Deck 2: Molecules of Life
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
Match between columns
سؤال
سؤال
Match between columns
سؤال
سؤال
Match between columns
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
Match between columns
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/100
العب
ملء الشاشة (f)
Deck 2: Molecules of Life
1
Helium, neon and argon are _____.
A)extremely stable because they have vacancies in their outer shells
B)extremely stable because they don't have any vacancies in their outer shells
C)extremely unstable because they have vacancies in their outer shells
D)extremely unstable because they don't have any vacancies in their outer shells
E)extremely unstable because they have vacancies in their inner shells
A)extremely stable because they have vacancies in their outer shells
B)extremely stable because they don't have any vacancies in their outer shells
C)extremely unstable because they have vacancies in their outer shells
D)extremely unstable because they don't have any vacancies in their outer shells
E)extremely unstable because they have vacancies in their inner shells
B
2
Oxygen has an atomic number of 8. This means that oxygen has ____.
A)8 electrons in its outer most shell
B)8 neutrons in its nucleus
C)4 protons and 4 neutrons in its nucleus
D)8 protons in its nucleus
E)8 protons and 8 neutrons in its nucleus
A)8 electrons in its outer most shell
B)8 neutrons in its nucleus
C)4 protons and 4 neutrons in its nucleus
D)8 protons in its nucleus
E)8 protons and 8 neutrons in its nucleus
D
3
Fats are major components of the cell's ____.
A)membranes
B)cytoplasm
C)proteins
D)ribosomes
E)DNA
A)membranes
B)cytoplasm
C)proteins
D)ribosomes
E)DNA
A
4
In ____ bonds, atoms share electrons equally.
A)double
B)ionic
C)polar covalent
D)nonpolar covalent
E)hydrogen
A)double
B)ionic
C)polar covalent
D)nonpolar covalent
E)hydrogen

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
5
The neutral subatomic particle is the ____.
A)neutron
B)proton
C)electron
D)quark
E)Higg's boson
A)neutron
B)proton
C)electron
D)quark
E)Higg's boson

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
6
Carbon 14 radioisotopes decay into stable ____.
Nitrogen 15 isotopes
A)carbon 13 isotopes
B)nitrogen atoms
C)carbon atoms
D)nitrogen 15 isotopes
E)sodium atoms
Nitrogen 15 isotopes
A)carbon 13 isotopes
B)nitrogen atoms
C)carbon atoms
D)nitrogen 15 isotopes
E)sodium atoms

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
7
The atomic number is determined by the number of ____.
A)protons
B)neutrons
C)electrons
D)protons plus neutrons
E)protons plus electrons
A)protons
B)neutrons
C)electrons
D)protons plus neutrons
E)protons plus electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
8
The bond in table salt (NaCl)is ____.
A)polar
B)ionic
C)covalent
D)double
E)nonpolar
A)polar
B)ionic
C)covalent
D)double
E)nonpolar

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which invention led to trans fats being marketed as a solid cooking fat?
A)the electric light
B)the telephone
C)the automobile
D)the microwave oven
E)the refrigerator
A)the electric light
B)the telephone
C)the automobile
D)the microwave oven
E)the refrigerator

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
10
The negative subatomic particle is the ____.
A)neutron
B)proton
C)electron
D)quark
E)Higg's boson
A)neutron
B)proton
C)electron
D)quark
E)Higg's boson

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
11
Hydrogenation is a _____.
A)manufacturing process that adds hydrogen atoms to carbohydrates
B)natural process that that adds hydrogen atoms to carbohydrates
C)manufacturing process that adds hydrogen atoms to oils
D)natural process that removes hydrogen atoms from fats
E)manufacturing process that removes hydrogen atoms from fats
A)manufacturing process that adds hydrogen atoms to carbohydrates
B)natural process that that adds hydrogen atoms to carbohydrates
C)manufacturing process that adds hydrogen atoms to oils
D)natural process that removes hydrogen atoms from fats
E)manufacturing process that removes hydrogen atoms from fats

