Deck 13: Electrochemistry
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/45
العب
ملء الشاشة (f)
Deck 13: Electrochemistry
1
Electrolysis can be used to electroplate metals onto surfaces.
True
2
A positive voltage in the standard reduction potential means that the half-reaction proceeds as a reduction when connected to the standard hydrogen electrode (SHE)because the SHE is serving as the anode .
True
3
Galvanic corrosion occurs only when two different metals contact each other in the presence of an appropriate electrolyte.
True
4
If a chemical species gains electrons in a redox reaction, the species is said to have undergone oxidation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which of the following species undergoes oxidation in the formation of iron (III)oxide?
A)FeO
B)Fe2O3
C)Fe
D)O2
A)FeO
B)Fe2O3
C)Fe
D)O2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
6
Consider the following reaction: Cu → Cu 2+ + 2e -
Which of the following best describes the reaction?
A)Oxidation half-reaction
B)Reduction half-reaction
C)Hydrolysis
D)Excitation
Which of the following best describes the reaction?
A)Oxidation half-reaction
B)Reduction half-reaction
C)Hydrolysis
D)Excitation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
7
If an electrochemical half-reaction includes the production or consumption of a gas, the standard state is a pressure of 1 atm.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
8
In the context of electrolysis, if the electrodes are chemically inert materials that simply provide a path for electrons, the process is called active electrolysis.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
9
All dry cell batteries are rechargeable.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
10
Consider the following reaction: Na++ e − → Na
Which of the following best describes the reaction?
A)Oxidation half-reaction
B)Reduction half-reaction
C)Ionization
D)Dissociation
Which of the following best describes the reaction?
A)Oxidation half-reaction
B)Reduction half-reaction
C)Ionization
D)Dissociation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
11
The species undergoing reduction is referred to as an oxidizing agent.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
12
A galvanic cell is an electrochemical cell in which a spontaneous chemical reaction can be used to generate an electrical current.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
13
Reduction occurs at the anode of a galvanic cell.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of the following species undergoes reduction in the formation of iron (III)oxide?
A)FeO
B)Fe2O3
C)Fe
D)O2
A)FeO
B)Fe2O3
C)Fe
D)O2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
15
The rusting of automobile bodies is an example of uniform corrosion.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
16
Secondary cells are rechargeable.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
17
The standard hydrogen electrode (SHE)involves a platinum wire or foil that is the conducting source of electrons; hydrogen gas is bubbled over the electrode at a pressure of 1 atm, and the electrolyte solution is 1 M HCl(aq).

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
18
Electrolysis involves using an external electric current to drive an electrochemical reaction in a nonspontaneous direction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
19
The Nernst equation describes the pH required for a spontaneous electrolytic reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
20
Identify a true statement about a redox reaction.
A)It involves a transfer of protons from one species to another.
B)It creates electrons.
C)It involves a transfer of electrons between two species.
D)It is always nonspontaneous.
A)It involves a transfer of protons from one species to another.
B)It creates electrons.
C)It involves a transfer of electrons between two species.
D)It is always nonspontaneous.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
21
The rusting of the sheet metal of a car is an example of:
A)uniform corrosion.
B)galvanic corrosion.
C)electrolysis.
D)galvanized steel.
A)uniform corrosion.
B)galvanic corrosion.
C)electrolysis.
D)galvanized steel.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
22
By convention, the standard hydrogen electrode (SHE)is assigned a voltage of _____ V .
A)− 1.000
B)1.000
C)0.150
D)0.000
A)− 1.000
B)1.000
C)0.150
D)0.000

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
23
What is the cell potential (E0) f or a galvanic cell formed from the following two half-reactions? Assume that the cell temperature is 38°C and the operating pressure is 0.04 atm. 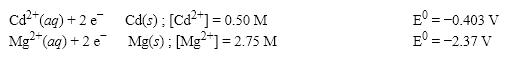
A)1.90 V
B)1.95 V
C)1.99 V
D)2.20 V
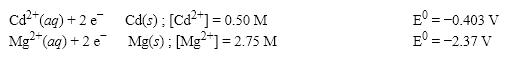
A)1.90 V
B)1.95 V
C)1.99 V
D)2.20 V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
24
What is the cell potential (E 0 )for a galvanic cell formed from the following two half- reactions ? 
A)−1 .43 V
B)+1.43 V
C)−0 .93 V
D)+0.93 V

