Deck 2: Atoms, Molecules, and Ions
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/132
العب
ملء الشاشة (f)
Deck 2: Atoms, Molecules, and Ions
1
Which subatomic particles listed below are considered charged?
I. neutron
II. proton
III. electron
A) I only
B) I and II
C) I and III
D) II and III
E) All of these
I. neutron
II. proton
III. electron
A) I only
B) I and II
C) I and III
D) II and III
E) All of these
II and III
2
An atom is best defined as a:
A) pure substance composed of two or more elements.
B) pure substance that can be decomposed by chemical means.
C) homogeneous uniform mixture.
D) smallest unit of an element that enters into a chemical combination.
E) chemical combination of two or more elements.
A) pure substance composed of two or more elements.
B) pure substance that can be decomposed by chemical means.
C) homogeneous uniform mixture.
D) smallest unit of an element that enters into a chemical combination.
E) chemical combination of two or more elements.
smallest unit of an element that enters into a chemical combination.
3
The mass of an atom is mainly contained in which of the below particles?
A) protons
B) protons and neutrons
C) protons and electrons
D) neutrons and electrons
E) neutrons
A) protons
B) protons and neutrons
C) protons and electrons
D) neutrons and electrons
E) neutrons
protons and neutrons
4
Which of the following subatomic particles has a negative charge associated with it?
I. a proton
II. an electron
III. a neutron
A) I only
B) II only
C) III only
D) I and II
E) All of these
I. a proton
II. an electron
III. a neutron
A) I only
B) II only
C) III only
D) I and II
E) All of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
5
J) J. Thomson used the deflection of electrons by magnetic and electric fields to measure
A) the volume of the electron.
B) the mass of the electron.
C) the charge of the electron.
D) the mass to charge ratio of the electron.
E) none of these.
A) the volume of the electron.
B) the mass of the electron.
C) the charge of the electron.
D) the mass to charge ratio of the electron.
E) none of these.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
6
What was the result of the Rutherford experiment of directing a beam of alpha particles at a thin gold foil?
A) X-rays were discovered.
B) The neutron was discovered.
C) The nucleus in atoms was discovered.
D) The charge of a proton was measured.
E) None of these.
A) X-rays were discovered.
B) The neutron was discovered.
C) The nucleus in atoms was discovered.
D) The charge of a proton was measured.
E) None of these.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
7
The mass number of the only stable isotope of F is:
A) 19
B) 18
C) 10
D) 9
E) none of these
A) 19
B) 18
C) 10
D) 9
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
8
The atomic number of P is:
A) 14
B) 15
C) 30
D) 31
E) none of these
A) 14
B) 15
C) 30
D) 31
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
9
By observing the scattering of alpha particles from gold foil, Rutherford determined that atoms have:
A) positive charges distributed approximately evenly throughout the atom.
B) a dense positively charged core.
C) different isotopes having different masses.
D) wave-like particles of very small dimensions.
E) none of these.
A) positive charges distributed approximately evenly throughout the atom.
B) a dense positively charged core.
C) different isotopes having different masses.
D) wave-like particles of very small dimensions.
E) none of these.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
10
The atomic number of F is:
A) 19
B) 18
C) 10
D) 9
E) none of these
A) 19
B) 18
C) 10
D) 9
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
11
Which statements below are part of Dalton's Atomic Theory?
I. Matter is composed of small indivisible particles called atoms.
II. An element is composed entirely of one type of atom.
III. Atoms of two or more elements can combine to form compounds.
A) I and II but not III
B) I and III but not II
C) II and III but not I
D) All three are part of Dalton's Theory.
E) None are part of Dalton's Theory.
I. Matter is composed of small indivisible particles called atoms.
II. An element is composed entirely of one type of atom.
III. Atoms of two or more elements can combine to form compounds.
A) I and II but not III
B) I and III but not II
C) II and III but not I
D) All three are part of Dalton's Theory.
E) None are part of Dalton's Theory.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
12
The mass number of the only stable isotope of P is:
A) 15
B) 16
C) 30
D) 31
E) none of these
A) 15
B) 16
C) 30
D) 31
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which of the subatomic particles listed below contribute(s) most significantly to the overall mass of a single atom?
A) a proton
B) an electron
C) a neutron
D) a proton and a neutron
E) a proton and an electron
A) a proton
B) an electron
C) a neutron
D) a proton and a neutron
E) a proton and an electron

