Deck 11: Chemical Kinetics
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/40
العب
ملء الشاشة (f)
Deck 11: Chemical Kinetics
1
A reaction mechanism is a collection of elementary steps.
True
2
According to the differential rate law: rate = k[X]m[Y]n. If m = 2 and n = 1, the overall reaction order is:
A) 3
B) 2
C) 1
D) 0
A) 3
B) 2
C) 1
D) 0
3
3
For a 1st order reaction, a plot of concentration with respect to time produces a plot that is:
A) linear
B) non-linear
C) parabolic
D) linear; m = 0
A) linear
B) non-linear
C) parabolic
D) linear; m = 0
non-linear
4
If a reaction is first order with respect to [A], doubling the concentration of [A] will result in:
A) a doubling of the rate
B) a four-fold increase in rate
C) an eight-fold increase in rate
D) no change in the rate of reaction
A) a doubling of the rate
B) a four-fold increase in rate
C) an eight-fold increase in rate
D) no change in the rate of reaction

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
5
The instantaneous rate of a reaction is determined using the slope of a line tangent to the curve of the change of concentration versus time.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
6
For a first-order reaction, t1/2 = ln 2/ k

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
7
The production of ozone begins with:
A) a UV photon dissociating a diatomic oxygen molecule
B) a reaction between O2 and a CFC
C) the formation of a VOC radical
D) the formation of an O4 intermediate
A) a UV photon dissociating a diatomic oxygen molecule
B) a reaction between O2 and a CFC
C) the formation of a VOC radical
D) the formation of an O4 intermediate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
8
A termolecular step in a reaction mechanism is a step that involves two molecules.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
9
The rate of a reaction may be expressed as the disappearance of reactants with respect to time.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
10
O3, not O2, is the thermodynamically favored allotrope of oxygen for the conditions found at the Earth's surface.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
11
When two concentrations are measured at times separated by a finite difference, the ____ may be calculated:
A) entropic rate
B) induced rate
C) average rate
D) instantaneous rate
A) entropic rate
B) induced rate
C) average rate
D) instantaneous rate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
12
Stirring a reaction increases the rate of the reaction by raising the energy of activation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
13
For a first-order reaction, a plot of concentration vs time provides a line with a slope equal to − k.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
14
If a reaction is 1st order with respect to [A] and 2nd order with respect to [B], doubling the concentration of reactant [B] will result in a four-fold increase in the rate of reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
15
If a reaction is zero order with respect to [B], tripling the concentration of [B] will result in:
A) a tripling of the rate
B) a four-fold increase in rate
C) a nine-fold increase in rate
D) no change in the rate of reaction
A) a tripling of the rate
B) a four-fold increase in rate
C) a nine-fold increase in rate
D) no change in the rate of reaction

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
16
Endothermic reactions do not require activation energy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
17
A plot of a reactant concentration vs. time is linear for a zeroth order reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
18
If a reaction is second order with respect to [A], doubling the concentration of [A] will result in:
A) a doubling of the rate
B) a four-fold increase in rate
C) an eight-fold increase in rate
D) no change in the rate of reaction
A) a doubling of the rate
B) a four-fold increase in rate
C) an eight-fold increase in rate
D) no change in the rate of reaction

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
19
The rate constant "k" will vary with changes in concentration of 1st order reactants.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which statement best describes the rate of reaction for the production of ammonia via the Haber reaction ( 3 H2 + N2 → 2 NH3)
A) rate = 2 Δ [NH3] / Δ t
B) rate = Δ [NH3] / 2 Δ t
C) rate = 2 Δ [NH3] / 2 Δ t
D) There is too little information to assess the change.
A) rate = 2 Δ [NH3] / Δ t
B) rate = Δ [NH3] / 2 Δ t
C) rate = 2 Δ [NH3] / 2 Δ t
D) There is too little information to assess the change.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
21
Consider the reaction: 2A + B → C,
And a kinetics study on this reaction yielded:
![<strong>Consider the reaction: 2A + B → C, And a kinetics study on this reaction yielded: What order is the reaction with respect to ([A], [B])?</strong> A) 1, 2 B) 2, 1 C) 2, 0 D) 0, 2](https://d2lvgg3v3hfg70.cloudfront.net/TBX8709/11ebe889_6d7f_6109_b264_a9f7e71db46a_TBX8709_00.jpg)
What order is the reaction with respect to ([A], [B])?
A) 1, 2
B) 2, 1
C) 2, 0
D) 0, 2
And a kinetics study on this reaction yielded:
![<strong>Consider the reaction: 2A + B → C, And a kinetics study on this reaction yielded: What order is the reaction with respect to ([A], [B])?</strong> A) 1, 2 B) 2, 1 C) 2, 0 D) 0, 2](https://d2lvgg3v3hfg70.cloudfront.net/TBX8709/11ebe889_6d7f_6109_b264_a9f7e71db46a_TBX8709_00.jpg)
What order is the reaction with respect to ([A], [B])?
A) 1, 2
B) 2, 1
C) 2, 0
D) 0, 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
22
The decomposition of hydrogen peroxide (H2O2) into O2( g ) and liquid H2O produced the following data:
 What is the average rate in mL/min for the third ten-minute period of the reaction?
What is the average rate in mL/min for the third ten-minute period of the reaction?
A) 6.72 mL/min
B) 10.1 mL/min
C) 7.98 mL/min
D) 15.7 mL/min
 What is the average rate in mL/min for the third ten-minute period of the reaction?
What is the average rate in mL/min for the third ten-minute period of the reaction?A) 6.72 mL/min
B) 10.1 mL/min
C) 7.98 mL/min
D) 15.7 mL/min