A)−1 .43 V
B)+1.43 V
C)−0 .93 V
D)+0.93 V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
25
A salt bridge between half-reactions maintains the electrical balance of a galvanic cell. This bridge is filled with:
A)a strong electrolyte.
B)inert carbon.
C)a weak electrolyte.
D)inert helium.
A)a strong electrolyte.
B)inert carbon.
C)a weak electrolyte.
D)inert helium.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
26
In the following galvanic cell: 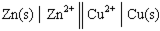 , the right-hand side of this notation represents the:
, the right-hand side of this notation represents the:
A)spontaneous half of the reaction.
B)oxidation half-reaction.
C)anode of the cell.
D)reduction half-reaction.
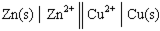 , the right-hand side of this notation represents the:
, the right-hand side of this notation represents the:A)spontaneous half of the reaction.
B)oxidation half-reaction.
C)anode of the cell.
D)reduction half-reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
27
Suppose that you have an iron rod that requires copper coating. You have calculated that you need to deposit 4.00 g of copper to achieve an adequate coating. If your electrolysis cell (using Cu 2+)runs at 1.5 A, how long must you operate the cell to obtain the desired coating?
A)1 hour and 12 minutes
B)2 hours and 15 minutes
C)3 hours and 22 minutes
D)4 hours and 35 minutes
A)1 hour and 12 minutes
B)2 hours and 15 minutes
C)3 hours and 22 minutes
D)4 hours and 35 minutes

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
28
What is the cell potential (E 0 )for a galvanic cell formed from the following two half- reactions? 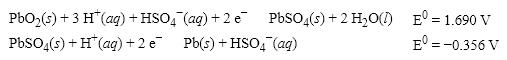
A)+1.334 V
B)− 2.046 V
C)+2.046 V
D)+2.758 V
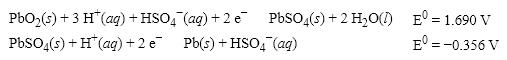
A)+1.334 V
B)− 2.046 V
C)+2.046 V
D)+2.758 V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
29
How many grams of silver are deposited at a platinum cathode in the electrolysis of AgNO 3 (aq)by 5.30 A of electric current in 4.0 hours?
A)85.3 g
B)42.6 g
C)121 g
D)188 g
A)85.3 g
B)42.6 g
C)121 g
D)188 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
30
In the context of cell notations in galvanic cells, a phase boundary is denoted by _____.
A)|
B)||
C)-
D)=
A)|
B)||
C)-
D)=

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
31
In a galvanic cell, oxidation occurs at _____.
A)the cathode
B)a salt bridge
C)the anode
D)the external circuit
A)the cathode
B)a salt bridge
C)the anode
D)the external circuit

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
32
The most prevalent type of primary battery in use today is _____.
A)the rechargeable batter y
B)the lead-acid storage battery
C)the alkaline batter y
D)the nickel-metal-hydride battery
A)the rechargeable batter y
B)the lead-acid storage battery
C)the alkaline batter y
D)the nickel-metal-hydride battery

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
33
Identify the balanced chemical equation for the following cell: 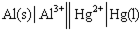
A)Al( s )+ Hg2+( aq )→ Al3+( aq )+ Hg( l )
B)2 Al( s )+ Hg2+( aq )→ 2 Al3+( aq )+ Hg( l )
C)Al( s )+ 3 Hg2+( aq )→ Al3+( aq )+ 3 Hg( l )
D)2 Al( s )+ 3 Hg2+( aq )→ 2 Al3+( aq )+ 3 Hg( l )
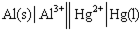
A)Al( s )+ Hg2+( aq )→ Al3+( aq )+ Hg( l )
B)2 Al( s )+ Hg2+( aq )→ 2 Al3+( aq )+ Hg( l )
C)Al( s )+ 3 Hg2+( aq )→ Al3+( aq )+ 3 Hg( l )
D)2 Al( s )+ 3 Hg2+( aq )→ 2 Al3+( aq )+ 3 Hg( l )

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
34
In a galvanic cell, reduction occurs at _____.
A)the cathode
B)a salt bridge
C)the anode
D)the external circuit
A)the cathode
B)a salt bridge
C)the anode
D)the external circuit

