Deck 20: The Chemistry of Hydrogen, Elements in Groups 3A Through 6A, and the Noble Gases
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/41
العب
ملء الشاشة (f)
Deck 20: The Chemistry of Hydrogen, Elements in Groups 3A Through 6A, and the Noble Gases
1
Given the three statements below, pick the correct answer.
I. CH4 is an important industrial source of H2.
II. The reaction of coke (C) and H2O is an important industrial source of H2.
III. The reaction of sodium and H2O is an important industrial source of H2.
A) I and II are true, III is false
B) I and III are true, II is false
C) II and III are true, I is false
D) all are true
E) only I is true
I. CH4 is an important industrial source of H2.
II. The reaction of coke (C) and H2O is an important industrial source of H2.
III. The reaction of sodium and H2O is an important industrial source of H2.
A) I and II are true, III is false
B) I and III are true, II is false
C) II and III are true, I is false
D) all are true
E) only I is true
I and II are true, III is false
2
The main difference that determines if a substance will be an insulator or a semiconductor is:
A) trace impurities in the semiconductor.
B) the size of the energy gap between bands.
C) the number of valence electrons.
D) the atomic weight of the species.
E) none of these
A) trace impurities in the semiconductor.
B) the size of the energy gap between bands.
C) the number of valence electrons.
D) the atomic weight of the species.
E) none of these
the size of the energy gap between bands.
3
Which element listed below melts just above room temperature?
A) gallium
B) aluminum
C) thallium
D) indium
E) none of these
A) gallium
B) aluminum
C) thallium
D) indium
E) none of these
gallium
4
What is the explanation for the facts that nitrogen has the elemental form N2 and for phosphorus the elemental forms are P4 or forms based on P4 polymers?
A) Phosphorus has more valence orbitals.
B) Nitrogen can form stable pi bonds with itself, but phosphorus cannot.
C) Phosphorus is more reactive.
D) Nitrogen is more electronegative.
E) none of these
A) Phosphorus has more valence orbitals.
B) Nitrogen can form stable pi bonds with itself, but phosphorus cannot.
C) Phosphorus is more reactive.
D) Nitrogen is more electronegative.
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
5
What is(are) the products of the reaction involving BCl3 and NH3?
A) BH3 and NCl3
B) Cl3B - NH3
C) HCl, B and N2
D) H2, N2, B and Cl2
E) BN and HCl
A) BH3 and NCl3
B) Cl3B - NH3
C) HCl, B and N2
D) H2, N2, B and Cl2
E) BN and HCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
6
Pick the choice below that is an example of a material or process that does not involve alumina.
A) ruby
B) water purification
C) catalyst support
D) corundum
E) alumina is involved in all of these.
A) ruby
B) water purification
C) catalyst support
D) corundum
E) alumina is involved in all of these.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
7
The largest scale commercial use of hydrogen gas is:
A) the production of ammonia.
B) as an energy source.
C) to float lighter-than-air ships.
D) reaction with oxygen to form water in desert regions.
E) none of these
A) the production of ammonia.
B) as an energy source.
C) to float lighter-than-air ships.
D) reaction with oxygen to form water in desert regions.
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
8
What is the most abundant metallic element in the earth's crust?
A) iron
B) cobalt
C) aluminum
D) gallium
E) none of these
A) iron
B) cobalt
C) aluminum
D) gallium
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
9
Which reaction shown below represents the Thermite reaction?
A) 2 Al (s) + Fe2 O3(s) Al 2 O3(s) + 2 Fe (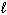 )
)
B) B2H6 (g) + 3 O2 (g)→ B2O3 (s) + 3 H2O (g)
C) 4 Al (s) + 3 O2 (g)→ 2 Al2O3 (s)
D) N2 (g) + 3 H2 (g)→ 2 NH3 (g)
E) CO (g) + H2O→ H2 (g) + CO2 (g)
A) 2 Al (s) + Fe2 O3(s) Al 2 O3(s) + 2 Fe (
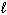 )
)B) B2H6 (g) + 3 O2 (g)→ B2O3 (s) + 3 H2O (g)
C) 4 Al (s) + 3 O2 (g)→ 2 Al2O3 (s)
D) N2 (g) + 3 H2 (g)→ 2 NH3 (g)
E) CO (g) + H2O→ H2 (g) + CO2 (g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
10
The reason that Be can conduct an electrical current even though the 2s band is filled is:
A) trace impurities in the Be.
B) the small size of Be allows for conduction.
C) the 2s and 2p bands do not overlap.
D) overlap of the 2s and 2p bands.
E) none of these
A) trace impurities in the Be.
B) the small size of Be allows for conduction.
C) the 2s and 2p bands do not overlap.
D) overlap of the 2s and 2p bands.
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
11
Name the shape of the arrangement of boron atoms in its elemental forms.
A) dodecahedron
B) tetrahedron
C) icosahedron
D) octahedron
E) none of these
A) dodecahedron
B) tetrahedron
C) icosahedron
D) octahedron
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
12
Why can an oxygen atom only form one compound with fluorine, OF2, but sulfur forms the compounds SF2, SF4, and SF6?
A) Oxygen has a higher electronegativity than sulfur.
B) Sulfur is larger than oxygen.
C) The difference in electronegativity is smaller between O and F, than it is between S and F.
D) Sulfur has d valence orbitals whereas oxygen does not.
E) none of these
A) Oxygen has a higher electronegativity than sulfur.
B) Sulfur is larger than oxygen.
C) The difference in electronegativity is smaller between O and F, than it is between S and F.
D) Sulfur has d valence orbitals whereas oxygen does not.
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which compound listed below would have its bonding explained by the inert pair effect?
A) Pb(CH2CH3)4
B) InCl3
C) TlNO3
D) SnCl4
E) none of these
A) Pb(CH2CH3)4
B) InCl3
C) TlNO3
D) SnCl4
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
14
From the list below, pick the choice that is not an allotrope of carbon.
A) diamond
B) graphite
C) buckminsterfullerene
D) carbonate
E) all of these are allotropes of carbon
A) diamond
B) graphite
C) buckminsterfullerene
D) carbonate
E) all of these are allotropes of carbon