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which type of radioactivity is a high energy electron?
A) Beta ray
B) Alpha particle
C) Gamma ray
D) x-ray
E) none of these
A) Beta ray
B) Alpha particle
C) Gamma ray
D) x-ray
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
15
Isotopes are atoms of the same element that have the same number of ____, but different number of ____.
A) protons, electrons
B) neutrons, protons
C) protons, neutrons
D) electrons, protons
E) neutrons, electron
A) protons, electrons
B) neutrons, protons
C) protons, neutrons
D) electrons, protons
E) neutrons, electron

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
16
The nucleus of an atom contains what subatomic particle(s)?
A) neutrons
B) protons
C) electrons
D) neutrons and protons
E) neutrons, protons and electrons
A) neutrons
B) protons
C) electrons
D) neutrons and protons
E) neutrons, protons and electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
17
Which particle listed below fits the description of a completely ionized helium atom?
A) Beta ray
B) Alpha particle
C) Gamma ray
D) x-ray
E) none of these
A) Beta ray
B) Alpha particle
C) Gamma ray
D) x-ray
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
18
An atom is electrically neutral because
A) it contains equal numbers of protons and electrons.
B) it contains equal numbers of neutrons and electrons.
C) no charged particles are present in atoms.
D) the mass number and atomic number are equal.
E) none of these.
A) it contains equal numbers of protons and electrons.
B) it contains equal numbers of neutrons and electrons.
C) no charged particles are present in atoms.
D) the mass number and atomic number are equal.
E) none of these.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
19
The Millikan oil drop experiment measured:
A) the charge of a proton.
B) the charge of an electron.
C) the ratio of charge to mass for a proton.
D) the mass of the neutron.
E) none of these.
A) the charge of a proton.
B) the charge of an electron.
C) the ratio of charge to mass for a proton.
D) the mass of the neutron.
E) none of these.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
20
The main difference between a proton and a neutron is:
A) a proton has considerably more mass than a neutron.
B) a proton is charged, a neutron is not.
C) a neutron is much larger than a proton.
D) a neutron is located in the nucleus of an atom, a proton is not.
E) a proton is much larger than a neutron.
A) a proton has considerably more mass than a neutron.
B) a proton is charged, a neutron is not.
C) a neutron is much larger than a proton.
D) a neutron is located in the nucleus of an atom, a proton is not.
E) a proton is much larger than a neutron.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
21
The symbol for the species that contains 22 protons, 26 neutrons, and 18 electrons is:
A) "48Ti4 - "
B) "22Ti4+"
C) "26Ti2 - "
D) "48Ti4+"
E) "none of these"
A) "48Ti4 - "
B) "22Ti4+"
C) "26Ti2 - "
D) "48Ti4+"
E) "none of these"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which species listed below has 15 protons, 18 electrons, and 16 neutrons?
A) "31P"
B) "18Ar"
C) "16S2 - "
D) "31P3 - "
E) "31Ga3+"
A) "31P"
B) "18Ar"
C) "16S2 - "
D) "31P3 - "
E) "31Ga3+"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
23
A species that contains 16 protons, 17 neutrons, and 16 electrons would be represented by the symbol:
A) "3317Cl+"
B) "3316S+"
C) "3216S"
D) "3316S - "
E) "3316S"
A) "3317Cl+"
B) "3316S+"
C) "3216S"
D) "3316S - "
E) "3316S"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
24
The species containing 6 protons and 5 electrons (and some number of neutrons) would be written as:
A) C
B) C -
C) B+
D) C+
E) none of these
A) C
B) C -
C) B+
D) C+
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
25
What isotope has 45 neutrons and 35 protons?
A) Bromine - 35
B) Bromine - 45
C) Bromine - 80
D) Rhodium - 45
E) Rhodium - 80
A) Bromine - 35
B) Bromine - 45
C) Bromine - 80
D) Rhodium - 45
E) Rhodium - 80