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
12
A(n)____ is a type of chemical bond in which a strong mutual attraction forms between ions of opposite charge.
A)hydrogen bond
B)nonpolar bond
C)polar bond
D)covalent bond
E)ionic bond
A)hydrogen bond
B)nonpolar bond
C)polar bond
D)covalent bond
E)ionic bond

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
13
Tracers are used in what form of medical test?
A)PET scans
B)CT scans
C)sonograms
D)x-rays
E)MRI
A)PET scans
B)CT scans
C)sonograms
D)x-rays
E)MRI

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
14
The human body requires about ____ of fat each day to stay healthy.
A)1 teaspoon
B)4 teaspoons
C)1 tablespoon
D)4 tablespoons
E)1 cup
A)1 teaspoon
B)4 teaspoons
C)1 tablespoon
D)4 tablespoons
E)1 cup

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
15
The positive subatomic particle is the ____.
A)neutron
B)proton
C)electron
D)positron
E)quark
A)neutron
B)proton
C)electron
D)positron
E)quark

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
16
An atom that carries a charge is called a(n)____.
A)ion
B)molecule
C)compound
D)element
E)microelement
A)ion
B)molecule
C)compound
D)element
E)microelement

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
17
Carbon has an atomic number of 6. Carbon-14 has _____.
A)6 neutrons and 6 protons
B)6 neutrons and 8 protons
C)8 neutrons and 6 protons
D)14 neutrons and 6 protons
E)14 protons and 6 neutrons
A)6 neutrons and 6 protons
B)6 neutrons and 8 protons
C)8 neutrons and 6 protons
D)14 neutrons and 6 protons
E)14 protons and 6 neutrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
18
A typical fat molecule has ____ fatty acid tails.
A)one
B)two
C)three
D)four
E)five
A)one
B)two
C)three
D)four
E)five

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
19
We can accurately determine the age of a rock or fossil by measuring its ____.
A)proton concentration
B)electron concentration
C)neutron concentration
D)isotope concentration
E)ion concentration
A)proton concentration
B)electron concentration
C)neutron concentration
D)isotope concentration
E)ion concentration

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
20
The nucleus of an atom contains ____.
A)protons only
B)electrons only
C)neutrons only
D)protons and neutrons
E)protons and electrons
A)protons only
B)electrons only
C)neutrons only
D)protons and neutrons
E)protons and electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
21
When water molecules form into ice, ____.
A)the water molecules jiggle more
B)their structure becomes less rigid
C)the water molecules pack less densely
D)hydrogen bonds between water molecules readily break
E)evaporation of water molecules happens more readily
A)the water molecules jiggle more
B)their structure becomes less rigid
C)the water molecules pack less densely
D)hydrogen bonds between water molecules readily break
E)evaporation of water molecules happens more readily

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which substance is hydrophobic?
A)canola oil
B)sodium chloride
C)sugar
D)water
E)the potassium ion
A)canola oil
B)sodium chloride
C)sugar
D)water
E)the potassium ion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
23
The positively charged ion, potassium, and the negatively charged ion, fluoride, will form what kind of bond?
A)ionic
B)polar covalent
C)nonpolar covalent
D)hydrogen
E)isotonic
A)ionic
B)polar covalent
C)nonpolar covalent
D)hydrogen
E)isotonic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
24
Fats will dissolve in ethanol. Ethanol is an example of a ____.
A)solute
B)solution
C)solvent
D)salt
E)ion
A)solute
B)solution
C)solvent
D)salt
E)ion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
25
A solution is a uniform mixture in which a ____ is dissolved completely in a ____.
A)salt; solute
B)solute; salt
C)solute; solvent
D)solvent; salt
E)solvent; solute
A)salt; solute
B)solute; salt
C)solute; solvent
D)solvent; salt
E)solvent; solute

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
26
Surface tension is an example of ____.
A)hydrophobicity
B)concentration
C)evaporation
D)cohesion
E)polarity
A)hydrophobicity
B)concentration
C)evaporation
D)cohesion
E)polarity

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
27
The structural formula for molecular oxygen is depicted as O=O. What kind of bond holds molecular oxygen together?
A)ionic
B)polar covalent
C)single covalent
D)double covalent
E)triple covalent
A)ionic
B)polar covalent
C)single covalent
D)double covalent
E)triple covalent