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
23
A catalyst elevates the rate of a reaction by:
A) lowering the activation energy
B) decreasing the effectiveness of molecular collisions
C) increasing the number of molecular collisions
D) none of the above
A) lowering the activation energy
B) decreasing the effectiveness of molecular collisions
C) increasing the number of molecular collisions
D) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
24
The synthesis of ammonia from hydrogen and nitrogen produced the following kinetic data:
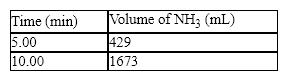 What is the average rate in mL/min for the second five-minute period of the reaction?
What is the average rate in mL/min for the second five-minute period of the reaction?
A) 167 mL/min
B) 85.3 mL/min
C) 249 mL/min
D) 429 mL/min
 What is the average rate in mL/min for the second five-minute period of the reaction?
What is the average rate in mL/min for the second five-minute period of the reaction?A) 167 mL/min
B) 85.3 mL/min
C) 249 mL/min
D) 429 mL/min

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
25
Consider the reaction: 2A + B → C, 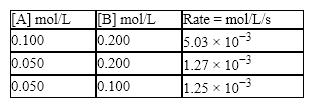 What is the value of the rate constant?
What is the value of the rate constant?
A) 3.20 mol L − 1s
B) 19.8 mol L − 1s
C) 0.530 mol L − 1s
D) 4.60 mol L − 1s
 What is the value of the rate constant?
What is the value of the rate constant?A) 3.20 mol L − 1s
B) 19.8 mol L − 1s
C) 0.530 mol L − 1s
D) 4.60 mol L − 1s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
26
Consider the reaction: A + 2B → C,
And a kinetics study on this reaction yielded:
![<strong>Consider the reaction: A + 2B → C, And a kinetics study on this reaction yielded: What order is the reaction with respect to ([A], [B])?</strong> A) 1, 2 B) 2, 2 C) 2, 1 D) 0, 2](https://d2lvgg3v3hfg70.cloudfront.net/TBX8709/11ebe889_6d7f_39f8_b264_37d48e692754_TBX8709_00.jpg)
What order is the reaction with respect to ([A], [B])?
A) 1, 2
B) 2, 2
C) 2, 1
D) 0, 2
And a kinetics study on this reaction yielded:
![<strong>Consider the reaction: A + 2B → C, And a kinetics study on this reaction yielded: What order is the reaction with respect to ([A], [B])?</strong> A) 1, 2 B) 2, 2 C) 2, 1 D) 0, 2](https://d2lvgg3v3hfg70.cloudfront.net/TBX8709/11ebe889_6d7f_39f8_b264_37d48e692754_TBX8709_00.jpg)
What order is the reaction with respect to ([A], [B])?
A) 1, 2
B) 2, 2
C) 2, 1
D) 0, 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
27
During the formation of MgO from the burning of Mg metal, what steps will result in an increase in the rate of reaction?
A) increasing the metal surface area by milling
B) selecting tarnished pieces of magnesium for the experiment
C) increasing the amount of metal by selecting a larger cube of magnesium
D) none of the above.
A) increasing the metal surface area by milling
B) selecting tarnished pieces of magnesium for the experiment
C) increasing the amount of metal by selecting a larger cube of magnesium
D) none of the above.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
28
Raising the temperature of a reaction elevates the rate of reaction by:
A) increasing the energy of activation
B) creating more molecules in the reaction.
C) increasing the number of molecules moving at a speed sufficiently high enough to produce a reactive collision.
D) decreasing the entropy of the system.
A) increasing the energy of activation
B) creating more molecules in the reaction.
C) increasing the number of molecules moving at a speed sufficiently high enough to produce a reactive collision.
D) decreasing the entropy of the system.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
29
The synthesis of ammonia from hydrogen and nitrogen produced the following kinetic data:
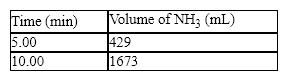
What is the average rate in mL/min for the first five minutes of the reaction?
A) 167 mL/min
B) 85.3 mL/min
C) 249 mL/min
D) 429 mL/min

What is the average rate in mL/min for the first five minutes of the reaction?
A) 167 mL/min
B) 85.3 mL/min
C) 249 mL/min
D) 429 mL/min