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
26
How does a neutral atom become a charged ion?
I. By adjusting its number of electrons.
II. By adjusting its number of protons.
III. By adjusting its number of neutrons.
A) I only
B) II only
C) III only
D) I and II
E) All of these
I. By adjusting its number of electrons.
II. By adjusting its number of protons.
III. By adjusting its number of neutrons.
A) I only
B) II only
C) III only
D) I and II
E) All of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
27
The species 35Cl - has:
A) 35 protons, 35 neutrons, and 35 electrons.
B) 18 protons, 17 neutrons, and 17 electrons.
C) 17 protons, 18 neutrons, and 17 electrons.
D) 18 protons, 17 neutrons, and 19 electrons.
E) 17 protons, 18 neutrons, and 18 electrons.
A) 35 protons, 35 neutrons, and 35 electrons.
B) 18 protons, 17 neutrons, and 17 electrons.
C) 17 protons, 18 neutrons, and 17 electrons.
D) 18 protons, 17 neutrons, and 19 electrons.
E) 17 protons, 18 neutrons, and 18 electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
28
The symbol for the species that contains 28 protons, 30 neutrons, and 26 electrons is:
A) "58Ni2 - "
B) "30Ni2+"
C) "56Ni2 - "
D) "58Ni2+"
E) "none of these"
A) "58Ni2 - "
B) "30Ni2+"
C) "56Ni2 - "
D) "58Ni2+"
E) "none of these"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
29
The isotope 31P3 - has how many protons, neutrons and electrons?
A) 31 protons, 15 neutrons and 34 electrons
B) 15 protons, 16 neutrons and 18 electrons
C) 15 protons, 31 neutrons and 12 electrons
D) 15 protons, 31 neutrons and 15 electrons
E) 16 protons, 15 neutrons, and 19 electrons
A) 31 protons, 15 neutrons and 34 electrons
B) 15 protons, 16 neutrons and 18 electrons
C) 15 protons, 31 neutrons and 12 electrons
D) 15 protons, 31 neutrons and 15 electrons
E) 16 protons, 15 neutrons, and 19 electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
30
The species written as S2 - has:
A) 16 protons and 18 electrons.
B) 18 protons and 16 electrons.
C) 16 protons and 14 electrons.
D) 18 protons and 20 electrons.
E) none of these.
A) 16 protons and 18 electrons.
B) 18 protons and 16 electrons.
C) 16 protons and 14 electrons.
D) 18 protons and 20 electrons.
E) none of these.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
31
A Sulfur - 34 isotope has how many protons and how many neutrons?
A) 16 protons and 34 neutrons
B) 16 protons and 18 neutrons
C) 34 protons and 16 neutrons
D) 34 protons and 18 neutrons
E) 18 protons and 16 neutrons
A) 16 protons and 34 neutrons
B) 16 protons and 18 neutrons
C) 34 protons and 16 neutrons
D) 34 protons and 18 neutrons
E) 18 protons and 16 neutrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
32
How many protons and neutrons are in the Platinum - 195 isotope, 195Pt?
A) 195 protons, 78 neutrons
B) 78 protons, 195 neutrons
C) 117 protons, 78 neutrons
D) 78 protons, 117 neutrons
E) 117 protons, 195 neutrons
A) 195 protons, 78 neutrons
B) 78 protons, 195 neutrons
C) 117 protons, 78 neutrons
D) 78 protons, 117 neutrons
E) 117 protons, 195 neutrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which symbol listed below describes a particle with 15 protons, 16 neutrons, and 16 electrons?
A)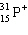
B)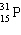
C)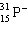
D)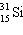
E) none of these
A)
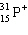
B)
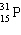
C)
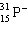
D)
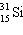
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
34
A particle that contains 8 protons, 9 neutrons, and 7 electrons could be written as:
A) "168O"
B) "179O+"
C) "178O"
D) "178O - "
E) "178O+"
A) "168O"
B) "179O+"
C) "178O"
D) "178O - "
E) "178O+"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
35
A Silicon - 29 isotope has how many protons and how many neutrons?
A) 16 protons and 13 neutrons
B) 16 protons and 29 neutrons
C) 29 protons and 29 neutrons
D) 14 protons and 29 neutrons
E) 14 protons and 15 neutrons
A) 16 protons and 13 neutrons
B) 16 protons and 29 neutrons
C) 29 protons and 29 neutrons
D) 14 protons and 29 neutrons
E) 14 protons and 15 neutrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
36
A species containing 16 protons, 17 neutrons, and 15 electrons would be designated as:
A)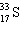
B)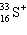
C)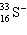
D)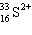
E) none of these
A)
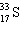
B)
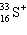
C)
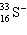
D)
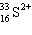
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
37
The symbol 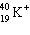 stands for an isotope of potassium containing:
stands for an isotope of potassium containing:
A) 19 electrons, 19 neutrons, 20 protons.
B) 18 electrons, 19 neutrons, 20 protons.
C) 19 electrons, 20 neutrons, 19 protons.
D) 18 electrons, 19 neutrons, 19 protons.
E) 18 electrons, 21 neutrons, 19 protons.
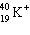 stands for an isotope of potassium containing:
stands for an isotope of potassium containing:A) 19 electrons, 19 neutrons, 20 protons.
B) 18 electrons, 19 neutrons, 20 protons.
C) 19 electrons, 20 neutrons, 19 protons.
D) 18 electrons, 19 neutrons, 19 protons.
E) 18 electrons, 21 neutrons, 19 protons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
38
The species 28Si2 - has:
A) 14 protons, 14 neutrons, and 16 electrons.
B) 14 protons, 14 neutrons, and 14 electrons.
C) 14 protons, 14 neutrons, and 12 electrons.
D) 28 protons, 28 neutrons, and 26 electrons.
E) none of these.
A) 14 protons, 14 neutrons, and 16 electrons.
B) 14 protons, 14 neutrons, and 14 electrons.
C) 14 protons, 14 neutrons, and 12 electrons.
D) 28 protons, 28 neutrons, and 26 electrons.
E) none of these.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
39
How many protons , neutrons and electrons are in the iodide anion, 127I - 1?
A) 53 protons, 74 neutrons, and 53 electrons
B) 52 protons, 74 neutrons, and 53 electrons
C) 53 protons, 74 neutrons, and 54 electrons
D) 53 protons, 127 neutrons, and 54 electrons
E) 52 protons, 75 neutrons, and 51 electrons
A) 53 protons, 74 neutrons, and 53 electrons
B) 52 protons, 74 neutrons, and 53 electrons
C) 53 protons, 74 neutrons, and 54 electrons
D) 53 protons, 127 neutrons, and 54 electrons
E) 52 protons, 75 neutrons, and 51 electrons

