Deck 14: Nuclear Chemistry Available Through Custom Publishing
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/40
العب
ملء الشاشة (f)
Deck 14: Nuclear Chemistry Available Through Custom Publishing
1
In the context of radioactive decay, _____ is the emission of a high-energy photon.
A)alpha decay
B)beta decay
C)gamma decay
D)electron capture
A)alpha decay
B)beta decay
C)gamma decay
D)electron capture
gamma decay
2
Radioactive decay is a nonspontaneous process.
False
3
The energy of the sun originates in a fission reaction in which a helium nucleus splits into four hydrogen nuclei.
False
4
Which of the following statements is true of an alpha particle?
A)It is less massive than a beta particle.
B)It is less massive than a neutron.
C)It is a hydrogen nucleus.
D)It is positively charged.
A)It is less massive than a beta particle.
B)It is less massive than a neutron.
C)It is a hydrogen nucleus.
D)It is positively charged.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
5
In the context of radioactive decay, the electrons emitted from the nucleus are usually called _____.
A)alpha particles
B)beta particles
C)neutrinos
D)antineutrinos
A)alpha particles
B)beta particles
C)neutrinos
D)antineutrinos

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
6
When a nucleus undergoes alpha decay, an electron is ejected from the nucleus.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following statements is true of the beta decay of a nucleus?
A)It results in an increase in the mass number.
B)It results in a decrease in the atomic number.
C)It results in the emission of an antineutrino.
D)It results in the emission of a high-energy photon.
A)It results in an increase in the mass number.
B)It results in a decrease in the atomic number.
C)It results in the emission of an antineutrino.
D)It results in the emission of a high-energy photon.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
8
In the context of the penetrating power of various forms of ionizing radiation, alpha particles can pass completely through the human body and can damage internal organs.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
9
A positron is a negatively charged proton.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
10
_____ are the most prevalent elements in cosmic rays.
A)Hydrogen and helium
B)Carbon and oxygen
C)Nitrogen and neon
D)Magnesium and silicon
A)Hydrogen and helium
B)Carbon and oxygen
C)Nitrogen and neon
D)Magnesium and silicon

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
11
Cosmic rays are photons travelling at high speeds.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
12
When a nucleus undergoes alpha decay, _____. its atomic number increases by 2
A)its mass number decreases by 4
B)its atomic number increases by 2
C)it emits a positron
D)it emits an electron
A)its mass number decreases by 4
B)its atomic number increases by 2
C)it emits a positron
D)it emits an electron

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
13
Fusion does not produce the high-level radioactive waste that fission generates.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
14
When a nucleus undergoes beta decay, _____.
A)its mass number decreases by 2
B)its atomic number increases by 1
C)it emits a proton
D)it emits a high-energy photon
A)its mass number decreases by 2
B)its atomic number increases by 1
C)it emits a proton
D)it emits a high-energy photon

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
15
Which of the following statements is true of an antineutrino emitted in beta decay?
A)Its mass is equivalent to the mass of a neutron.
B)It has no electric charge.
C)Its mass is equivalent to the mass of a proton.
D)It has a negative charge.
A)Its mass is equivalent to the mass of a neutron.
B)It has no electric charge.
C)Its mass is equivalent to the mass of a proton.
D)It has a negative charge.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
16
Commercial nuclear reactors rely on controlled nuclear fusion reactions.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which of the following statements is true of the isotopes of carbon?
A)Naturally occurring terrestrial carbon is 98.9% carbon-14.
B)Carbon-13 is unstable, and it undergoes spontaneous radioactive decay.
C)Carbon-14 is unstable, and it undergoes spontaneous radioactive decay.
D)Naturally occurring terrestrial carbon is 98.9% carbon-13.
A)Naturally occurring terrestrial carbon is 98.9% carbon-14.
B)Carbon-13 is unstable, and it undergoes spontaneous radioactive decay.
C)Carbon-14 is unstable, and it undergoes spontaneous radioactive decay.
D)Naturally occurring terrestrial carbon is 98.9% carbon-13.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
18
In the context of measuring the dose of radiation, the exposure measures the amount of radiation actually absorbed by a particular material.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
19
In the context of energetics of nuclear reactions, the greater the nuclear binding energy, the less stable the nucleus.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which of the following completes the nuclear decay equation given below? 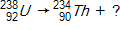
A)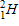
B)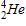
C)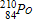
D)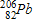
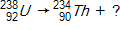
A)
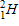
B)
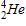
C)
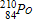
D)
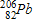

