Deck 6: Organic Chemistry
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/191
العب
ملء الشاشة (f)
Deck 6: Organic Chemistry
1
Passage
A limited number of cellular functions exist for D-amino acids, such as the structural role of D-alanine and D-glutamate in bacterial cell walls. Because genetically encoded amino acids are synthesized in the L form, production of D-amino acids depends on enzymes called racemases. With the exception of cysteine, conversion of an L-amino acid to a D-amino acid corresponds to the conversion of an S stereoisomer to an R stereoisomer. The mechanism of conversion requires the formation of a high-energy carbanion intermediate. Although pyridoxal phosphate (PLP)-dependent amino acid racemases such as glutamate racemase use PLP as a coenzyme to stabilize this intermediate, the reaction catalyzed by PLP-independent racemases such as alanine racemase proceeds through the enolate intermediate shown in Figure 1.
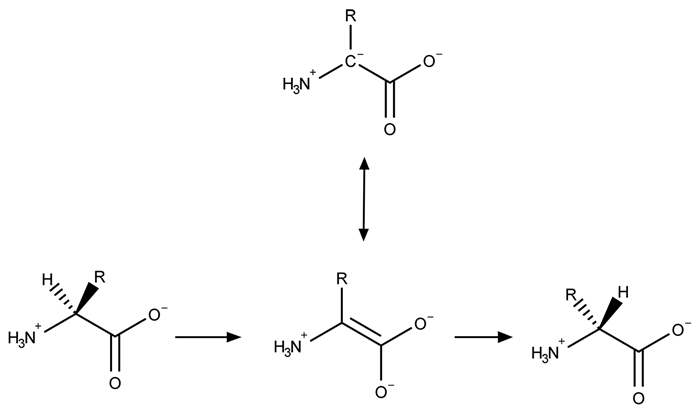 Figure 1 PLP-independent amino acid racemase reactionThe first step in most PLP-dependent reactions is the condensation of an aldehyde group on the coenzyme PLP. This forms a Schiff base linking PLP to a lysine side chain on the enzyme's active site. The lysine is then substituted with the amino acid to be converted from L to D configuration (Figure 2).
Figure 1 PLP-independent amino acid racemase reactionThe first step in most PLP-dependent reactions is the condensation of an aldehyde group on the coenzyme PLP. This forms a Schiff base linking PLP to a lysine side chain on the enzyme's active site. The lysine is then substituted with the amino acid to be converted from L to D configuration (Figure 2).
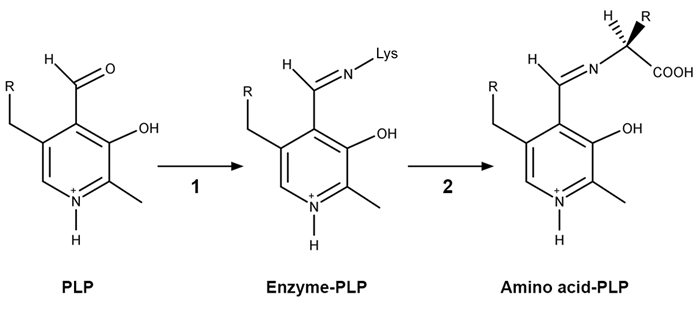 Figure 2 Formation of an amino acid-PLP adductThe next step in PLP-dependent reactions is the removal of the amino acid's α-hydrogen by a base in the enzyme's active site. A quinonoid intermediate and two additional intermediates are formed, as shown in Figure 3. The final step includes the rebonding of PLP to the enzyme's active site and the release of the D-amino acid.
Figure 2 Formation of an amino acid-PLP adductThe next step in PLP-dependent reactions is the removal of the amino acid's α-hydrogen by a base in the enzyme's active site. A quinonoid intermediate and two additional intermediates are formed, as shown in Figure 3. The final step includes the rebonding of PLP to the enzyme's active site and the release of the D-amino acid.
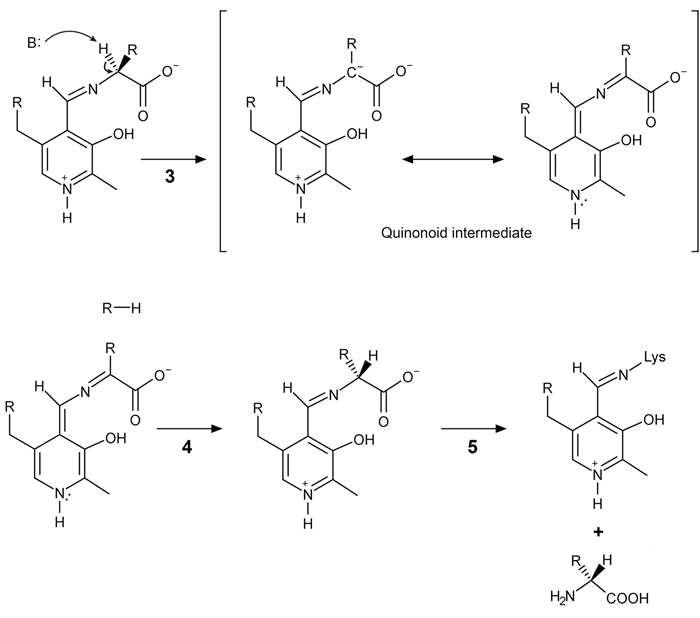 Figure 3 Intermediates of a PLP-dependent amino acid racemase-catalyzed reactionOther PLP-dependent enzymes participate in the decarboxylation of amino acids via a similar mechanism. Such enzymes break the bond between the carboxylate carbon and α-carbon in an amino acid by forming a quinonoid intermediate, which helps stabilize the carbanion intermediate of the reaction.
Figure 3 Intermediates of a PLP-dependent amino acid racemase-catalyzed reactionOther PLP-dependent enzymes participate in the decarboxylation of amino acids via a similar mechanism. Such enzymes break the bond between the carboxylate carbon and α-carbon in an amino acid by forming a quinonoid intermediate, which helps stabilize the carbanion intermediate of the reaction.
Adapted from Cava F, Lam H, De pedro MA, Waldor MK. Emerging knowledge of regulatory roles of D-amino acids in bacteria. Cell Mol Life Sci. 2011.
Which of the following structures can result from the PLP-dependent decarboxylation of an α-amino acid?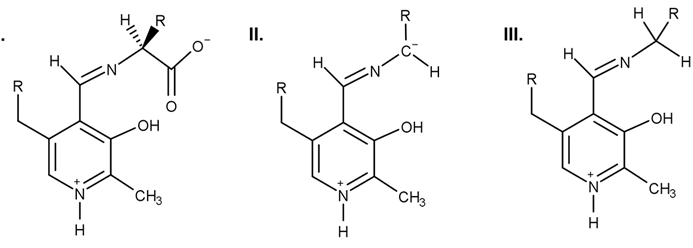
A)I only
B)II only
C)II and III only
D)I, II, and III
A limited number of cellular functions exist for D-amino acids, such as the structural role of D-alanine and D-glutamate in bacterial cell walls. Because genetically encoded amino acids are synthesized in the L form, production of D-amino acids depends on enzymes called racemases. With the exception of cysteine, conversion of an L-amino acid to a D-amino acid corresponds to the conversion of an S stereoisomer to an R stereoisomer. The mechanism of conversion requires the formation of a high-energy carbanion intermediate. Although pyridoxal phosphate (PLP)-dependent amino acid racemases such as glutamate racemase use PLP as a coenzyme to stabilize this intermediate, the reaction catalyzed by PLP-independent racemases such as alanine racemase proceeds through the enolate intermediate shown in Figure 1.
 Figure 1 PLP-independent amino acid racemase reactionThe first step in most PLP-dependent reactions is the condensation of an aldehyde group on the coenzyme PLP. This forms a Schiff base linking PLP to a lysine side chain on the enzyme's active site. The lysine is then substituted with the amino acid to be converted from L to D configuration (Figure 2).
Figure 1 PLP-independent amino acid racemase reactionThe first step in most PLP-dependent reactions is the condensation of an aldehyde group on the coenzyme PLP. This forms a Schiff base linking PLP to a lysine side chain on the enzyme's active site. The lysine is then substituted with the amino acid to be converted from L to D configuration (Figure 2). Figure 2 Formation of an amino acid-PLP adductThe next step in PLP-dependent reactions is the removal of the amino acid's α-hydrogen by a base in the enzyme's active site. A quinonoid intermediate and two additional intermediates are formed, as shown in Figure 3. The final step includes the rebonding of PLP to the enzyme's active site and the release of the D-amino acid.
Figure 2 Formation of an amino acid-PLP adductThe next step in PLP-dependent reactions is the removal of the amino acid's α-hydrogen by a base in the enzyme's active site. A quinonoid intermediate and two additional intermediates are formed, as shown in Figure 3. The final step includes the rebonding of PLP to the enzyme's active site and the release of the D-amino acid. Figure 3 Intermediates of a PLP-dependent amino acid racemase-catalyzed reactionOther PLP-dependent enzymes participate in the decarboxylation of amino acids via a similar mechanism. Such enzymes break the bond between the carboxylate carbon and α-carbon in an amino acid by forming a quinonoid intermediate, which helps stabilize the carbanion intermediate of the reaction.
Figure 3 Intermediates of a PLP-dependent amino acid racemase-catalyzed reactionOther PLP-dependent enzymes participate in the decarboxylation of amino acids via a similar mechanism. Such enzymes break the bond between the carboxylate carbon and α-carbon in an amino acid by forming a quinonoid intermediate, which helps stabilize the carbanion intermediate of the reaction.Adapted from Cava F, Lam H, De pedro MA, Waldor MK. Emerging knowledge of regulatory roles of D-amino acids in bacteria. Cell Mol Life Sci. 2011.
Which of the following structures can result from the PLP-dependent decarboxylation of an α-amino acid?

A)I only
B)II only
C)II and III only
D)I, II, and III
II and III only
2
Passage
Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1.