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
40
The symbol 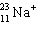 represents a particle that contains:
represents a particle that contains:
A) 11 protons, 22 neutrons, 11 electrons.
B) 11 protons, 23 neutrons, 10 electrons.
C) 11 protons, 12 neutrons, 11 electrons.
D) 12 protons, 11 neutrons, 10 electrons.
E) 11 protons, 12 neutrons, 10 electrons.
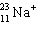 represents a particle that contains:
represents a particle that contains:A) 11 protons, 22 neutrons, 11 electrons.
B) 11 protons, 23 neutrons, 10 electrons.
C) 11 protons, 12 neutrons, 11 electrons.
D) 12 protons, 11 neutrons, 10 electrons.
E) 11 protons, 12 neutrons, 10 electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
41
A new element is prepared that has two isotopes. One isotope is 32.00% abundant and has a mass of 103.2 u. The other has a mass of 105.2 u. What is the atomic mass of this element?
A) 103.8
B) 104.6
C) 104.2
D) 104.9
E) none of these
A) 103.8
B) 104.6
C) 104.2
D) 104.9
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
42
Which of the following elements is non-metallic ?
A) K
B) Fe
C) Na
D) Ce
E) none of these
A) K
B) Fe
C) Na
D) Ce
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
43
Which of the elements from the list below is considered a nonmetallic element?
A) Barium
B) Iodine
C) Calcium
D) Chromium
E) Platinum
A) Barium
B) Iodine
C) Calcium
D) Chromium
E) Platinum

