your answer is correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
42
Determine the change in length of a 20-m railroad track made of steel if the temperature is changed from −15°C to +35°C. (αSTEEL = 1.1 × 10−5/°C)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
43
A gold ring has an inner diameter of 2.168 cm at a temperature of 15.0°C. Determine its diameter at 100.0°C. (αGOLD = 1.42 × 10−5/°C)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
44
Suppose the ends of a 30-m-long steel beam are rigidly clamped at 0°C to prevent expansion. The beam has a cross-sectional area of 30 cm2. What force against the clamps does the beam exert when it is heated to 40°C? (αSteel = 1.1 × 10−5/°C, Ysteel = 20 × 1010N/m2).

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
45
At what Fahrenheit temperature are the Kelvin and Fahrenheit temperatures numerically equal?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck