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
21
The radioactive decay of uranium-238to form a stable lead-206product involves _____ beta decays.
A)two
B)four
C)six
D)eight
A)two
B)four
C)six
D)eight

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which of the following statements is true of nuclear fusion?
A)It generates high-level radioactive waste similar to that of nuclear fission.
B)It involves splitting a heavier nucleus into smaller nuclei.
C)It yields more energy per nucleus than nuclear fission.
D)Its primary product is deuterium.
A)It generates high-level radioactive waste similar to that of nuclear fission.
B)It involves splitting a heavier nucleus into smaller nuclei.
C)It yields more energy per nucleus than nuclear fission.
D)Its primary product is deuterium.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
23
invariant mass In the context of fission reactions, the amount of material required for a sustainable chain reaction is called the _____.
A)inertial mass
B)relativistic mass
C)critical mass
D)invariant mass
A)inertial mass
B)relativistic mass
C)critical mass
D)invariant mass

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
24
The radioactive decay of uranium-238to form a stable lead-206product involves _____ alpha decays.
A)two
B)four
C)six
D)eight
A)two
B)four
C)six
D)eight

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which of the following statements is true of gamma decay?
A)It leads to the emission of positrons.
B)It is the process by which a nucleus captures an electron from the first shell in an atom.
C)It accompanies the alpha decay of most nuclei.
D)It changes neither the mass number nor the atomic number of a nuclide.
A)It leads to the emission of positrons.
B)It is the process by which a nucleus captures an electron from the first shell in an atom.
C)It accompanies the alpha decay of most nuclei.
D)It changes neither the mass number nor the atomic number of a nuclide.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
26
In electron capture, a nucleus captures an electron from the _____ shell in an atom.
A)first
B)second
C)third
D)fourth
A)first
B)second
C)third
D)fourth

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
27
The decay series starting with uranium-238involves a series of alpha and beta emissions before it eventually produces a stable _____ product.
A)thallium-204
B)lead-206
C)polonium-210
D)thorium-234
A)thallium-204
B)lead-206
C)polonium-210
D)thorium-234

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
28
Which of the following statements is true of the nuclear binding energy of a nucleus?
A)It is directly proportional to the stability of a nucleus.
B)It is inversely proportional to the stability of a nucleus.
C)It is independent of the mass of a nucleus.
D)It is independent of the mass of an atom.
A)It is directly proportional to the stability of a nucleus.
B)It is inversely proportional to the stability of a nucleus.
C)It is independent of the mass of a nucleus.
D)It is independent of the mass of an atom.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
29
In the context of the ionizing and penetrating power of radiation, _____ penetrate into the skin but generally cannot reach internal organs.
A)gamma rays
B)radio waves
C)alpha particles
D)beta particles
A)gamma rays
B)radio waves
C)alpha particles
D)beta particles

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
30
Calculate the energy released by a nucleus of uranium-235if it splits into an antimony-132nucleus and a niobium-101nucleus according to the equation below. 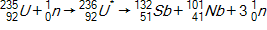 Consider the atomic masses of
Consider the atomic masses of 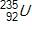 ,
, 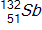 ,
, 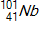 , and
, and 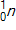 to be 235.044 u, 131.915 u, 100.915 u, and 1.00866 u, respectively.
to be 235.044 u, 131.915 u, 100.915 u, and 1.00866 u, respectively.
A)-1.53 × 10- 11 J
B)-2.94 × 10-11J
C)1.53 × 10-11J
D)2.94 × 10-11J
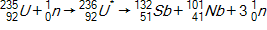 Consider the atomic masses of
Consider the atomic masses of 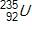 ,
, 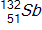 ,
, 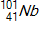 , and
, and 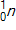 to be 235.044 u, 131.915 u, 100.915 u, and 1.00866 u, respectively.
to be 235.044 u, 131.915 u, 100.915 u, and 1.00866 u, respectively.A)-1.53 × 10- 11 J
B)-2.94 × 10-11J
C)1.53 × 10-11J
D)2.94 × 10-11J