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
44
What is the chemical symbol for the element, Iron ?
A) I
B) Ir
C) Fe
D) In
E) None of these
A) I
B) Ir
C) Fe
D) In
E) None of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
45
Gold is a metal. Which property listed below is not consistent with Gold being a metal?
A) Gold is a thermal conductor.
B) Gold is an electrical insulator.
C) Gold is a bright, shiny lustrous solid.
D) Gold is a malleable and pliable solid.
E) None of these are consistent with Gold being a metal.
A) Gold is a thermal conductor.
B) Gold is an electrical insulator.
C) Gold is a bright, shiny lustrous solid.
D) Gold is a malleable and pliable solid.
E) None of these are consistent with Gold being a metal.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
46
A specially prepared sample of nitrogen contains 30% 14N (mass = 14.00 u) and 70% 15N (mass = 15.00 u). What is the atomic mass of the nitrogen in this sample?
A) 14.00
B) 14.30
C) 14.50
D) 14.70
E) 15.00
A) 14.00
B) 14.30
C) 14.50
D) 14.70
E) 15.00

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
47
Which describes a set of elements on the periodic table?
A) Hominide
B) Period
C) Moeity
D) Thoride
E) Transplatonic
A) Hominide
B) Period
C) Moeity
D) Thoride
E) Transplatonic

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
48
Cations are formed when neutral atoms:
A) Gain protons
B) Lose protons
C) Gain electrons
D) Lose electrons
E) Adjusting the number of neutrons.
A) Gain protons
B) Lose protons
C) Gain electrons
D) Lose electrons
E) Adjusting the number of neutrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
49
Which of the following matched pairs of elemental names and chemical symbols is not properly labeled?
I. Silicon, S
II. Nitrogen, N
III. Tungsten, T
IV. Gold, Au
V. Mercury, Me
A) I and V
B) I, II and III
C) III, IV and V
D) I, III and V
E) II and IV
I. Silicon, S
II. Nitrogen, N
III. Tungsten, T
IV. Gold, Au
V. Mercury, Me
A) I and V
B) I, II and III
C) III, IV and V
D) I, III and V
E) II and IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
50
If the 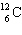 isotope had been assigned a mass of 6.0 u instead of 12 u, the weight of sulfur would be:
isotope had been assigned a mass of 6.0 u instead of 12 u, the weight of sulfur would be:
A) 32 u
B) 16 u
C) 8.0 u
D) 64 u
E) none of these
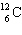 isotope had been assigned a mass of 6.0 u instead of 12 u, the weight of sulfur would be:
isotope had been assigned a mass of 6.0 u instead of 12 u, the weight of sulfur would be:A) 32 u
B) 16 u
C) 8.0 u
D) 64 u
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
51
Which of the following elements is considered a non-metal ?
I. Carbon
II. Potassium
III. Magnesium
A) I only
B) I and II
C) I and III
D) II and III
E) None of these
I. Carbon
II. Potassium
III. Magnesium
A) I only
B) I and II
C) I and III
D) II and III
E) None of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
52
What is the chemical symbol for the element, potassium ?
A) K
B) P
C) Pt
D) W
E) Po
A) K
B) P
C) Pt
D) W
E) Po

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
53
Which of the following elements is considered a metal ?
A) Sulfur
B) Chlorine
C) Phosphorous
D) Magnesium
E) Nitrogen
A) Sulfur
B) Chlorine
C) Phosphorous
D) Magnesium
E) Nitrogen

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
54
What isotope is given by the following model for an atom?
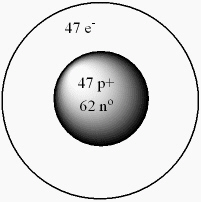
A) "6247Sm"
B) "10947Ag"
C) "10962Sm"
D) "6247Ag"
E) "10947Mt"

A) "6247Sm"
B) "10947Ag"
C) "10962Sm"
D) "6247Ag"
E) "10947Mt"