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
15
A special type of bonding in the boranes (BnHm) is known as:
A) highly polarized bonds.
B) electron rich bonds.
C) unsaturated bonds.
D) three-center, two-electron bonds.
E) none of these
A) highly polarized bonds.
B) electron rich bonds.
C) unsaturated bonds.
D) three-center, two-electron bonds.
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
16
Reaction of red hot carbon with steam produces hydrogen gas as shown in equation 1 below. The carbon monoxide that is produced in equation 1 can react further with steam to produce more hydrogen as shown in equation 2. Why are these two reactions undesirable methods for producing hydrogen gas?
(1) C (s) + H2O (g)→H2 (g) + CO (g) (2) CO (g) + H2O (g)→H2 (g) + CO2 (g)
A) The carbon monoxide produced in equation 1 is a poisonous gas.
B) The carbon dioxide produced in equation 2 is a greenhouse gas.
C) The carbon dioxide produced in equation 2 is a poisonous gas.
D) Elemental carbon is very expensive.
E) Both answers a and b are correct.
(1) C (s) + H2O (g)→H2 (g) + CO (g) (2) CO (g) + H2O (g)→H2 (g) + CO2 (g)
A) The carbon monoxide produced in equation 1 is a poisonous gas.
B) The carbon dioxide produced in equation 2 is a greenhouse gas.
C) The carbon dioxide produced in equation 2 is a poisonous gas.
D) Elemental carbon is very expensive.
E) Both answers a and b are correct.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
17
What is the hybridization of silicon in silicates?
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
18
What are the products of the reaction involving calcium hydride, CaH2, and water, H2O?
A) Ca, H2 and O2
B) CaO, H3O+
C) Ca(OH)2 and H2
D) Ca and H2O2
E) CaO2 and H2
A) Ca, H2 and O2
B) CaO, H3O+
C) Ca(OH)2 and H2
D) Ca and H2O2
E) CaO2 and H2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
19
What is the main reason that is generally given to explain the fact that the second row element carbon forms discrete molecules, CO2, with two oxygen atoms, but the third row element silicon forms a network solid, (SiO2)n, when it bonds to two oxygen atoms?
A) Silicon forms stronger bonds with oxygen than carbon does.
B) The electronegativity of carbon and oxygen are closer than that of silicon and oxygen.
C) Third row elements are too large to form stable pi bonds.
D) Oxygen lies to the right of the periodic table.
E) none of these
A) Silicon forms stronger bonds with oxygen than carbon does.
B) The electronegativity of carbon and oxygen are closer than that of silicon and oxygen.
C) Third row elements are too large to form stable pi bonds.
D) Oxygen lies to the right of the periodic table.
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
20
Which of the following elements is considered a metalloid?
A) gallium
B) nitrogen
C) arsenic
D) indium
E) chlorine
A) gallium
B) nitrogen
C) arsenic
D) indium
E) chlorine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
21
The conductivity of silicon can be increased by introducing trace quantities of another element as an impurity in a process referred to as doping. Which element listed below when introduced as an impurity into silicon would provide a n-type conductor?
A) P
B) B
C) Al
D) C
E) Ge
A) P
B) B
C) Al
D) C
E) Ge