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
31
The SI unit of nuclear activity is the _____.
A)curie
B)becquerel
C)candela
D)lux
A)curie
B)becquerel
C)candela
D)lux

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
32
In the context of the ionizing and penetrating power of radiation, _____ can pass completely through a human body and damage internal organs.
A)gamma rays
B)radio waves
C)alpha particles
D)beta particles
A)gamma rays
B)radio waves
C)alpha particles
D)beta particles

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
33
The half-life of uranium-238is 4.5 billion years. What is the decay constant for uranium-238?
A)1.72 × 10-7yr-1
B)1.65 × 10-8yr-1
C)1.44 × 10-9yr-1
D)1.54 × 10-10yr-1
A)1.72 × 10-7yr-1
B)1.65 × 10-8yr-1
C)1.44 × 10-9yr-1
D)1.54 × 10-10yr-1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
34
In the context of the nuclear binding energy, which of the following is the most stable nuclei?
A)Chromium- 52
B)Iron-56
C)Krypton-84
D)Tin-119
A)Chromium- 52
B)Iron-56
C)Krypton-84
D)Tin-119

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
35
A tiny sample of a fabric is burned to CO2,and the 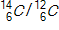 ratio is 0.350 times the ratio in today's atmosphere. How old is the fabric if the half-life of carbon-14is 5730 years?
ratio is 0.350 times the ratio in today's atmosphere. How old is the fabric if the half-life of carbon-14is 5730 years?
A)8676 years
B)9252 years
C)10,320 years
D)11,425 years
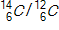 ratio is 0.350 times the ratio in today's atmosphere. How old is the fabric if the half-life of carbon-14is 5730 years?
ratio is 0.350 times the ratio in today's atmosphere. How old is the fabric if the half-life of carbon-14is 5730 years?A)8676 years
B)9252 years
C)10,320 years
D)11,425 years

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
36
Electron capture leads to the:
A)emission of an electron from the second shell in an atom.
B)conversion of a proton in the nucleus to a neutron.
C)emission of a beta particle.
D)conversion of a neutron in the nucleus to a proton.
A)emission of an electron from the second shell in an atom.
B)conversion of a proton in the nucleus to a neutron.
C)emission of a beta particle.
D)conversion of a neutron in the nucleus to a proton.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
37
Which of the following statements is true of nuclear stability?
A)The nuclear binding energy is directly proportional to nuclear stability.
B)The nuclear stability increases with an increase in mass number (Z)till Z = 93.
C)The nuclear stability decreases with an increase in mass number (Z)till Z = 83.
D)The nuclear binding energy is inversely proportional to nuclear stability.
A)The nuclear binding energy is directly proportional to nuclear stability.
B)The nuclear stability increases with an increase in mass number (Z)till Z = 93.
C)The nuclear stability decreases with an increase in mass number (Z)till Z = 83.
D)The nuclear binding energy is inversely proportional to nuclear stability.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
38
In the context of transmutation, 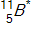 in the equation below is called a(n)_____.
in the equation below is called a(n)_____. 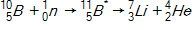
A)alpha particle
B)isotope
C)compound nucleus
D)beta particle
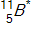 in the equation below is called a(n)_____.
in the equation below is called a(n)_____. 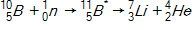
A)alpha particle
B)isotope
C)compound nucleus
D)beta particle

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
39
In the context of nuclear stability, when the atomic number _____ no number of neutrons can stabilize the nucleus and its large collection of positive charges.
A)less than 33
B)more than 33 but less than 52
C)more than 52 but less than 83
D)more than 83
A)less than 33
B)more than 33 but less than 52
C)more than 52 but less than 83
D)more than 83

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck
40
A(n)_____ is a positively charged electron.
A)neutrino
B)photon
C)positron
D)antineutrino
A)neutrino
B)photon
C)positron
D)antineutrino

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 40 في هذه المجموعة.
فتح الحزمة
k this deck