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
55
Which of the following elements is a metal ?
A) Br
B) C
C) Se
D) Si
E) none of these
A) Br
B) C
C) Se
D) Si
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
56
What is the chemical symbol for the element, Silicon ?
A) S
B) Si
C) Sn
D) Sc
E) None of these
A) S
B) Si
C) Sn
D) Sc
E) None of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
57
Which of the following is considered a general property of nonmetals ?
A) They are malleable solids.
B) They are thermal conductors.
C) They are electrical conductors.
D) Those that are solids are typically dull colored substances that lack luster and are brittle.
E) They can be drawn into wires.
A) They are malleable solids.
B) They are thermal conductors.
C) They are electrical conductors.
D) Those that are solids are typically dull colored substances that lack luster and are brittle.
E) They can be drawn into wires.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
58
A new element is found that has an isotope that occurs naturally in 75% abundance and has a mass oF48.0 u and another isotope that occurs naturally in 25% abundance with a mass of 50.0 u. What is the atomic mass of the element?
A) 48.0 u
B) 48.5 u
C) 49.0 u
D) not enough data available to work this problem
E) none of these is correct
A) 48.0 u
B) 48.5 u
C) 49.0 u
D) not enough data available to work this problem
E) none of these is correct

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
59
Which of the elements from the list below is considered a metallic element ?
A) Zinc
B) Sulfur
C) Carbon
D) Silicon
E) Iodine
A) Zinc
B) Sulfur
C) Carbon
D) Silicon
E) Iodine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
60
Which of the following elements is considered a non-metal ?
I. Calcium
II. Phosphorous
III. Iodine
A) I and II
B) I and III
C) II and III
D) All of these
E) None of these
I. Calcium
II. Phosphorous
III. Iodine
A) I and II
B) I and III
C) II and III
D) All of these
E) None of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
61
Uranium is a member of what region on the periodic table?
A) Noble gas
B) Lanthanide
C) Actinide
D) Transition metal
E) Representative element
A) Noble gas
B) Lanthanide
C) Actinide
D) Transition metal
E) Representative element

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
62
Which set of elements would be expected to have nearly identical chemical properties?
A) C, N, O
B) N, P, As
C) Fe, Co, Ni
D) Ca, Na, Fe
E) Th, U, Am
A) C, N, O
B) N, P, As
C) Fe, Co, Ni
D) Ca, Na, Fe
E) Th, U, Am

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
63
Which of the following elements is a 2nd period, Alkaline Earth Metal?
A) Calcium
B) Beryllium
C) Strontium
D) Carbon
E) Boron
A) Calcium
B) Beryllium
C) Strontium
D) Carbon
E) Boron

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
64
What is the molecular mass (to three significant figures) of C12H10O?
A) 170 u
B) 150 u
C) 190 u
D) 210 u
E) none of these
A) 170 u
B) 150 u
C) 190 u
D) 210 u
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
65
What is the special name for the group on the periodic table that includes the elements Oxygen, Sulfur, Selenium, Tellurium, and Polonium?
A) Alkali Metals
B) Alkaline Earth Metals
C) Chalcogens
D) Halogens
E) Noble Gases
A) Alkali Metals
B) Alkaline Earth Metals
C) Chalcogens
D) Halogens
E) Noble Gases

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
66
Which element listed below is not classified as a lanthanide or actinide?
A) Ce
B) Sm
C) U
D) Fr
E) all are lanthanides or actinides
A) Ce
B) Sm
C) U
D) Fr
E) all are lanthanides or actinides

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
67
The notation 4 SO2 is used to represent:
A) 4 molecules, each containing 1 sulfur and 2 oxygen atoms.
B) 4 sulfur atoms and 4 O2 molecules.
C) 4 sulfur atoms and 8 oxygen atoms.
D) a molecule containing 4 S atoms and 8 O atoms.
E) none of these.
A) 4 molecules, each containing 1 sulfur and 2 oxygen atoms.
B) 4 sulfur atoms and 4 O2 molecules.
C) 4 sulfur atoms and 8 oxygen atoms.
D) a molecule containing 4 S atoms and 8 O atoms.
E) none of these.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
68
What is the molecular mass (to two significant figures) of C6H12O?
A) 84 u
B) 62 u
C) 110 u
D) 100 u
E) none of these
A) 84 u
B) 62 u
C) 110 u
D) 100 u
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
69
What is the special name for the group on the periodic table that includes the elements Fluorine, Chlorine, Bromine, Iodine, and Astatine?
A) Alkali Metals
B) Alkaline Earth Metals
C) Chalcogens
D) Halogens
E) Noble Gases
A) Alkali Metals
B) Alkaline Earth Metals
C) Chalcogens
D) Halogens
E) Noble Gases

