Deck 10: Atomic Physics
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/134
العب
ملء الشاشة (f)
Deck 10: Atomic Physics
1
In an energy level transition from the n = 2 state to the n =1 state for a hydrogen atom a photon will be emitted.
True
2
The energy of an oscillating atom in a blackbody can be any value within a certain limited range.
False
3
The peak of the radiation curve of a blackbody moves upward toward higher intensity as its temperature increases.
True
4
The peak of the radiation curve of a blackbody moves toward larger wavelength as its temperature increases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
5
An excited atom can lose energy by emitting an electron.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
6
In a blackbody, the energy of the oscillating atoms is quantized.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
7
The element helium was discovered by studying the Sun's spectrum.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
8
An x-ray can be emitted when an electron undergoes an energy level transition.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
9
A 100 eV electron and a 100 eV proton have equal de Broglie wavelengths.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
10
In the Bohr model of the hydrogen atom both the energy and orbital angular momentum of the electron are quantized.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
11
Electrons in atoms move about the nucleus in well-defined circular orbits.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
12
The uncertainty principle states that a particle cannot be precisely localized.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
13
The early Solvay Conferences had a great effect upon the development of quantum mechanics.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
14
Perfect absorbers of electromagnetic radiation are also perfect emitters.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
15
There is a direct correlation between the absorption and emission spectra of a particular gas.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
16
An atom can be excited into a higher energy state by absorbing a photon.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
17
A photon is quantum of electromagnetic radiation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
18
The highest energy level in an atom corresponds to n = 1.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
19
The photoelectric effect occurs when electrons are emitted when light strikes the surface of a metal.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
20
The ground state is the lowest energy level in an atom.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
21
When the speed of an electron is increased the de Broglie wavelength is decreased.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
22
Two different gases may have the same emission line spectrum.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
23
Characteristic peaks occur in an x-ray spectrum of an atom because electrons in the atom make transitions to lower energy levels after being bombarded with high energy electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
24
The energy level diagram for hydrogen 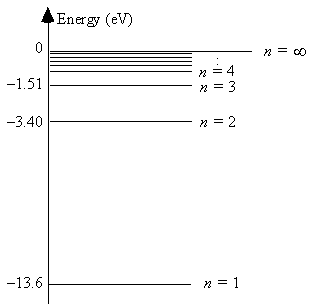
The energy of a photon emitted in a transition from the n = 3 state to the n = 2 state is 1.9 eV.
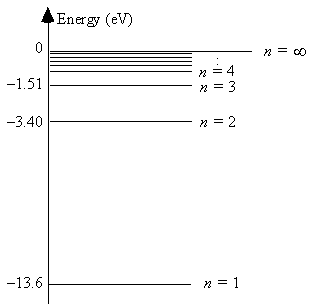
The energy of a photon emitted in a transition from the n = 3 state to the n = 2 state is 1.9 eV.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
25
If an electron acquires more than the ionization energy, the electron will return to the ground state.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
26
The energy level diagram for hydrogen 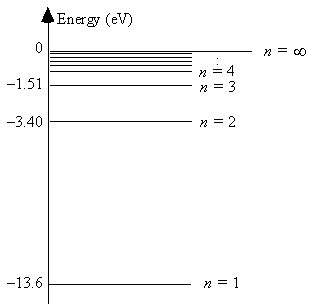
The ionization energy of hydrogen is 13.6 eV.
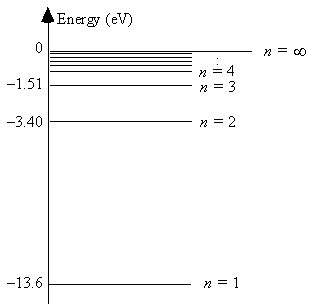
The ionization energy of hydrogen is 13.6 eV.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
27
When the frequency of the light is increased in a photoelectric effect experiment, the energy of the emitted electrons increases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
28
The energy level diagram for hydrogen 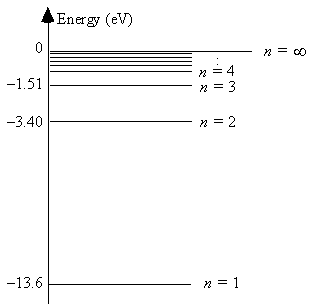
The smallest energy of an absorbed photon that will raise a hydrogen atom from its ground state to its first excited state is 13.6 eV.