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
22
The equation that describes the hydrolysis of P4O10 is:
A) P4O10 (s) + 10 H₂ (g) 4 P (s) + 10 H₂ O (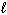 )
)
B) P4O10 (s) + 16 H₂ (g) 4 PH ₃(g) + 10 H₂ O (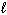 )
)
C) P4O10 (s) + 6 H₂ O (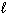 ) 4 H ₃PO4 (aq)
) 4 H ₃PO4 (aq)
D) P4O10 (s) + 3 H₂ O (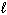 ) 2 H ₃PO3(aq) + 2 O₂(g)
) 2 H ₃PO3(aq) + 2 O₂(g)
E) none of these
A) P4O10 (s) + 10 H₂ (g) 4 P (s) + 10 H₂ O (
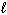 )
)B) P4O10 (s) + 16 H₂ (g) 4 PH ₃(g) + 10 H₂ O (
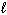 )
)C) P4O10 (s) + 6 H₂ O (
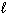 ) 4 H ₃PO4 (aq)
) 4 H ₃PO4 (aq)D) P4O10 (s) + 3 H₂ O (
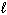 ) 2 H ₃PO3(aq) + 2 O₂(g)
) 2 H ₃PO3(aq) + 2 O₂(g)E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
23
Which statement listed below is true?
A) The N - N - O bond angle in N2O is 120 ° .
B) The gas N2O is a serious cause of air pollution.
C) NO is paramagnetic.
D) NO2 is a linear molecule.
E) Choices a-d are false.
A) The N - N - O bond angle in N2O is 120 ° .
B) The gas N2O is a serious cause of air pollution.
C) NO is paramagnetic.
D) NO2 is a linear molecule.
E) Choices a-d are false.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
24
Given the three statements below, pick the best answer.
I. Elemental sulfur is generally found as S8 molecules.
II. SO2 is a linear molecule.
III. The O - S - O bond angles in SO3 are 120 ° .
A) I and II are true, III is false
B) I and III are true, II is false
C) II and III are true, I is false
D) all three are true
E) only I is true
I. Elemental sulfur is generally found as S8 molecules.
II. SO2 is a linear molecule.
III. The O - S - O bond angles in SO3 are 120 ° .
A) I and II are true, III is false
B) I and III are true, II is false
C) II and III are true, I is false
D) all three are true
E) only I is true