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
28
Nearly all of life's chemistry occurs near a pH of ____.
A)1
B)3
C)5
D)7
E)9
A)1
B)3
C)5
D)7
E)9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
29
A solution at a pH of 10 contains how many times more hydrogen ions than a solution at a pH of 7?
A)2
B)3
C)10
D)100
E)1,000
A)2
B)3
C)10
D)100
E)1,000

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
30
Which bond is weakest?
A)ionic
B)double covalent
C)polar covalent
D)nonpolar covalent
E)hydrogen
A)ionic
B)double covalent
C)polar covalent
D)nonpolar covalent
E)hydrogen

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
31
A pH value of ____ has the highest concentration of hydrogen ions.
A)1
B)3
C)5
D)7
E)9
A)1
B)3
C)5
D)7
E)9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
32
Which type of chemical bonds are found within a water molecule?
A)hydrogen
B)ionic
C)polar covalent
D)nonpolar covalent
E)triple
A)hydrogen
B)ionic
C)polar covalent
D)nonpolar covalent
E)triple

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which property of water molecules is responsible for movement of water from roots to leaves in a plant?
A)hydrophobicity
B)temperature stability
C)fusion
D)solvent polarity
E)cohesion
A)hydrophobicity
B)temperature stability
C)fusion
D)solvent polarity
E)cohesion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
34
Water molecules are attracted to one another because the ____.
A)slightly positive charge of the hydrogen atom from one molecule of water attracts the slightly negative charge of the oxygen atom from another molecule
B)slightly negative charge of the hydrogen atom from one molecule of water attracts the slightly negative charge of the oxygen atom from another molecule
C)slightly positive charge of the hydrogen atom attracts the oxygen within the same molecule of water, which leads to an increase in its polarity
D)water molecules participate in non-polar covalent bonds, which increase the attraction of the molecules to each other
E)water molecules bind to each other through their mutual attraction to ionic compounds
A)slightly positive charge of the hydrogen atom from one molecule of water attracts the slightly negative charge of the oxygen atom from another molecule
B)slightly negative charge of the hydrogen atom from one molecule of water attracts the slightly negative charge of the oxygen atom from another molecule
C)slightly positive charge of the hydrogen atom attracts the oxygen within the same molecule of water, which leads to an increase in its polarity
D)water molecules participate in non-polar covalent bonds, which increase the attraction of the molecules to each other
E)water molecules bind to each other through their mutual attraction to ionic compounds

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
35
____ is the tendency of water molecules to stay attached to one another.
A)Adhesion
B)Cohesion
C)Fusion
D)Interaction
E)Junction
A)Adhesion
B)Cohesion
C)Fusion
D)Interaction
E)Junction

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
36
Glucose dissolves in water because it ____.
A)ionizes
B)is a polysaccharide
C)is a polar and forms many hydrogen bonds with water molecules
D)has a very reactive primary structure
E)is an isotope
A)ionizes
B)is a polysaccharide
C)is a polar and forms many hydrogen bonds with water molecules
D)has a very reactive primary structure
E)is an isotope

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
37
Hydrogen bonding ____ the movement of molecules, therefore, substances that form a lot of hydrogen bonds, like water, will require ____ energy to increase their temperature by one degree Celsius.
A)decreases; less
B)decreases; more
C)doesn't affect; no additional
D)increases; less
E)increases; more
A)decreases; less
B)decreases; more
C)doesn't affect; no additional
D)increases; less
E)increases; more

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
38
Hydrophobic molecules are ____ water.
A)attracted by
B)absorbed by
C)repelled by
D)mixed with
E)polarized by
A)attracted by
B)absorbed by
C)repelled by
D)mixed with
E)polarized by

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
39
Sweating to keep cool in the summer is the result of ____.
A)hydrogen bonds breaking to release energy
B)hydrogen bonds forming, which requires energy
C)evaporation of water giving off energy
D)cohesion of water molecules giving off energy
E)cohesion of water molecules requiring energy
A)hydrogen bonds breaking to release energy
B)hydrogen bonds forming, which requires energy
C)evaporation of water giving off energy
D)cohesion of water molecules giving off energy
E)cohesion of water molecules requiring energy