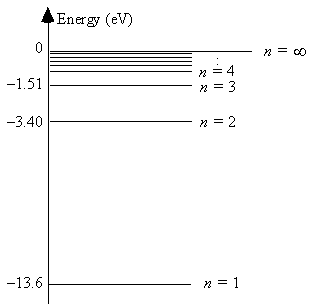
The smallest energy of an absorbed photon that will raise a hydrogen atom from its ground state to its first excited state is 13.6 eV.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
29
A laser emits monochromatic and coherent light.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
30
At any instant in time, it is possible to specify simultaneously the position and the momentum of a particle to arbitrarily high precision.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
31
The aurora borealis is an example of atoms and molecules in the atmosphere absorbing photons and exciting the atoms and molecules to emit spectra.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
32
The chemical composition of stars can be determined from lines in their spectra.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
33
Photocopiers make use of the photoelectric effect.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
34
The characteristic peaks in an x-ray spectrum of an atom occur because of rapid deceleration of the bombarding electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
35
A laser can be used for very accurate measurements of distances between objects.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
36
Light being amplified by stimulated emission of radiation is the principle of the photoelectric effect.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
37
The energy level diagram for hydrogen 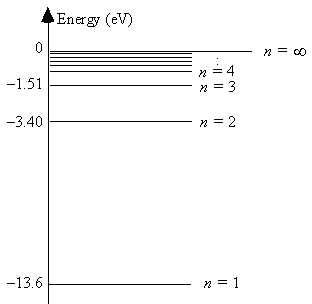
The energy of the most energetically possible photon emitted is 10.2 eV.
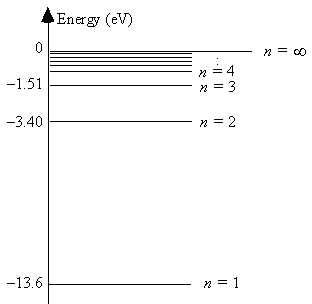
The energy of the most energetically possible photon emitted is 10.2 eV.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
38
As the intensity of light is increased in a photoelectric effect experiment, the energy of the emitted electrons increases.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
39
The fact that two electrons cannot occupy the same quantum state at the same time is the Heisenberg exclusion principle.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
40
UPC price scanners at supermarket checkouts use laser holography to read the bar codes because the packages are three dimensional.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
41
What is the symbol used for Planck's constant?
A) p
B)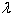
C) h
D) none of the above
A) p
B)
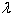
C) h
D) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
42
Electrons emitted when light strikes the surface of a metal is
A) photon emission.
B) photon absorption.
C) the photoelectric effect.
D) a laser.
E) an emission spectrum.
A) photon emission.
B) photon absorption.
C) the photoelectric effect.
D) a laser.
E) an emission spectrum.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
43
An atom can be excited into a higher energy state by
A) emitting a photon.
B) absorbing a photon.
C) undergoing the photoelectric effect.
D) decreasing its de Broglie wavelength.
E) the uncertainty principle.
A) emitting a photon.
B) absorbing a photon.
C) undergoing the photoelectric effect.
D) decreasing its de Broglie wavelength.
E) the uncertainty principle.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
44
In the Bohr model of the hydrogen atom
A) energy is quantized.
B) the electron's angular momentum is quantized.
C) the emission spectrum is quantized.
D) the absorption spectrum is quantized.
E) all of the above
A) energy is quantized.
B) the electron's angular momentum is quantized.
C) the emission spectrum is quantized.
D) the absorption spectrum is quantized.
E) all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
45
The fact that a particle cannot be precisely localized is the
A) photoelectric effect.
B) uncertainty principle.
C) principle of a hologram.
D) principle of a laser.
E) reason why photons are emitted.
A) photoelectric effect.
B) uncertainty principle.
C) principle of a hologram.
D) principle of a laser.
E) reason why photons are emitted.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
46
If the speed of an electron increases, its de Broglie wavelength
A) increases.
B) decreases.
C) stays the same.
D) may increase or decrease.
A) increases.
B) decreases.
C) stays the same.
D) may increase or decrease.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
47
Which of these is not involved in the operation of lasers?
A) stimulated emission
B) population inversion
C) metastable state
D) blackbody radiation
A) stimulated emission
B) population inversion
C) metastable state
D) blackbody radiation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
48
The highest energy level in an atom is its
A) ionization level.
B) emission level.
C) absorption level.
D) photon level.
E) ground state.
A) ionization level.
B) emission level.
C) absorption level.
D) photon level.
E) ground state.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
49
What is "excluded" by the Pauli exclusion principle?
A) certain values of angular momentum
B) precise values of both position and momentum
C) electrons in the same quantum state
D) none of the above
A) certain values of angular momentum
B) precise values of both position and momentum
C) electrons in the same quantum state
D) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
50
What quantities are plotted on a blackbody radiation curve?
A) total radiated energy vs. temperature
B) radiation intensity vs. wavelength
C) wavelength of peak emission vs. temperature
D) none of the above
A) total radiated energy vs. temperature
B) radiation intensity vs. wavelength
C) wavelength of peak emission vs. temperature
D) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
51
The quantity that is quantized in the oscillating atoms of a blackbody is
A) angular momentum.
B) energy.
C) the emission spectrum.
D) the absorption spectrum.
E) the wave function.
A) angular momentum.
B) energy.
C) the emission spectrum.
D) the absorption spectrum.
E) the wave function.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
52
An excited atom can lose energy by
A) emitting a photon.
B) absorbing a photon.
C) undergoing the photoelectric effect.
D) increasing its de Broglie wavelength.
E) the uncertainty principle.
A) emitting a photon.
B) absorbing a photon.
C) undergoing the photoelectric effect.
D) increasing its de Broglie wavelength.
E) the uncertainty principle.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
53
The lowest energy level in an atom is its
A) ionization level.
B) emission level.
C) absorption level.
D) photon level.
E) ground state.
A) ionization level.
B) emission level.
C) absorption level.
D) photon level.
E) ground state.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
54
A three-dimensional image made using a laser is a(n)
A) emission spectrum.
B) absorption spectrum.
C) wave function.
D) hologram.
E) photoelectric effect.
A) emission spectrum.
B) absorption spectrum.
C) wave function.
D) hologram.
E) photoelectric effect.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
55
What does it mean for something to be "quantized?"
A) It can have only certain discrete values.
B) It can have only integer values.
C) It can have values only between certain limits.
D) none of the above
A) It can have only certain discrete values.
B) It can have only integer values.
C) It can have values only between certain limits.
D) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
56
What is coherent light?
A) light of a single wavelength
B) light that is all in phase
C) light that is polarized in one direction
D) none of the above
A) light of a single wavelength
B) light that is all in phase
C) light that is polarized in one direction
D) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
57
A quantum of electromagnetic radiation is a
A) wave function.
B) photon.
C) de Broglie wave.
D) laser.
E) hologram.
A) wave function.
B) photon.
C) de Broglie wave.
D) laser.
E) hologram.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
58
The person who first explained the blackbody spectrum was
A) Max Planck.
B) Albert Einstein.
C) Neils Bohr.
D) Erwin Schrödinger.
E) Werner Heisenberg.
A) Max Planck.
B) Albert Einstein.
C) Neils Bohr.
D) Erwin Schrödinger.
E) Werner Heisenberg.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
59
The energy associated with a photon of blue light is ________________ the energy associated with a photon of red light.
A) greater than
B) less than
C) equal to
D) unrelated to
A) greater than
B) less than
C) equal to
D) unrelated to

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
60
Electron diffraction shows
A) that electrons behave like waves.
B) the photoelectric effect.
C) characteristic x-ray spectra.
D) that electrons in atoms have only certain allowed energies.
A) that electrons behave like waves.
B) the photoelectric effect.
C) characteristic x-ray spectra.
D) that electrons in atoms have only certain allowed energies.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
61
The continuous part of an x-ray spectrum of an atom occurs because
A) of photon absorption.
B) of the photoelectric effect.
C) electrons in the atom make transitions to lower energy levels.
D) of the uncertainty principle.
E) of rapid deceleration of the bombarding electrons.
A) of photon absorption.
B) of the photoelectric effect.
C) electrons in the atom make transitions to lower energy levels.
D) of the uncertainty principle.
E) of rapid deceleration of the bombarding electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
62
The person who expounded the uncertainty principle was
A) Max Planck.
B) Albert Einstein.
C) Neils Bohr.
D) Erwin Schrödinger.
E) Werner Heisenberg.
A) Max Planck.
B) Albert Einstein.
C) Neils Bohr.
D) Erwin Schrödinger.
E) Werner Heisenberg.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
63
The aurora borealis (northern lights) are caused by
A) sunlight reflecting from polar ice.
B) emissions from atoms and molecules in the upper atmosphere excited by electrons from the Sun.
C) thunderstorms.
D) an unknown process.
A) sunlight reflecting from polar ice.
B) emissions from atoms and molecules in the upper atmosphere excited by electrons from the Sun.
C) thunderstorms.
D) an unknown process.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
64
The energy level diagram for hydrogen 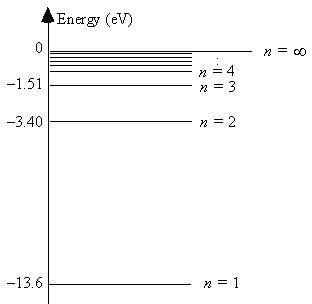 The energy of a photon emitted in a transition from the n = 3 state to the n = 2 state is
The energy of a photon emitted in a transition from the n = 3 state to the n = 2 state is
A) 1.5 eV.
B) 1.9 eV.
C) 3.4 eV.
D) 10.2 eV.
E) 13.6 eV.
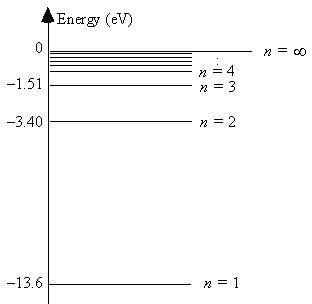 The energy of a photon emitted in a transition from the n = 3 state to the n = 2 state is
The energy of a photon emitted in a transition from the n = 3 state to the n = 2 state isA) 1.5 eV.
B) 1.9 eV.
C) 3.4 eV.
D) 10.2 eV.
E) 13.6 eV.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
65
Lasers are used
A) to produce holograms.
B) in surgery.
C) to measure distances between objects accurately.
D) in DVD and compact disc players.
E) all of the above
A) to produce holograms.
B) in surgery.
C) to measure distances between objects accurately.
D) in DVD and compact disc players.
E) all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
66
The person who first explained the photoelectric effect was
A) Max Planck.
B) Albert Einstein.
C) Neils Bohr.
D) Erwin Schrödinger.
E) Werner Heisenberg.
A) Max Planck.
B) Albert Einstein.
C) Neils Bohr.
D) Erwin Schrödinger.
E) Werner Heisenberg.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
67
The energy level diagram for hydrogen 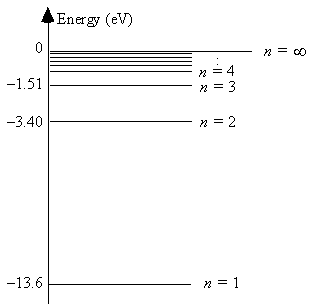 The energy of the most energetically possible photon emitted is
The energy of the most energetically possible photon emitted is
A) 1.5 eV.
B) 1.9 eV.
C) 3.4 eV.
D) 10.2 eV.
E) 13.6 eV.
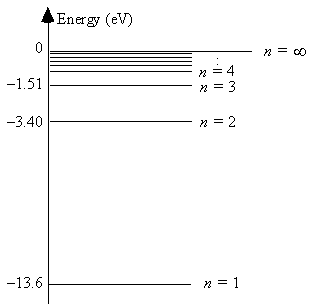 The energy of the most energetically possible photon emitted is
The energy of the most energetically possible photon emitted isA) 1.5 eV.
B) 1.9 eV.
C) 3.4 eV.
D) 10.2 eV.
E) 13.6 eV.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
68
What is meant by a line spectrum?
A) a spectrum which is straight
B) a spectrum made of bright lines in certain places, separated by dark gaps
C) a spectrum made by a hot filament stretched into a straight line
D) none of the above
A) a spectrum which is straight
B) a spectrum made of bright lines in certain places, separated by dark gaps
C) a spectrum made by a hot filament stretched into a straight line
D) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
69
How small an object can a scanning tunneling microscope "see?"
A) about the size of a mushroom spore
B) about the size of a living cell
C) as small as a single atom
D) as small as the nucleus of an atom
A) about the size of a mushroom spore
B) about the size of a living cell
C) as small as a single atom
D) as small as the nucleus of an atom

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
70
The energy level diagram for hydrogen 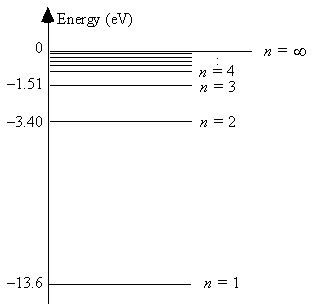 The ionization energy of hydrogen is
The ionization energy of hydrogen is
A) 1.5 eV.
B) 1.9 eV.
C) 3.4 eV.
D) 10.2 eV.
E) 13.6 eV.
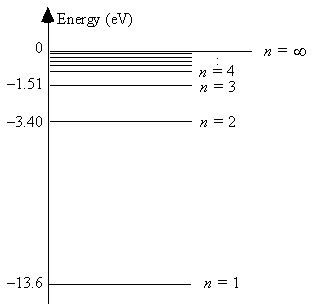 The ionization energy of hydrogen is
The ionization energy of hydrogen isA) 1.5 eV.
B) 1.9 eV.
C) 3.4 eV.
D) 10.2 eV.
E) 13.6 eV.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
71
Which of the following devices make use of the photoelectric effect?
A) digital cameras
B) photocopiers
C) laser printers
D) solar cells
E) all of the above
A) digital cameras
B) photocopiers
C) laser printers
D) solar cells
E) all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
72
The characteristic peaks in an x-ray spectrum of an atom occur because
A) of photon absorption.
B) of the photoelectric effect.
C) electrons in the atom make transitions to lower energy levels.
D) of the uncertainty principle.
E) of rapid deceleration of the bombarding electrons.
A) of photon absorption.
B) of the photoelectric effect.
C) electrons in the atom make transitions to lower energy levels.
D) of the uncertainty principle.
E) of rapid deceleration of the bombarding electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
73
The energy of a photon that ionizes a hydrogen atom from the ground state will be _________ the energy of a photon that ionizes a different hydrogen atom from the first excited state (n - 2).
A) larger than
B) smaller than
C) equal to
D) twice
A) larger than
B) smaller than
C) equal to
D) twice

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
74
In a laser
A) light is amplified by stimulated emission of radiation.
B) the emitted light is monochromatic.
C) the emitted light is coherent.
D) there is a population inversion of electrons in atoms.
E) all of the above
A) light is amplified by stimulated emission of radiation.
B) the emitted light is monochromatic.
C) the emitted light is coherent.
D) there is a population inversion of electrons in atoms.
E) all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
75
When the intensity of the light is increased in a photoelectric effect experiment, the energy of the emitted electrons
A) increases.
B) decreases.
C) stays the same.
D) becomes more quantized.
E) becomes less quantized.
A) increases.
B) decreases.
C) stays the same.
D) becomes more quantized.
E) becomes less quantized.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
76
When the frequency of the light is increased in a photoelectric effect experiment, the energy of the emitted electrons
A) increases.
B) decreases.
C) stays the same.
D) becomes more quantized.
E) becomes less quantized.
A) increases.
B) decreases.
C) stays the same.
D) becomes more quantized.
E) becomes less quantized.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
77
The energy level diagram for hydrogen 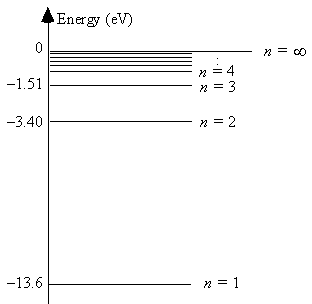 The smallest energy of an absorbed photon that will raise a hydrogen atom from its ground state to its first excited state is
The smallest energy of an absorbed photon that will raise a hydrogen atom from its ground state to its first excited state is
A) 1.5 eV.
B) 1.9 eV.
C) 3.4 eV.
D) 10.2 eV.
E) 13.6 eV.
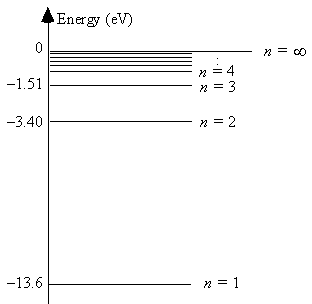 The smallest energy of an absorbed photon that will raise a hydrogen atom from its ground state to its first excited state is
The smallest energy of an absorbed photon that will raise a hydrogen atom from its ground state to its first excited state isA) 1.5 eV.
B) 1.9 eV.
C) 3.4 eV.
D) 10.2 eV.
E) 13.6 eV.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
78
The person who first explained the discrete hydrogen atom spectrum was
A) Max Planck.
B) Albert Einstein.
C) Neils Bohr.
D) Erwin Schrödinger.
E) Werner Heisenberg.
A) Max Planck.
B) Albert Einstein.
C) Neils Bohr.
D) Erwin Schrödinger.
E) Werner Heisenberg.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
79
The spectra of different elements can be distinguished because
A) they have the same spectral lines, but the lines are of different intensities.
B) they have spectral lines in different positions.
C) every element has one unique spectral line.
D) none of the above
A) they have the same spectral lines, but the lines are of different intensities.
B) they have spectral lines in different positions.
C) every element has one unique spectral line.
D) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck
80
The person who developed the concept of a wave function was
A) Max Planck.
B) Albert Einstein.
C) Neils Bohr.
D) Erwin Schrödinger.
E) Werner Heisenberg.
A) Max Planck.
B) Albert Einstein.
C) Neils Bohr.
D) Erwin Schrödinger.
E) Werner Heisenberg.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 134 في هذه المجموعة.
فتح الحزمة
k this deck








