Deck 12: Chemical Equilibrium
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/40
العب
ملء الشاشة (f)
Deck 12: Chemical Equilibrium
1
A large "K" value (>0) indicates that the reaction tends to favor the forward direction and the formation of products.
True
2
According to the Brønsted-Lowry theory of acids and bases, acids are proton (hydronium) acceptors.
False
3
Exothermic reactions in equilibrium will favor the reactants if the system is cooled.
False
4
Which of following species is a Brønsted-Lowry base?
A) NaOH
B) C4H10
C) CaCl2
D) H2SO4
A) NaOH
B) C4H10
C) CaCl2
D) H2SO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
5
If NaCl is added to a saturated solution of AgCl, the equilibrium will shift back towards the reactants; and the solubility of AgCl will decrease.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
6
Which "K" value indicates a reaction most favoring the formation of products?
A) K = 5.31 × 103
B) K = 4.99 × 106
C) K = 8.2 × 10 − 3
D) K = 1.7 × 10 − 6
A) K = 5.31 × 103
B) K = 4.99 × 106
C) K = 8.2 × 10 − 3
D) K = 1.7 × 10 − 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of following species is a Brønsted-Lowry acid?
A) NaOH
B) C4H10
C) CaCl2
D) H2SO4
A) NaOH
B) C4H10
C) CaCl2
D) H2SO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
8
If a reaction is in a state where Q > K,
A) the rate of the reaction will always double.
B) more products will form.
C) more reactants will form.
D) the reaction will remain in a steady-state.
A) the rate of the reaction will always double.
B) more products will form.
C) more reactants will form.
D) the reaction will remain in a steady-state.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
9
The reaction: 2 A( s ) + B( g ) 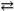 2 C( s ) represents a(n):
2 C( s ) represents a(n):
A) homogeneous equilibrium
B) heterogeneous equilibrium
C) instantaneous equilibrium
D) reaction that will not come to an equilibrium
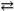 2 C( s ) represents a(n):
2 C( s ) represents a(n):A) homogeneous equilibrium
B) heterogeneous equilibrium
C) instantaneous equilibrium
D) reaction that will not come to an equilibrium

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
10
The production of ammonia N2( g ) + 3 H2( g ) 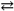 2 NH3( g ) represents a(n):
2 NH3( g ) represents a(n):
A) homogeneous equilibrium
B) heterogeneous equilibrium
C) instantaneous equilibrium
D) reaction that will not come to an equilibrium
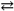 2 NH3( g ) represents a(n):
2 NH3( g ) represents a(n):A) homogeneous equilibrium
B) heterogeneous equilibrium
C) instantaneous equilibrium
D) reaction that will not come to an equilibrium

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
11
The higher the Ksp of the salt, the less soluble the salt.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
12
Unlike traditional concrete, engineered cementitious composites (ECC) do not contain gravel but instead comtain fly ash.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
13
Products appear in the numerator of the reaction quotient

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
14
Cememt structures with many small cracks are more likely to fail than similar structure with fewer, larger cracks.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
15
In a dynamic equilibrium:
A) the rate of the forward reaction goes to zero
B) the rate of the forward and reverse reactions go to zero
C) the rates of the forward and reverse reactions become equal
D) the reaction begins to favor the reverse direction
A) the rate of the forward reaction goes to zero
B) the rate of the forward and reverse reactions go to zero
C) the rates of the forward and reverse reactions become equal
D) the reaction begins to favor the reverse direction

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
16
Hydrogen is produced industrially by reacting propane and liquid water:
C3H8( g ) + 3 H2O( l ) → 3 CO( g ) + 7 H2( g )
If the denominator of the equilibrium expression is expressed as: [C3H8]m[ H2O]n, what are the values of "m" and "n"?
A) 3, 7
B) 1, 0
C) 1, 3
D) 7, 3
C3H8( g ) + 3 H2O( l ) → 3 CO( g ) + 7 H2( g )
If the denominator of the equilibrium expression is expressed as: [C3H8]m[ H2O]n, what are the values of "m" and "n"?
A) 3, 7
B) 1, 0
C) 1, 3
D) 7, 3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
17
In a dynamic equilibrium the rates of the forward and reverse reactions go to zero once the equilibrium is reached.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
18
"Insoluble" salts are actually "sparingly" soluble with equilibrium expressions noted as Ka

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
19
A catalyst will increase the rate of the forward and reverse reactions equally.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
20
The term "strong acid" refers to any acid of 0.100 M concentration or higher.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
21
The conjugate acid of sodium acetate (Na+CH3COO − ) is:
A) NaOH
B) CH3COOH
C) HCl
D) K+CH3COO −
A) NaOH
B) CH3COOH
C) HCl
D) K+CH3COO −