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. The addition of metallic ions to FIX causes a conformational change that leads to induction of FIX activity, as shown in Figure 2. The 1 ppm (parts per million) proton peak in the inactivated FIX most likely represents:</strong> A)a comparatively large number of deshielded protons. B)a large amount of hydrogen bonding. C)a nearby atom with high electronegativity. D)a portion of FIX with comparatively shielded protons.](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_33d3_a3bf_614cec085188_MD0008_00.jpg) Figure 1 Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2.
Figure 1 Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2.
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. The addition of metallic ions to FIX causes a conformational change that leads to induction of FIX activity, as shown in Figure 2. The 1 ppm (parts per million) proton peak in the inactivated FIX most likely represents:</strong> A)a comparatively large number of deshielded protons. B)a large amount of hydrogen bonding. C)a nearby atom with high electronegativity. D)a portion of FIX with comparatively shielded protons.](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_5ae4_a3bf_119cf7513bca_MD0008_00.jpg) Figure 2 Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K1 and a trans-epoxy vitamin K1, as shown in Figure 3.
Figure 2 Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K1 and a trans-epoxy vitamin K1, as shown in Figure 3.
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. The addition of metallic ions to FIX causes a conformational change that leads to induction of FIX activity, as shown in Figure 2. The 1 ppm (parts per million) proton peak in the inactivated FIX most likely represents:</strong> A)a comparatively large number of deshielded protons. B)a large amount of hydrogen bonding. C)a nearby atom with high electronegativity. D)a portion of FIX with comparatively shielded protons.](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_5ae5_a3bf_fb8c7e084374_MD0008_00.jpg) Figure 3 Results of HPLC separation
Figure 3 Results of HPLC separation
Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995.
The addition of metallic ions to FIX causes a conformational change that leads to induction of FIX activity, as shown in Figure 2. The 1 ppm (parts per million) proton peak in the inactivated FIX most likely represents:
A)a comparatively large number of deshielded protons.
B)a large amount of hydrogen bonding.
C)a nearby atom with high electronegativity.
D)a portion of FIX with comparatively shielded protons.
Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1.
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. The addition of metallic ions to FIX causes a conformational change that leads to induction of FIX activity, as shown in Figure 2. The 1 ppm (parts per million) proton peak in the inactivated FIX most likely represents:</strong> A)a comparatively large number of deshielded protons. B)a large amount of hydrogen bonding. C)a nearby atom with high electronegativity. D)a portion of FIX with comparatively shielded protons.](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_33d3_a3bf_614cec085188_MD0008_00.jpg) Figure 1 Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2.
Figure 1 Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2.![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. The addition of metallic ions to FIX causes a conformational change that leads to induction of FIX activity, as shown in Figure 2. The 1 ppm (parts per million) proton peak in the inactivated FIX most likely represents:</strong> A)a comparatively large number of deshielded protons. B)a large amount of hydrogen bonding. C)a nearby atom with high electronegativity. D)a portion of FIX with comparatively shielded protons.](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_5ae4_a3bf_119cf7513bca_MD0008_00.jpg) Figure 2 Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K1 and a trans-epoxy vitamin K1, as shown in Figure 3.
Figure 2 Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K1 and a trans-epoxy vitamin K1, as shown in Figure 3.![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. The addition of metallic ions to FIX causes a conformational change that leads to induction of FIX activity, as shown in Figure 2. The 1 ppm (parts per million) proton peak in the inactivated FIX most likely represents:</strong> A)a comparatively large number of deshielded protons. B)a large amount of hydrogen bonding. C)a nearby atom with high electronegativity. D)a portion of FIX with comparatively shielded protons.](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_5ae5_a3bf_fb8c7e084374_MD0008_00.jpg) Figure 3 Results of HPLC separation
Figure 3 Results of HPLC separationAdapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995.
The addition of metallic ions to FIX causes a conformational change that leads to induction of FIX activity, as shown in Figure 2. The 1 ppm (parts per million) proton peak in the inactivated FIX most likely represents:
A)a comparatively large number of deshielded protons.
B)a large amount of hydrogen bonding.
C)a nearby atom with high electronegativity.
D)a portion of FIX with comparatively shielded protons.
a portion of FIX with comparatively shielded protons.
3
Passage
Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1.
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. What are the bond angles of the water molecule formed during Reaction 1, the methylated carbon atom of the product from Reaction 1, and the alkene formed in Reaction 2, respectively?</strong> A)104.5°, 104.5°, and 120° B)104.5°, 109.5°, and 120° C)109.5°, 109.5°, and 180° D)180°, 109.5°, and 120°](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_33d3_a3bf_614cec085188_MD0008_00.jpg) Figure 1 Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2.
Figure 1 Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2.
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. What are the bond angles of the water molecule formed during Reaction 1, the methylated carbon atom of the product from Reaction 1, and the alkene formed in Reaction 2, respectively?</strong> A)104.5°, 104.5°, and 120° B)104.5°, 109.5°, and 120° C)109.5°, 109.5°, and 180° D)180°, 109.5°, and 120°](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_5ae4_a3bf_119cf7513bca_MD0008_00.jpg) Figure 2 Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K1 and a trans-epoxy vitamin K1, as shown in Figure 3.
Figure 2 Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K1 and a trans-epoxy vitamin K1, as shown in Figure 3.