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
25
Which statement below properly describes the predicted (by VSEPR) and experimental bond angles in PH3?
A) predicted: 109 ° measured: 107 °
B) predicted: 120 ° measured: 107 °
C) predicted: 109 ° measured: 94 °
D) predicted: 120 ° measured: 94 °
E) none of these
A) predicted: 109 ° measured: 107 °
B) predicted: 120 ° measured: 107 °
C) predicted: 109 ° measured: 94 °
D) predicted: 120 ° measured: 94 °
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
26
Given the three statements below, pick the best answer.
I. sulfuric acid has the formula H2SO4
II. sulfuric acid is a strong dehydrating agent
III. sulfuric acid reacts endothermically with water
A) I and II are true, III is false
B) I and III are true, II is false
C) II and III are true, I is false
D) only II is true
E) all three statements are true
I. sulfuric acid has the formula H2SO4
II. sulfuric acid is a strong dehydrating agent
III. sulfuric acid reacts endothermically with water
A) I and II are true, III is false
B) I and III are true, II is false
C) II and III are true, I is false
D) only II is true
E) all three statements are true

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
27
What product(s) is(are) formed when sulfuric acid, H2SO4, is allowed to react with carbohydrates such as sucrose, C12H22O11?
A) C, H2O
B) C, H2 and O2
C) CO2 and H2
D) CO and H2
E) CO2 and H2O2
A) C, H2O
B) C, H2 and O2
C) CO2 and H2
D) CO and H2
E) CO2 and H2O2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
28
A property of O2 that is explained by molecular orbital theory, but not Lewis structures, is:
A) O2 is paramagnetic.
B) O2 is a good oxidizing agent.
C) O2 is very abundant in the universe.
D) The O - O bond order is 2.
E) O2 is a gas at room temperature.
A) O2 is paramagnetic.
B) O2 is a good oxidizing agent.
C) O2 is very abundant in the universe.
D) The O - O bond order is 2.
E) O2 is a gas at room temperature.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
29
A blanket of gaseous Nitrogen, N2, is commonly used to store reactive substances that would otherwise oxidize in the presence of oxygen. However, nitrogen reacts with magnesium metal. Which equation shown below represents the chemical reaction that results when magnesium is allowed to react with nitrogen?
A) Mg (s) + N2 (g)→ MgN2 (s)
B) 2 Mg (s) + N2 (g)→ 2 MgN (s)
C) 3 Mg (s) + N2 (g)→ Mg3N2 (s)
D) 4 Mg (s) + N2 (g)→ 2 Mg2N (s)
E) 6 Mg (s) + N2 (g)→ 2 Mg3N (s)
A) Mg (s) + N2 (g)→ MgN2 (s)
B) 2 Mg (s) + N2 (g)→ 2 MgN (s)
C) 3 Mg (s) + N2 (g)→ Mg3N2 (s)
D) 4 Mg (s) + N2 (g)→ 2 Mg2N (s)
E) 6 Mg (s) + N2 (g)→ 2 Mg3N (s)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
30
The O - O bond order in O2 - is:
A) 1
B) 2
C) 3
D) 1/2
E) 3/2
A) 1
B) 2
C) 3
D) 1/2
E) 3/2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which Lewis structure for N2O4 shown below obeys the octet rule for each atom and contains the correct number of valence electrons?
A)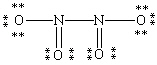
B)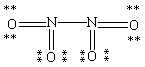
C)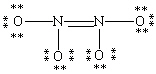
D)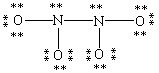
E)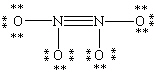
A)