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
40
What molecule would be considered a covalent compound?
A)oxygen (O2)
B)sodium chloride (NaCl)
C)water (H2O)
D)a diamond (C)
E)ozone (O3)
A)oxygen (O2)
B)sodium chloride (NaCl)
C)water (H2O)
D)a diamond (C)
E)ozone (O3)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
41
Which type of bonding allows the long, straight chains of cellulose to lock together tightly?
A)hydrogen
B)polar covalent
C)ionic
D)nonpolar covalent
E)metallic
A)hydrogen
B)polar covalent
C)ionic
D)nonpolar covalent
E)metallic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
42
The chemical reactions that cells use to acquire and use energy to live, grow and reproduce are called ____.
A)hydrolysis
B)condensation
C)phosphorylation
D)metabolism
E)oxidation
A)hydrolysis
B)condensation
C)phosphorylation
D)metabolism
E)oxidation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
43
Cellulose is ____.
A)the most complex of the organic compounds
B)a polymer of glucose and fructose
C)a polymer of glucose and galactose
D)a component of plasma membranes
E)a material found in plant cell walls
A)the most complex of the organic compounds
B)a polymer of glucose and fructose
C)a polymer of glucose and galactose
D)a component of plasma membranes
E)a material found in plant cell walls

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
44
Large polymers are formed from smaller subunits by which type of reaction?
A)oxidation
B)reduction
C)condensation
D)hydrolysis
E)decarboxylation
A)oxidation
B)reduction
C)condensation
D)hydrolysis
E)decarboxylation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
45
Humans do not contain the enzymes to break down ____.
A)cellulose
B)fructose
C)glycogen
D)starch
E)sucrose
A)cellulose
B)fructose
C)glycogen
D)starch
E)sucrose

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
46
What category of compounds helps our body fluids to stay within a consistent pH range?
A)solvents
B)buffers
C)solutes
D)acids
E)bases
A)solvents
B)buffers
C)solutes
D)acids
E)bases

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
47
Glucose monomers linked into a highly branched chain make up ____.
A)glycogen
B)cellulose
C)fructose
D)starch
E)sucrose
A)glycogen
B)cellulose
C)fructose
D)starch
E)sucrose

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which is an organic molecule?
A)carbon dioxide (CO2)
B)water (H2O)
C)methane (CH4)
D)hydrochloric acid (HCl)
E)oxygen (O2)
A)carbon dioxide (CO2)
B)water (H2O)
C)methane (CH4)
D)hydrochloric acid (HCl)
E)oxygen (O2)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
49
____ is one of the substances that maintains our blood pH between 7.35 and 7.45.
A)Water
B)Carbonic acid
C)Hydrochloric acid
D)Hydrogen peroxide
E)Sodium hydroxide
A)Water
B)Carbonic acid
C)Hydrochloric acid
D)Hydrogen peroxide
E)Sodium hydroxide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
50
In a cell membrane, the phospholipid heads are ____.
A)hydrophobic
B)nonpolar
C)dissolved in the cell's watery interior
D)sandwiched between the phospholipid tails
E)formed by fatty acids
A)hydrophobic
B)nonpolar
C)dissolved in the cell's watery interior
D)sandwiched between the phospholipid tails
E)formed by fatty acids

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
51
The breakdown of large molecules by enzymes and the addition of water is known as a ____ reaction.
A)oxidation
B)reduction
C)condensation
D)hydrolysis
E)decarboxylation
A)oxidation
B)reduction
C)condensation
D)hydrolysis
E)decarboxylation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
52
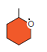
How many carbons are present in this figure?
A)0
B)4
C)5
D)6
E)7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
53
Plants store their excess carbohydrates in the form of ____.
A)cellulose
B)starch
C)glycogen
D)sucrose
E)galactose
A)cellulose
B)starch
C)glycogen
D)sucrose
E)galactose

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
54
Glycogen is a polysaccharide used for energy storage by ____.
A)plants
B)animals
C)protists
D)bacteria
E)archaea
A)plants
B)animals
C)protists
D)bacteria
E)archaea