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
35
Which of the following cells is most likely to be an example of a galvanic cell?
A)Zn(s)| Zn 2+ || Cu 2+ | Cu(s)
B)Cu(s)| Cu 2+ || Fe 2+ | Fe(s)
C)Au(s)| Au + || Ag + | Ag(s)
D)Sn(s)| Sn 2+ || Fe 2+ | Fe(s)
A)Zn(s)| Zn 2+ || Cu 2+ | Cu(s)
B)Cu(s)| Cu 2+ || Fe 2+ | Fe(s)
C)Au(s)| Au + || Ag + | Ag(s)
D)Sn(s)| Sn 2+ || Fe 2+ | Fe(s)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
36
Balance the following electrochemical reaction in acid: MnO 4 − ( aq )+ Zr( s )↔ Mn 2+( aq )+ Zr 2+( aq )
A)2 MnO4 − ( aq )+ 16 H+( aq )+ 5 Zr( s )→ 2 Mn2+( aq )+ 5 Zr2+( aq )+ 8 H2O( l )
B)2 MnO4 − ( aq )+ 5 Zr( s ) → 2 Mn2+( aq )+ 5 Zr2+( aq )
C)MnO4 − ( aq )+ Zr( s )→ Mn2+( aq )+ Zr2+( aq )+ 5 e −
D)2 MnO4 − ( aq )+ 8 H+( aq )+ 5 Zr( s )→ 2 Mn2+( aq )+ 5 Zr2+( aq )+ 8 H2O( l )
A)2 MnO4 − ( aq )+ 16 H+( aq )+ 5 Zr( s )→ 2 Mn2+( aq )+ 5 Zr2+( aq )+ 8 H2O( l )
B)2 MnO4 − ( aq )+ 5 Zr( s ) → 2 Mn2+( aq )+ 5 Zr2+( aq )
C)MnO4 − ( aq )+ Zr( s )→ Mn2+( aq )+ Zr2+( aq )+ 5 e −
D)2 MnO4 − ( aq )+ 8 H+( aq )+ 5 Zr( s )→ 2 Mn2+( aq )+ 5 Zr2+( aq )+ 8 H2O( l )

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
37
The most common fuel cells are based on the reaction of _____.
A)nickel and oxygen to produce nickel oxide
B)hydrogen and oxygen to produce water
C)lead and sulfuric acid to produce lead sulfate
D)zinc and air to produce zinc oxide
A)nickel and oxygen to produce nickel oxide
B)hydrogen and oxygen to produce water
C)lead and sulfuric acid to produce lead sulfate
D)zinc and air to produce zinc oxide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
38
What is the cell potential (E 0 )for a galvanic cell formed from the following two half- reactions? 
A)− 2.31 V
B)+4.33 V
C)− 1.32 V
D)+2.00 V

A)− 2.31 V
B)+4.33 V
C)− 1.32 V
D)+2.00 V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
39
Single use, non-rechargeable batteries are referred to as _____.
A)primary cells
B)secondary cells
C)tertiary cells
D)electrolytic cells
A)primary cells
B)secondary cells
C)tertiary cells
D)electrolytic cells

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
40
What is the cell potential (E0)for a galvanic cell formed from the following two half-reactions? Assume that the cell temperature is 45°C and the operating pressure is 0.06 atm. 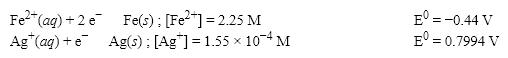
A)1.03 V
B)1.12 V
C)1.24 V
D)0.36 V
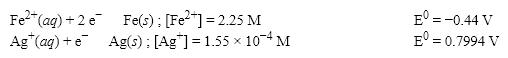
A)1.03 V
B)1.12 V
C)1.24 V
D)0.36 V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
41
In the context of the cell notation of galvanic cells, the anode is always written on the right and the cathode on the left.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
42
Which of the following represents the Nernst equation?
A)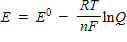
B)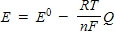
C)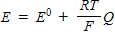
D)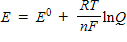
A)
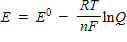
B)
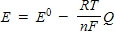
C)
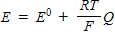
D)
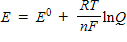

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
43
An electrolysis cell that deposits copper (from Cu 2+ ions)operates for 30 minutes at an electric current of 3.20 A. What mass of copper is deposited?
A)1.9 g
B)2.3 g
C)3.5 g
D)4.2 g
A)1.9 g
B)2.3 g
C)3.5 g
D)4.2 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
44
In general, a secondary cell has a much shorter life cycle than a primary cell of the same capacity.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck
45
In any galvanic cell, the half-reaction with the more positive reduction potential will be the cathode.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 45 في هذه المجموعة.
فتح الحزمة
k this deck