B)
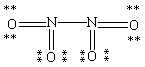
C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
32
What is the correct Lewis structure for the molecule XeF4?
A)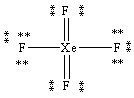
B)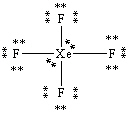
C)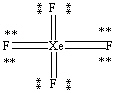
D)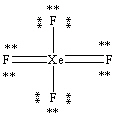
E)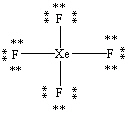
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which compound below has N in the +3 oxidation state?
A) N2H4
B) N2O
C) N2O3
D) N2O4
E) none of these
A) N2H4
B) N2O
C) N2O3
D) N2O4
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
34
Which Lewis structure shown below for XeF2 is correct?
A)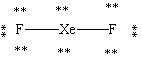
B)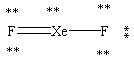
C)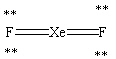
D)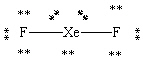
E)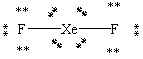
A)
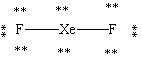
B)
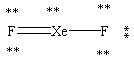
C)
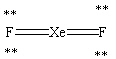
D)
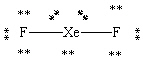
E)
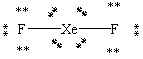

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
35
The conductivity of silicon can be increased by introducing trace quantities of another element as an impurity in a process referred to as doping. Which element listed below when introduced as an impurity into silicon would provide a p-type conductor?
A) S
B) B
C) N
D) C
E) Se
A) S
B) B
C) N
D) C
E) Se

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
36
Acid rain is a result of water reacting with what?
A) non-metal oxides
B) metal oxides
C) oxygen
D) non-metal oxides or metal oxides
E) non-metal oxides, metal oxides or oxygen
A) non-metal oxides
B) metal oxides
C) oxygen
D) non-metal oxides or metal oxides
E) non-metal oxides, metal oxides or oxygen

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
37
The O - O bond order in H2O2 is:
A) 1
B) 2
C) 3
D) 1/2
E) 3/2
A) 1
B) 2
C) 3
D) 1/2
E) 3/2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
38
What product(s) is(are) formed when sulfur trioxide is allowed to react with water?
A) H2, S8 and O2
B) H2S and O2
C) H2SO4
D) H2O2 and S8
E) none of these
A) H2, S8 and O2
B) H2S and O2
C) H2SO4
D) H2O2 and S8
E) none of these

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
39
What is the correct Lewis structure for white phosphorous, P4?
A)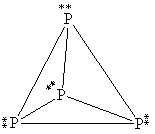
B)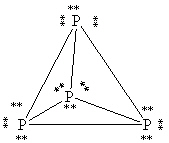
C)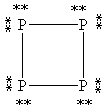
D)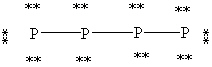
E)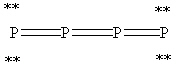
A)

B)

C)

D)

E)
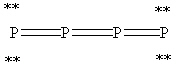

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
40
Three statements are listed below. Pick the correct answer.
I. The O - O - O bond angle in O3 is 180 ° .
II. O3 is very reactive.
III. O2 and O3 are allotropes.
A) I and II are true, III is false
B) I and III are true, II is false
C) II and III are true, I is false
D) only III is true
E) all three are true
I. The O - O - O bond angle in O3 is 180 ° .
II. O3 is very reactive.
III. O2 and O3 are allotropes.
A) I and II are true, III is false
B) I and III are true, II is false
C) II and III are true, I is false
D) only III is true
E) all three are true

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck
41
Given the three statements below, pick the best answer.
I. XeF2 is a linear molecule.
II. Xe is the only group VIIIA element to form molecules with other elements.
III. XeF4 is a planar molecule.
A) I and II are true, III is false
B) I and III are true, II is false
C) II and III are true, I is false
D) all three are true
E) only I is true
I. XeF2 is a linear molecule.
II. Xe is the only group VIIIA element to form molecules with other elements.
III. XeF4 is a planar molecule.
A) I and II are true, III is false
B) I and III are true, II is false
C) II and III are true, I is false
D) all three are true
E) only I is true

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 41 في هذه المجموعة.
فتح الحزمة
k this deck