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
30
Consider the production of oxygen from ozone. The mechanism includes two elementary steps:
O3 + Cl → ClO + O2 ClO + O3 → Cl + 2O2
Which species is a reactive intermediate?
A) O3
B) ClO
C) Cl
D) both ClO and Cl
O3 + Cl → ClO + O2 ClO + O3 → Cl + 2O2
Which species is a reactive intermediate?
A) O3
B) ClO
C) Cl
D) both ClO and Cl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
31
The production of nitric oxide is governed by the reaction:
4 NH3( g ) + 5 O2( g ) → 4 NO( g ) + 6 H2O( g )
If the rate at which NO is produced is 6.35 × 10 −2 mol L − 1s − 1, at what rate is O2 consumed?
A) 8.29 × 10 − 3 mol L − 1s − 1
B) 1.59 × 10 − 2 mol L − 1s − 1
C) 6.35 × 10 −2 mol L − 1s − 1
D) 7.94 × 10 −2 mol L − 1s − 1
4 NH3( g ) + 5 O2( g ) → 4 NO( g ) + 6 H2O( g )
If the rate at which NO is produced is 6.35 × 10 −2 mol L − 1s − 1, at what rate is O2 consumed?
A) 8.29 × 10 − 3 mol L − 1s − 1
B) 1.59 × 10 − 2 mol L − 1s − 1
C) 6.35 × 10 −2 mol L − 1s − 1
D) 7.94 × 10 −2 mol L − 1s − 1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
32
The catalytic converter on an automobile has a direct role in:
A) increasing the VOC emitted by the vehicle.
B) converting NO into O2 and N2
C) the conversion of ozone to oxygen
D) creating large amounts of PtN
A) increasing the VOC emitted by the vehicle.
B) converting NO into O2 and N2
C) the conversion of ozone to oxygen
D) creating large amounts of PtN

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
33
Lowering the activation energy of a reaction will increase the rate of reaction by:
A) removing potential energy from the reactants.
B) spontaneously increasing the amount of reactants.
C) packing the created products in a favored arrangement.
D) providing a new reaction mechanism.
A) removing potential energy from the reactants.
B) spontaneously increasing the amount of reactants.
C) packing the created products in a favored arrangement.
D) providing a new reaction mechanism.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
34
For a zero order reaction, a plot of concentration with respect to time produces a plot that is:
A) linear; m = − k
B) non-linear
C) parabolic
D) linear; m = 0
A) linear; m = − k
B) non-linear
C) parabolic
D) linear; m = 0

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
35
A chemical species that is not consumed by the reaction and increases the rate of the reaction is referred to as a(n):
A) reactive intermediate
B) entropic entity
C) spectator ion
D) catalyst
A) reactive intermediate
B) entropic entity
C) spectator ion
D) catalyst

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
36
The radioactive nuclide 14C decays with a t1/2 of 5730 yrs. An archaeologist is studying a woven mat using radiocarbon dating. If calculations related to the mat's original 14C content reveal that there was 5.83 × 1022 atoms of 14C present in the plant used to weave the mat, how long ago was the mat woven if modern spectroscopy was used to determine that 7.11 × 1020 atoms of 14C now exist in the sample?
A) 8.3 × 104 yrs
B) 4.1 × 102 yrs
C) 3.6 × 104 yrs
D) 5.0 × 106 yrs
A) 8.3 × 104 yrs
B) 4.1 × 102 yrs
C) 3.6 × 104 yrs
D) 5.0 × 106 yrs

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
37
Sulfur trioxide production follows the reaction:
2 SO2( g ) + O2( g ) → 2 SO3( g )
If the rate at which SO3 is produced is 4.28 × 10 − 4 mol L − 1s − 1, at what rate is SO2 consumed?
A) 4.28 × 10 − 4 mol L − 1s − 1
B) 1.08 × 10 − 3 mol L − 1s − 1
C) 1.07 × 10 − 4 mol L − 1s − 1
D) 2.14 × 10 − 4 mol L − 1s − 1
2 SO2( g ) + O2( g ) → 2 SO3( g )
If the rate at which SO3 is produced is 4.28 × 10 − 4 mol L − 1s − 1, at what rate is SO2 consumed?
A) 4.28 × 10 − 4 mol L − 1s − 1
B) 1.08 × 10 − 3 mol L − 1s − 1
C) 1.07 × 10 − 4 mol L − 1s − 1
D) 2.14 × 10 − 4 mol L − 1s − 1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
38
Consider the elementary step: A + B → C. What type of elementary step is this?
A) unimolecular
B) bimolecular
C) termolecular
D) none of the above
A) unimolecular
B) bimolecular
C) termolecular
D) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
39
If the decomposition of reactant A follows first-order kinetics, what concentration of A remains if:
[A]0 = 0.273 M, k = 1.53 × 10 − 2 minutes − 1, and the elapsed time = 1.61 hr?
A) 0.009 M
B) 0.0623 M
C) 0.109 M
D) 0.273 M
[A]0 = 0.273 M, k = 1.53 × 10 − 2 minutes − 1, and the elapsed time = 1.61 hr?
A) 0.009 M
B) 0.0623 M
C) 0.109 M
D) 0.273 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
40
Which of the following internal combustion engine products plays the largest role in the production of smog?
A) heat
B) NO2
C) CO2
D) H2O
A) heat
B) NO2
C) CO2
D) H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck