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
55
____ is a monosaccharide.
A)Cellulose
B)Fructose
C)Glycogen
D)Starch
E)Sucrose
A)Cellulose
B)Fructose
C)Glycogen
D)Starch
E)Sucrose

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
56
Sucrose is composed of ____.
A)two molecules of fructose
B)two molecules of glucose
C)a molecule of fructose and a molecule of glucose
D)a molecule of fructose and a molecule of galactose
E)two molecules of galactose
A)two molecules of fructose
B)two molecules of glucose
C)a molecule of fructose and a molecule of glucose
D)a molecule of fructose and a molecule of galactose
E)two molecules of galactose

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
57
A uniform mixture is called a ____.
A)concentration
B)salt
C)solute
D)solution
E)solvent
A)concentration
B)salt
C)solute
D)solution
E)solvent

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
58
Which two atoms are found in all organic compounds?
A)carbon and hydrogen
B)carbon and oxygen
C)oxygen and hydrogen
D)carbon and phosphorous
E)oxygen and sulfur
A)carbon and hydrogen
B)carbon and oxygen
C)oxygen and hydrogen
D)carbon and phosphorous
E)oxygen and sulfur

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
59
A triglyceride molecule is made up of ____.
A)one glycerol and two fatty acids
B)two fatty acids and two glycerols
C)one fatty acid and three glycerols
D)one glycerol and three fatty acids
E)one glycerol and two fatty acids
A)one glycerol and two fatty acids
B)two fatty acids and two glycerols
C)one fatty acid and three glycerols
D)one glycerol and three fatty acids
E)one glycerol and two fatty acids

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
60
Which organic molecule is a carbohydrate monomer?
A)triglyceride
B)fatty acids
C)nucleotide
D)amino acid
E)monosaccharide
A)triglyceride
B)fatty acids
C)nucleotide
D)amino acid
E)monosaccharide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
61
Primary protein structure is dependent upon ____.
A)hydrophobic interactions
B)hydrogen bonds between two amino acids
C)covalent linkages between carbons and nitrogens of adjacent amino acids
D)covalent linkages between carbons and oxygens of adjacent amino acids
E)covalent linkages between the polypeptide and sugars or lipids
A)hydrophobic interactions
B)hydrogen bonds between two amino acids
C)covalent linkages between carbons and nitrogens of adjacent amino acids
D)covalent linkages between carbons and oxygens of adjacent amino acids
E)covalent linkages between the polypeptide and sugars or lipids

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
62
Unsaturated fats ____.
A)are solid at room temperature
B)have at least one double bond in their fatty acid tail
C)are saturated with hydrogen atoms
D)mainly come from animals
E)consist of straight chain fatty acids
A)are solid at room temperature
B)have at least one double bond in their fatty acid tail
C)are saturated with hydrogen atoms
D)mainly come from animals
E)consist of straight chain fatty acids

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
63
A nucleotide consists of ____.
A)a five carbon sugar, a nitrogenous acid, and a phosphate group
B)a six carbon sugar, a nitrogenous base, and a phosphate group
C)a five carbon sugar, a nitrogenous base, and a phosphate group
D)a six carbon sugar, a nitrogenous acid, and a phosphate group
E)a four carbon sugar, a nitrogenous acid, and a phosphate group
A)a five carbon sugar, a nitrogenous acid, and a phosphate group
B)a six carbon sugar, a nitrogenous base, and a phosphate group
C)a five carbon sugar, a nitrogenous base, and a phosphate group
D)a six carbon sugar, a nitrogenous acid, and a phosphate group
E)a four carbon sugar, a nitrogenous acid, and a phosphate group

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
64
Match between columns

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
65
Fats that contain ____ double bonds are liquids at room temperature, whereas fats that contain ____ double bonds are solids at room temperature.
A)trans ; cis
B)cis ; trans
C)hydrogenated; partially hydrogenated
D)partially hydrogenated; hydrogenated
E)unsaturated; saturated
A)trans ; cis
B)cis ; trans
C)hydrogenated; partially hydrogenated
D)partially hydrogenated; hydrogenated
E)unsaturated; saturated