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
22
Consider the weak base ClO − .
ClO − + H2O![<strong>Consider the weak base ClO<sup> − </sup>. ClO<sup> − </sup> + H<sub>2</sub>O HClO + OH<sup> − </sup> If a dilute HCl is slowly titrated into a sample of ClO<sup> − </sup>, the [ClO<sup> − </sup>] concentration:</strong> A) decreases B) remains constant C) increases D) there is insufficient information to solve this problem](https://d2lvgg3v3hfg70.cloudfront.net/TBX8709/11ebe889_6d80_e7b1_b264_253759d1b45c_TBX8709_11.jpg) HClO + OH −
HClO + OH −
If a dilute HCl is slowly titrated into a sample of ClO − , the [ClO − ] concentration:
A) decreases
B) remains constant
C) increases
D) there is insufficient information to solve this problem
ClO − + H2O
![<strong>Consider the weak base ClO<sup> − </sup>. ClO<sup> − </sup> + H<sub>2</sub>O HClO + OH<sup> − </sup> If a dilute HCl is slowly titrated into a sample of ClO<sup> − </sup>, the [ClO<sup> − </sup>] concentration:</strong> A) decreases B) remains constant C) increases D) there is insufficient information to solve this problem](https://d2lvgg3v3hfg70.cloudfront.net/TBX8709/11ebe889_6d80_e7b1_b264_253759d1b45c_TBX8709_11.jpg) HClO + OH −
HClO + OH − If a dilute HCl is slowly titrated into a sample of ClO − , the [ClO − ] concentration:
A) decreases
B) remains constant
C) increases
D) there is insufficient information to solve this problem

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
23
What change in reaction direction occurs if dilute HCl is added to a H2PO4 − solution?
H2PO4 1− + H2O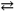 HPO42 − + H3O+
HPO42 − + H3O+
A) shifts to the right
B) shifts to the left
C) no change in the reaction
D) there is insufficient information to solve this problem
H2PO4 1− + H2O
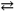 HPO42 − + H3O+
HPO42 − + H3O+ A) shifts to the right
B) shifts to the left
C) no change in the reaction
D) there is insufficient information to solve this problem

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
24
What is the pH of a 0.150 M NaOH solution?
A) 0.82
B) 3.05
C) 11.66
D) 13.18
A) 0.82
B) 3.05
C) 11.66
D) 13.18

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
25
What is the pH of a 0.0250 M pyridine solution? (Kb = 1.5 × 10 − 9)?
A) 8.79
B) 5.21
C) 13.1
D) 4.72
A) 8.79
B) 5.21
C) 13.1
D) 4.72

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
26
What is the molar solubility of CaF2 (Ksp = 3.9 × 10 − 11)?
A) 6.24 × 10 − 6 M
B) 4.41 × 10 − 6 M
C) 2.14 × 10 − 4 M
D) 9.27 × 10 − 5 M
A) 6.24 × 10 − 6 M
B) 4.41 × 10 − 6 M
C) 2.14 × 10 − 4 M
D) 9.27 × 10 − 5 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
27
Which of the following is a strong base?
A) HClO4
B) HNO2
C) HCl
D) KOH
A) HClO4
B) HNO2
C) HCl
D) KOH

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
28
What change in the reaction direction occurs if dilute NaOH is added to a H2PO41- solution?
H2PO4 1− + H2O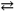 HPO42 − + H3O+
HPO42 − + H3O+
A) shifts to the right
B) shifts to the left
C) no change in the reaction
D) there is insufficient information to solve this problem
H2PO4 1− + H2O
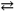 HPO42 − + H3O+
HPO42 − + H3O+A) shifts to the right
B) shifts to the left
C) no change in the reaction
D) there is insufficient information to solve this problem

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
29
What is the pH of a 0.200 M solution of HNO3?
A) 0.70
B) 1.31
C) 6.18
D) 8.53
A) 0.70
B) 1.31
C) 6.18
D) 8.53

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
30
The synthesis of the pesticide thionyl chloride (OSCl2) proceeds according to:
SO3( g ) + SCl2( l )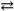 OSCl2( l ) + SO2( g )
OSCl2( l ) + SO2( g )
If Δ G0 = − 106 kJ/mol for this reaction at 298K, what is the Keq at this temperature? Recall: R = 8.314 J mol − 1 K − 1.
A) 7.21 × 1012
B) 3.50 × 1014
C) 3.81 × 1018
D) 1.19 × 1020
SO3( g ) + SCl2( l )
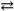 OSCl2( l ) + SO2( g )
OSCl2( l ) + SO2( g ) If Δ G0 = − 106 kJ/mol for this reaction at 298K, what is the Keq at this temperature? Recall: R = 8.314 J mol − 1 K − 1.
A) 7.21 × 1012
B) 3.50 × 1014
C) 3.81 × 1018
D) 1.19 × 1020