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
70
Which of the following elements is a 4th period, Alkaline Earth Metal?
A) Calcium
B) Beryllium
C) Strontium
D) Carbon
E) Boron
A) Calcium
B) Beryllium
C) Strontium
D) Carbon
E) Boron

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
71
Which of the following sets of elements would be expected to have very similar chemical properties for all elements?
Br, Cl, Co, Cu, F, Fe, Ni, S, Zn
A) F, Cl, S, Br
B) F, Cl, Br
C) Fe, Co, Ni, Cu, Zn
D) Fe, Ni, Zn
E) Co, Cu, Zn
Br, Cl, Co, Cu, F, Fe, Ni, S, Zn
A) F, Cl, S, Br
B) F, Cl, Br
C) Fe, Co, Ni, Cu, Zn
D) Fe, Ni, Zn
E) Co, Cu, Zn

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
72
Which of the formula(s) from the following list is(are) that of a diatomic molecular compound ?
I. NO2
II. N2
III. NO
A) I only
B) both I and III
C) both II and III
D) III only
E) none of these
I. NO2
II. N2
III. NO
A) I only
B) both I and III
C) both II and III
D) III only
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
73
Which of the following groups of elements are considered representative elements or Main Group Elements?
A) Alkali metals
B) Alkaline Earth metals
C) Halogens
D) Noble Gas
E) All of these
A) Alkali metals
B) Alkaline Earth metals
C) Halogens
D) Noble Gas
E) All of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
74
Which element listed below is a member of the alkali metal family?
A) Br
B) Ca
C) K
D) S
E) none of these
A) Br
B) Ca
C) K
D) S
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
75
From the list of the following elements, which one would most likely have nearly identical properties as sodium metal?
A) Mg
B) K
C) Ne
D) Cl
E) All of these
A) Mg
B) K
C) Ne
D) Cl
E) All of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
76
Which of the following is the fifth period, halogen?
A) B
B) I
C) At
D) Xe
E) Rb
A) B
B) I
C) At
D) Xe
E) Rb

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
77
Which of the following matched pair of elements would be expected to have the most similar chemical properties?
A) Calcium and Potassium
B) Flourine and Bromine
C) Oxygen and Nitrogen
D) Boron and Beryllium
E) Gold and Iron
A) Calcium and Potassium
B) Flourine and Bromine
C) Oxygen and Nitrogen
D) Boron and Beryllium
E) Gold and Iron

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
78
Copper is a metal . Which property listed below is not consistent with copper being a metal?
A) Copper conducts heat.
B) Copper conducts electricity.
C) Copper is a red shiny colored substance.
D) Copper is a brittle powdery solid.
E) Copper can be drawn into a wire.
A) Copper conducts heat.
B) Copper conducts electricity.
C) Copper is a red shiny colored substance.
D) Copper is a brittle powdery solid.
E) Copper can be drawn into a wire.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
79
What is the special name for the group on the periodic table that includes the elements Beryllium, Magnesium, Calcium, Strontium, and Barium?
A) Alkali Metals
B) Alkaline Earth Metals
C) Chalcogens
D) Halogens
E) Noble Gases
A) Alkali Metals
B) Alkaline Earth Metals
C) Chalcogens
D) Halogens
E) Noble Gases

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck
80
Each molecule of ethene contain S2atoms of carbon and 4 atoms of hydrogen. Its molecular formula is
A) 2 C+H2
B) 2 CH2
C) C2H4
D) Ca2H4
E) none of these
A) 2 C+H2
B) 2 CH2
C) C2H4
D) Ca2H4
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 132 في هذه المجموعة.
فتح الحزمة
k this deck