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
66
Match between columns

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
67
Which food product would likely contain the largest amount of unsaturated fat?
A)butter
B)lard
C)salami
D)olives
E)cheese
A)butter
B)lard
C)salami
D)olives
E)cheese

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
68
Match between columns

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
69
Nucleotides are monomers of ____.
A)complex lipids
B)proteins
C)polysaccharides
D)nucleic acids
E)cellulose
A)complex lipids
B)proteins
C)polysaccharides
D)nucleic acids
E)cellulose

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
70
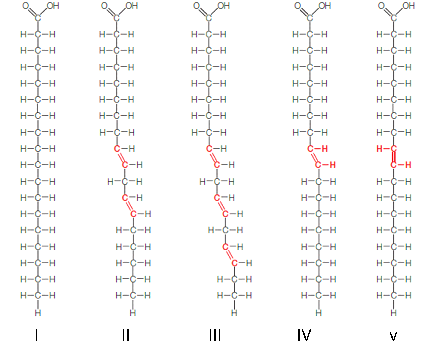
In the figure above, which fatty acids are most likely to be solid at room temperature?
A)I
B)II, III and IV
C)II, III, IV and V
D)I and IV
E)I and V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
71
A(n)____ is a protein monomer.
A)nucleotide
B)monosaccharide
C)simple sugar
D)amino acid
E)ribose
A)nucleotide
B)monosaccharide
C)simple sugar
D)amino acid
E)ribose

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
72
All steroids have ____.
A)the same number of double bonds
B)double bonds in the same positions
C)four carbon rings
D)the same functional groups
E)the same number and positions of double bonds
A)the same number of double bonds
B)double bonds in the same positions
C)four carbon rings
D)the same functional groups
E)the same number and positions of double bonds

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
73
Which type of bonds hold the two chains of DNA together in a DNA molecule?
A)hydrogen
B)polar covalent
C)nonpolar covalent
D)ionic
E)peptide
A)hydrogen
B)polar covalent
C)nonpolar covalent
D)ionic
E)peptide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
74
Protein misfolding causes ____.
A)Creutzfeldt-Jakob disease
B)arthritis
C)immunodepression
D)schizophrenia
E)tuberculosis
A)Creutzfeldt-Jakob disease
B)arthritis
C)immunodepression
D)schizophrenia
E)tuberculosis

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
75
Match between columns

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
76
In a polymer of nucleotides, how does one nucleotide attach to another?
A)The base of one nucleotide is attached to the base of the next.
B)The base of one nucleotide it attached to the sugar of the next.
C)The sugar of one nucleotide is attached to the sugar of the next.
D)The phosphate group of one nucleotide is attached to the base of the next.
E)The phosphate group of one nucleotide is attached to the sugar of the next.
A)The base of one nucleotide is attached to the base of the next.
B)The base of one nucleotide it attached to the sugar of the next.
C)The sugar of one nucleotide is attached to the sugar of the next.
D)The phosphate group of one nucleotide is attached to the base of the next.
E)The phosphate group of one nucleotide is attached to the sugar of the next.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
77
When a protein denatures, which type of bonding is affected?
A)covalent
B)peptide
C)ionic
D)hydrogen
E)metallic
A)covalent
B)peptide
C)ionic
D)hydrogen
E)metallic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
78
Two amino acids are bonded together to form a dipeptide by which type of reaction?
A)condensation
B)oxidation reduction
C)hydrolysis
D)decomposition
E)acid-base
A)condensation
B)oxidation reduction
C)hydrolysis
D)decomposition
E)acid-base

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
79
Which type of bond exists between two amino acids in a protein?
A)peptide
B)ionic
C)hydrogen
D)amino
E)sulfhydryl
A)peptide
B)ionic
C)hydrogen
D)amino
E)sulfhydryl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck
80
A protein that is linked to a carbohydrate is known as a ____.
A)glycoprotein
B)lipoprotein
C)fibrous proteins
D)denatured proteins
E)prions
A)glycoprotein
B)lipoprotein
C)fibrous proteins
D)denatured proteins
E)prions

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 100 في هذه المجموعة.
فتح الحزمة
k this deck