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which of the following is a weak acid?
A) NH3
B) HClO
C) HCl
D) HNO3
A) NH3
B) HClO
C) HCl
D) HNO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
32
Consider the solubility of BaSO4. If Ba(NO3)2 is added to the solution, the solubility of the barium sulfate:
A) is unaffected
B) is moot because barium sulfate is a strong electrolyte
C) decreases
D) increases
A) is unaffected
B) is moot because barium sulfate is a strong electrolyte
C) decreases
D) increases

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
33
Potassium hydrogen phthalate (KHP) concentrations can be determined through titrating samples of KHP (a monoprotic acid) with bases such as NaOH in the presence of an indicator such as phenolphthalein. The indicator is colorless in an acidic solution and turns pink in an alkaline solution. Thus we can establish an equilibrium for the phenolphthalein as such:
HIn + H2O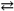 In − + H3O+
In − + H3O+
If the HIn species is the "acid color" or clear for the phenolphthalein and the In − species is the "base color" or pink for this particular indicator, what color will appear in a flask in which a 0.2993 gram sample of KHP has been totaled neutralized with an excess of NaOH?
A) the flask is colorless
B) the flask is pink
C) the flask is white from KCl precipitation
D) there is insufficient information to solve this problem
HIn + H2O
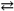 In − + H3O+
In − + H3O+ If the HIn species is the "acid color" or clear for the phenolphthalein and the In − species is the "base color" or pink for this particular indicator, what color will appear in a flask in which a 0.2993 gram sample of KHP has been totaled neutralized with an excess of NaOH?
A) the flask is colorless
B) the flask is pink
C) the flask is white from KCl precipitation
D) there is insufficient information to solve this problem

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which of the following solids will provide the greatest concentration of Cl1 − ions if one mole of the salt is placed in one liter of deionized water?
A) AgCl( s )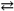 Ag1+( aq ) + Cl1 − ( aq ) Ksp = 1.8 × 10 − 10
Ag1+( aq ) + Cl1 − ( aq ) Ksp = 1.8 × 10 − 10
B) PbCl2( s )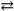 Pb2+( aq ) + 2 Cl1 − ( aq ) Ksp = 1.75 × 10 − 5
Pb2+( aq ) + 2 Cl1 − ( aq ) Ksp = 1.75 × 10 − 5
C) Hg2Cl2( s )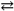 2 Hg1+( aq ) + 2 Cl1 − ( aq ) Ksp = 1.1 × 10 − 18
2 Hg1+( aq ) + 2 Cl1 − ( aq ) Ksp = 1.1 × 10 − 18
D) NaCl( s ) → Na1+( aq ) + Cl1 − ( aq )
A) AgCl( s )
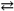 Ag1+( aq ) + Cl1 − ( aq ) Ksp = 1.8 × 10 − 10
Ag1+( aq ) + Cl1 − ( aq ) Ksp = 1.8 × 10 − 10B) PbCl2( s )
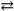 Pb2+( aq ) + 2 Cl1 − ( aq ) Ksp = 1.75 × 10 − 5
Pb2+( aq ) + 2 Cl1 − ( aq ) Ksp = 1.75 × 10 − 5C) Hg2Cl2( s )
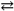 2 Hg1+( aq ) + 2 Cl1 − ( aq ) Ksp = 1.1 × 10 − 18
2 Hg1+( aq ) + 2 Cl1 − ( aq ) Ksp = 1.1 × 10 − 18D) NaCl( s ) → Na1+( aq ) + Cl1 − ( aq )

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
35
What is the pH of a 0.1055 M CH3COOH solution (Ka = 1.75 × 10 − 5)?
A) 2.770
B) 2.867
C) 3.188
D) 8.474
A) 2.770
B) 2.867
C) 3.188
D) 8.474

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
36
Which of following species is amphoteric?
A) NaOH
B) C4H10
C) H2O
D) H2SO4
A) NaOH
B) C4H10
C) H2O
D) H2SO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
37
The lower the pH of a weak acid, the:
A) higher the concentration of the weak acid.
B) higher the dissolved H2(g) concentration.
C) higher the Ka of the acid.
D) none of the above
A) higher the concentration of the weak acid.
B) higher the dissolved H2(g) concentration.
C) higher the Ka of the acid.
D) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
38
The conjugate base of ammonium chloride is:
A) NH3
B) HNO2
C) HCl
D) HNO3
A) NH3
B) HNO2
C) HCl
D) HNO3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
39
At equilibrium Δ G is:
A) = 0
B) > 0
C) can not be determined
A) = 0
B) > 0
C) can not be determined

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
40
What is the molar solubility of AgCl (Ksp = 1.8 × 10 − 10)?
A) 7.31 × 10 − 5 M
B) 5.13 × 10 − 6 M
C) 3.84 × 10 − 5 M
D) 1.34 × 10 − 5 M
A) 7.31 × 10 − 5 M
B) 5.13 × 10 − 6 M
C) 3.84 × 10 − 5 M
D) 1.34 × 10 − 5 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck