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. What are the bond angles of the water molecule formed during Reaction 1, the methylated carbon atom of the product from Reaction 1, and the alkene formed in Reaction 2, respectively?</strong> A)104.5°, 104.5°, and 120° B)104.5°, 109.5°, and 120° C)109.5°, 109.5°, and 180° D)180°, 109.5°, and 120°](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_5ae5_a3bf_fb8c7e084374_MD0008_00.jpg) Figure 3 Results of HPLC separation
Figure 3 Results of HPLC separation
Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995.
What are the bond angles of the water molecule formed during Reaction 1, the methylated carbon atom of the product from Reaction 1, and the alkene formed in Reaction 2, respectively?
A)104.5°, 104.5°, and 120°
B)104.5°, 109.5°, and 120°
C)109.5°, 109.5°, and 180°
D)180°, 109.5°, and 120°
Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1.
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. What are the bond angles of the water molecule formed during Reaction 1, the methylated carbon atom of the product from Reaction 1, and the alkene formed in Reaction 2, respectively?</strong> A)104.5°, 104.5°, and 120° B)104.5°, 109.5°, and 120° C)109.5°, 109.5°, and 180° D)180°, 109.5°, and 120°](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_33d3_a3bf_614cec085188_MD0008_00.jpg) Figure 1 Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2.
Figure 1 Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2.![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. What are the bond angles of the water molecule formed during Reaction 1, the methylated carbon atom of the product from Reaction 1, and the alkene formed in Reaction 2, respectively?</strong> A)104.5°, 104.5°, and 120° B)104.5°, 109.5°, and 120° C)109.5°, 109.5°, and 180° D)180°, 109.5°, and 120°](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_5ae4_a3bf_119cf7513bca_MD0008_00.jpg) Figure 2 Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K1 and a trans-epoxy vitamin K1, as shown in Figure 3.
Figure 2 Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K1 and a trans-epoxy vitamin K1, as shown in Figure 3.![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. What are the bond angles of the water molecule formed during Reaction 1, the methylated carbon atom of the product from Reaction 1, and the alkene formed in Reaction 2, respectively?</strong> A)104.5°, 104.5°, and 120° B)104.5°, 109.5°, and 120° C)109.5°, 109.5°, and 180° D)180°, 109.5°, and 120°](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_5ae5_a3bf_fb8c7e084374_MD0008_00.jpg) Figure 3 Results of HPLC separation
Figure 3 Results of HPLC separationAdapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995.
What are the bond angles of the water molecule formed during Reaction 1, the methylated carbon atom of the product from Reaction 1, and the alkene formed in Reaction 2, respectively?
A)104.5°, 104.5°, and 120°
B)104.5°, 109.5°, and 120°
C)109.5°, 109.5°, and 180°
D)180°, 109.5°, and 120°
104.5°, 109.5°, and 120°
4
Passage
The drug paracetamol, also known as acetaminophen, is used to relieve pain and fever, and is a metabolite of the antipyretic drug phenacetin (Figure 1). These two drugs have similar medicinal properties, but phenacetin has been shown to be carcinogenic and cause kidney damage. Acetaminophen is a safer alternative to phenacetin when taken in therapeutic doses.
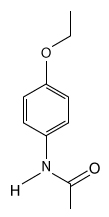 Figure 1 Structure of phenacetinA student synthesizes acetaminophen in a laboratory from phenol according to the reactions shown. The synthesis begins with the nitration of phenol by sodium nitrate (NaNO3) to produce the mixture of the isomers 2-nitrophenol and 4-nitrophenol (Reaction 1). These isomers can be easily separated by steam distillation to isolate the desired product, 4-nitrophenol. Reduction of the nitro group with sodium borohydride (NaBH4) (Reaction 2) is followed by acetylation of the amine with acetic anhydride (Reaction 3) to afford the final product, acetaminophen.
Figure 1 Structure of phenacetinA student synthesizes acetaminophen in a laboratory from phenol according to the reactions shown. The synthesis begins with the nitration of phenol by sodium nitrate (NaNO3) to produce the mixture of the isomers 2-nitrophenol and 4-nitrophenol (Reaction 1). These isomers can be easily separated by steam distillation to isolate the desired product, 4-nitrophenol. Reduction of the nitro group with sodium borohydride (NaBH4) (Reaction 2) is followed by acetylation of the amine with acetic anhydride (Reaction 3) to afford the final product, acetaminophen.
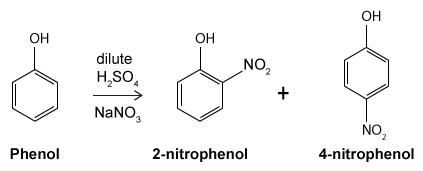 Reaction 1
Reaction 1
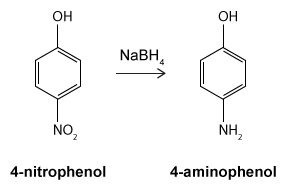 Reaction 2
Reaction 2
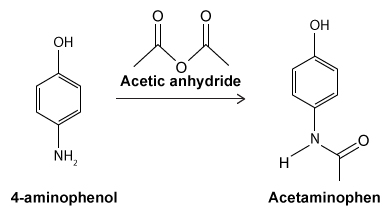 Reaction 3
Reaction 3
Which of the following statements most accurately describes the component that remains in the reaction flask during the steam distillation?
A)2-nitrophenol remains in the reaction flask because it has more intermolecular hydrogen bonding.
B)4-nitrophenol remains in the reaction flask because it has more intermolecular hydrogen bonding.
C)2-nitrophenol remains in the reaction flask because it has less intermolecular hydrogen bonding.
D)4-nitrophenol remains in the reaction flask because it has less intermolecular hydrogen bonding.
The drug paracetamol, also known as acetaminophen, is used to relieve pain and fever, and is a metabolite of the antipyretic drug phenacetin (Figure 1). These two drugs have similar medicinal properties, but phenacetin has been shown to be carcinogenic and cause kidney damage. Acetaminophen is a safer alternative to phenacetin when taken in therapeutic doses.
 Figure 1 Structure of phenacetinA student synthesizes acetaminophen in a laboratory from phenol according to the reactions shown. The synthesis begins with the nitration of phenol by sodium nitrate (NaNO3) to produce the mixture of the isomers 2-nitrophenol and 4-nitrophenol (Reaction 1). These isomers can be easily separated by steam distillation to isolate the desired product, 4-nitrophenol. Reduction of the nitro group with sodium borohydride (NaBH4) (Reaction 2) is followed by acetylation of the amine with acetic anhydride (Reaction 3) to afford the final product, acetaminophen.
Figure 1 Structure of phenacetinA student synthesizes acetaminophen in a laboratory from phenol according to the reactions shown. The synthesis begins with the nitration of phenol by sodium nitrate (NaNO3) to produce the mixture of the isomers 2-nitrophenol and 4-nitrophenol (Reaction 1). These isomers can be easily separated by steam distillation to isolate the desired product, 4-nitrophenol. Reduction of the nitro group with sodium borohydride (NaBH4) (Reaction 2) is followed by acetylation of the amine with acetic anhydride (Reaction 3) to afford the final product, acetaminophen. Reaction 1
Reaction 1 Reaction 2
Reaction 2 Reaction 3
Reaction 3Which of the following statements most accurately describes the component that remains in the reaction flask during the steam distillation?
A)2-nitrophenol remains in the reaction flask because it has more intermolecular hydrogen bonding.
B)4-nitrophenol remains in the reaction flask because it has more intermolecular hydrogen bonding.
C)2-nitrophenol remains in the reaction flask because it has less intermolecular hydrogen bonding.
D)4-nitrophenol remains in the reaction flask because it has less intermolecular hydrogen bonding.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
5
Passage
Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1.
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. HPLC is repeated after accidental contamination of the sample containing the cis/trans vitamin K<sub>1</sub> isomers and trans-epoxy vitamin K<sub>1</sub> in Figure 3. The most recent results demonstrate a new compound with a broad base of 405 mAU and retention time of 23.421 min. This contaminant likely:</strong> A)has a lower affinity for the stationary phase than cis vitamin K<sub>1</sub>. B)has a lower affinity for the stationary phase than trans-epoxy vitamin K<sub>1</sub>. C)has a lower affinity for the mobile phase than trans-epoxy vitamin K<sub>1</sub>. D)has a lower affinity for the mobile phase than trans vitamin K<sub>1</sub>.](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_33d3_a3bf_614cec085188_MD0008_00.jpg) Figure 1 Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2.
Figure 1 Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2.
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. HPLC is repeated after accidental contamination of the sample containing the cis/trans vitamin K<sub>1</sub> isomers and trans-epoxy vitamin K<sub>1</sub> in Figure 3. The most recent results demonstrate a new compound with a broad base of 405 mAU and retention time of 23.421 min. This contaminant likely:</strong> A)has a lower affinity for the stationary phase than cis vitamin K<sub>1</sub>. B)has a lower affinity for the stationary phase than trans-epoxy vitamin K<sub>1</sub>. C)has a lower affinity for the mobile phase than trans-epoxy vitamin K<sub>1</sub>. D)has a lower affinity for the mobile phase than trans vitamin K<sub>1</sub>.](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_5ae4_a3bf_119cf7513bca_MD0008_00.jpg) Figure 2 Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K1 and a trans-epoxy vitamin K1, as shown in Figure 3.
Figure 2 Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K1 and a trans-epoxy vitamin K1, as shown in Figure 3.
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. HPLC is repeated after accidental contamination of the sample containing the cis/trans vitamin K<sub>1</sub> isomers and trans-epoxy vitamin K<sub>1</sub> in Figure 3. The most recent results demonstrate a new compound with a broad base of 405 mAU and retention time of 23.421 min. This contaminant likely:</strong> A)has a lower affinity for the stationary phase than cis vitamin K<sub>1</sub>. B)has a lower affinity for the stationary phase than trans-epoxy vitamin K<sub>1</sub>. C)has a lower affinity for the mobile phase than trans-epoxy vitamin K<sub>1</sub>. D)has a lower affinity for the mobile phase than trans vitamin K<sub>1</sub>.](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_5ae5_a3bf_fb8c7e084374_MD0008_00.jpg) Figure 3 Results of HPLC separation
Figure 3 Results of HPLC separation
Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995.
HPLC is repeated after accidental contamination of the sample containing the cis/trans vitamin K1 isomers and trans-epoxy vitamin K1 in Figure 3. The most recent results demonstrate a new compound with a broad base of 405 mAU and retention time of 23.421 min. This contaminant likely:
A)has a lower affinity for the stationary phase than cis vitamin K1.
B)has a lower affinity for the stationary phase than trans-epoxy vitamin K1.
C)has a lower affinity for the mobile phase than trans-epoxy vitamin K1.
D)has a lower affinity for the mobile phase than trans vitamin K1.
Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1.
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. HPLC is repeated after accidental contamination of the sample containing the cis/trans vitamin K<sub>1</sub> isomers and trans-epoxy vitamin K<sub>1</sub> in Figure 3. The most recent results demonstrate a new compound with a broad base of 405 mAU and retention time of 23.421 min. This contaminant likely:</strong> A)has a lower affinity for the stationary phase than cis vitamin K<sub>1</sub>. B)has a lower affinity for the stationary phase than trans-epoxy vitamin K<sub>1</sub>. C)has a lower affinity for the mobile phase than trans-epoxy vitamin K<sub>1</sub>. D)has a lower affinity for the mobile phase than trans vitamin K<sub>1</sub>.](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_33d3_a3bf_614cec085188_MD0008_00.jpg) Figure 1 Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2.
Figure 1 Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2.![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. HPLC is repeated after accidental contamination of the sample containing the cis/trans vitamin K<sub>1</sub> isomers and trans-epoxy vitamin K<sub>1</sub> in Figure 3. The most recent results demonstrate a new compound with a broad base of 405 mAU and retention time of 23.421 min. This contaminant likely:</strong> A)has a lower affinity for the stationary phase than cis vitamin K<sub>1</sub>. B)has a lower affinity for the stationary phase than trans-epoxy vitamin K<sub>1</sub>. C)has a lower affinity for the mobile phase than trans-epoxy vitamin K<sub>1</sub>. D)has a lower affinity for the mobile phase than trans vitamin K<sub>1</sub>.](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_5ae4_a3bf_119cf7513bca_MD0008_00.jpg) Figure 2 Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K1 and a trans-epoxy vitamin K1, as shown in Figure 3.
Figure 2 Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K1 and a trans-epoxy vitamin K1, as shown in Figure 3.![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. HPLC is repeated after accidental contamination of the sample containing the cis/trans vitamin K<sub>1</sub> isomers and trans-epoxy vitamin K<sub>1</sub> in Figure 3. The most recent results demonstrate a new compound with a broad base of 405 mAU and retention time of 23.421 min. This contaminant likely:</strong> A)has a lower affinity for the stationary phase than cis vitamin K<sub>1</sub>. B)has a lower affinity for the stationary phase than trans-epoxy vitamin K<sub>1</sub>. C)has a lower affinity for the mobile phase than trans-epoxy vitamin K<sub>1</sub>. D)has a lower affinity for the mobile phase than trans vitamin K<sub>1</sub>.](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_5ae5_a3bf_fb8c7e084374_MD0008_00.jpg) Figure 3 Results of HPLC separation
Figure 3 Results of HPLC separationAdapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995.
HPLC is repeated after accidental contamination of the sample containing the cis/trans vitamin K1 isomers and trans-epoxy vitamin K1 in Figure 3. The most recent results demonstrate a new compound with a broad base of 405 mAU and retention time of 23.421 min. This contaminant likely:
A)has a lower affinity for the stationary phase than cis vitamin K1.
B)has a lower affinity for the stationary phase than trans-epoxy vitamin K1.
C)has a lower affinity for the mobile phase than trans-epoxy vitamin K1.
D)has a lower affinity for the mobile phase than trans vitamin K1.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
6
Passage
A limited number of cellular functions exist for D-amino acids, such as the structural role of D-alanine and D-glutamate in bacterial cell walls. Because genetically encoded amino acids are synthesized in the L form, production of D-amino acids depends on enzymes called racemases. With the exception of cysteine, conversion of an L-amino acid to a D-amino acid corresponds to the conversion of an S stereoisomer to an R stereoisomer. The mechanism of conversion requires the formation of a high-energy carbanion intermediate. Although pyridoxal phosphate (PLP)-dependent amino acid racemases such as glutamate racemase use PLP as a coenzyme to stabilize this intermediate, the reaction catalyzed by PLP-independent racemases such as alanine racemase proceeds through the enolate intermediate shown in Figure 1.
 Figure 1 PLP-independent amino acid racemase reactionThe first step in most PLP-dependent reactions is the condensation of an aldehyde group on the coenzyme PLP. This forms a Schiff base linking PLP to a lysine side chain on the enzyme's active site. The lysine is then substituted with the amino acid to be converted from L to D configuration (Figure 2).
Figure 1 PLP-independent amino acid racemase reactionThe first step in most PLP-dependent reactions is the condensation of an aldehyde group on the coenzyme PLP. This forms a Schiff base linking PLP to a lysine side chain on the enzyme's active site. The lysine is then substituted with the amino acid to be converted from L to D configuration (Figure 2).
 Figure 2 Formation of an amino acid-PLP adductThe next step in PLP-dependent reactions is the removal of the amino acid's α-hydrogen by a base in the enzyme's active site. A quinonoid intermediate and two additional intermediates are formed, as shown in Figure 3. The final step includes the rebonding of PLP to the enzyme's active site and the release of the D-amino acid.
Figure 2 Formation of an amino acid-PLP adductThe next step in PLP-dependent reactions is the removal of the amino acid's α-hydrogen by a base in the enzyme's active site. A quinonoid intermediate and two additional intermediates are formed, as shown in Figure 3. The final step includes the rebonding of PLP to the enzyme's active site and the release of the D-amino acid.
 Figure 3 Intermediates of a PLP-dependent amino acid racemase-catalyzed reactionOther PLP-dependent enzymes participate in the decarboxylation of amino acids via a similar mechanism. Such enzymes break the bond between the carboxylate carbon and α-carbon in an amino acid by forming a quinonoid intermediate, which helps stabilize the carbanion intermediate of the reaction.
Figure 3 Intermediates of a PLP-dependent amino acid racemase-catalyzed reactionOther PLP-dependent enzymes participate in the decarboxylation of amino acids via a similar mechanism. Such enzymes break the bond between the carboxylate carbon and α-carbon in an amino acid by forming a quinonoid intermediate, which helps stabilize the carbanion intermediate of the reaction.
Adapted from Cava F, Lam H, De pedro MA, Waldor MK. Emerging knowledge of regulatory roles of D-amino acids in bacteria. Cell Mol Life Sci. 2011.
During PLP-dependent reactions, reprotonation of the planar molecule results from the addition of a hydrogen atom:
A)in a nonstereospecific manner.
B)to either side of the planar intermediate with equal likelihood.
C)with an orientation opposite that of the hydrogen atom removed.
D)with the same orientation as the hydrogen atom removed.
A limited number of cellular functions exist for D-amino acids, such as the structural role of D-alanine and D-glutamate in bacterial cell walls. Because genetically encoded amino acids are synthesized in the L form, production of D-amino acids depends on enzymes called racemases. With the exception of cysteine, conversion of an L-amino acid to a D-amino acid corresponds to the conversion of an S stereoisomer to an R stereoisomer. The mechanism of conversion requires the formation of a high-energy carbanion intermediate. Although pyridoxal phosphate (PLP)-dependent amino acid racemases such as glutamate racemase use PLP as a coenzyme to stabilize this intermediate, the reaction catalyzed by PLP-independent racemases such as alanine racemase proceeds through the enolate intermediate shown in Figure 1.
 Figure 1 PLP-independent amino acid racemase reactionThe first step in most PLP-dependent reactions is the condensation of an aldehyde group on the coenzyme PLP. This forms a Schiff base linking PLP to a lysine side chain on the enzyme's active site. The lysine is then substituted with the amino acid to be converted from L to D configuration (Figure 2).
Figure 1 PLP-independent amino acid racemase reactionThe first step in most PLP-dependent reactions is the condensation of an aldehyde group on the coenzyme PLP. This forms a Schiff base linking PLP to a lysine side chain on the enzyme's active site. The lysine is then substituted with the amino acid to be converted from L to D configuration (Figure 2). Figure 2 Formation of an amino acid-PLP adductThe next step in PLP-dependent reactions is the removal of the amino acid's α-hydrogen by a base in the enzyme's active site. A quinonoid intermediate and two additional intermediates are formed, as shown in Figure 3. The final step includes the rebonding of PLP to the enzyme's active site and the release of the D-amino acid.
Figure 2 Formation of an amino acid-PLP adductThe next step in PLP-dependent reactions is the removal of the amino acid's α-hydrogen by a base in the enzyme's active site. A quinonoid intermediate and two additional intermediates are formed, as shown in Figure 3. The final step includes the rebonding of PLP to the enzyme's active site and the release of the D-amino acid. Figure 3 Intermediates of a PLP-dependent amino acid racemase-catalyzed reactionOther PLP-dependent enzymes participate in the decarboxylation of amino acids via a similar mechanism. Such enzymes break the bond between the carboxylate carbon and α-carbon in an amino acid by forming a quinonoid intermediate, which helps stabilize the carbanion intermediate of the reaction.
Figure 3 Intermediates of a PLP-dependent amino acid racemase-catalyzed reactionOther PLP-dependent enzymes participate in the decarboxylation of amino acids via a similar mechanism. Such enzymes break the bond between the carboxylate carbon and α-carbon in an amino acid by forming a quinonoid intermediate, which helps stabilize the carbanion intermediate of the reaction.Adapted from Cava F, Lam H, De pedro MA, Waldor MK. Emerging knowledge of regulatory roles of D-amino acids in bacteria. Cell Mol Life Sci. 2011.
During PLP-dependent reactions, reprotonation of the planar molecule results from the addition of a hydrogen atom:
A)in a nonstereospecific manner.
B)to either side of the planar intermediate with equal likelihood.
C)with an orientation opposite that of the hydrogen atom removed.
D)with the same orientation as the hydrogen atom removed.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
7
Passage
The drug paracetamol, also known as acetaminophen, is used to relieve pain and fever, and is a metabolite of the antipyretic drug phenacetin (Figure 1). These two drugs have similar medicinal properties, but phenacetin has been shown to be carcinogenic and cause kidney damage. Acetaminophen is a safer alternative to phenacetin when taken in therapeutic doses.
 Figure 1 Structure of phenacetinA student synthesizes acetaminophen in a laboratory from phenol according to the reactions shown. The synthesis begins with the nitration of phenol by sodium nitrate (NaNO3) to produce the mixture of the isomers 2-nitrophenol and 4-nitrophenol (Reaction 1). These isomers can be easily separated by steam distillation to isolate the desired product, 4-nitrophenol. Reduction of the nitro group with sodium borohydride (NaBH4) (Reaction 2) is followed by acetylation of the amine with acetic anhydride (Reaction 3) to afford the final product, acetaminophen.
Figure 1 Structure of phenacetinA student synthesizes acetaminophen in a laboratory from phenol according to the reactions shown. The synthesis begins with the nitration of phenol by sodium nitrate (NaNO3) to produce the mixture of the isomers 2-nitrophenol and 4-nitrophenol (Reaction 1). These isomers can be easily separated by steam distillation to isolate the desired product, 4-nitrophenol. Reduction of the nitro group with sodium borohydride (NaBH4) (Reaction 2) is followed by acetylation of the amine with acetic anhydride (Reaction 3) to afford the final product, acetaminophen.
 Reaction 1
Reaction 1
 Reaction 2
Reaction 2
 Reaction 3
Reaction 3
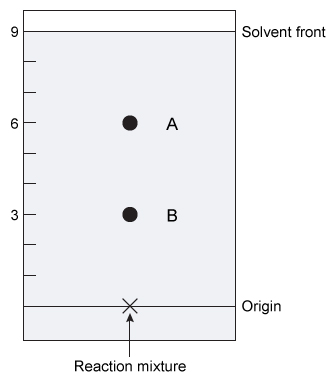 Normal phase thin layer chromatography (TLC) was used to monitor the formation of acetaminophen. According to the TLC plate shown, which spot has an Rf value of 0.33, and what compound most likely corresponds to this spot?
Normal phase thin layer chromatography (TLC) was used to monitor the formation of acetaminophen. According to the TLC plate shown, which spot has an Rf value of 0.33, and what compound most likely corresponds to this spot?
A)Spot A; acetaminophen
B)Spot B; acetaminophen
C)Spot A; 4-aminophenol
D)Spot B; 4-aminophenol
The drug paracetamol, also known as acetaminophen, is used to relieve pain and fever, and is a metabolite of the antipyretic drug phenacetin (Figure 1). These two drugs have similar medicinal properties, but phenacetin has been shown to be carcinogenic and cause kidney damage. Acetaminophen is a safer alternative to phenacetin when taken in therapeutic doses.
 Figure 1 Structure of phenacetinA student synthesizes acetaminophen in a laboratory from phenol according to the reactions shown. The synthesis begins with the nitration of phenol by sodium nitrate (NaNO3) to produce the mixture of the isomers 2-nitrophenol and 4-nitrophenol (Reaction 1). These isomers can be easily separated by steam distillation to isolate the desired product, 4-nitrophenol. Reduction of the nitro group with sodium borohydride (NaBH4) (Reaction 2) is followed by acetylation of the amine with acetic anhydride (Reaction 3) to afford the final product, acetaminophen.
Figure 1 Structure of phenacetinA student synthesizes acetaminophen in a laboratory from phenol according to the reactions shown. The synthesis begins with the nitration of phenol by sodium nitrate (NaNO3) to produce the mixture of the isomers 2-nitrophenol and 4-nitrophenol (Reaction 1). These isomers can be easily separated by steam distillation to isolate the desired product, 4-nitrophenol. Reduction of the nitro group with sodium borohydride (NaBH4) (Reaction 2) is followed by acetylation of the amine with acetic anhydride (Reaction 3) to afford the final product, acetaminophen. Reaction 1
Reaction 1 Reaction 2
Reaction 2 Reaction 3
Reaction 3 Normal phase thin layer chromatography (TLC) was used to monitor the formation of acetaminophen. According to the TLC plate shown, which spot has an Rf value of 0.33, and what compound most likely corresponds to this spot?
Normal phase thin layer chromatography (TLC) was used to monitor the formation of acetaminophen. According to the TLC plate shown, which spot has an Rf value of 0.33, and what compound most likely corresponds to this spot?A)Spot A; acetaminophen
B)Spot B; acetaminophen
C)Spot A; 4-aminophenol
D)Spot B; 4-aminophenol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
8
Passage
Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1.
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. During the oxidation of vitamin K in Reaction 1, an amino acid residue is carboxylated and results in the following structure: What was the most likely initial amino acid residue?</strong> A)Aspartic acid B)Asparagine C)Glutamine D)Glutamic acid](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_33d3_a3bf_614cec085188_MD0008_00.jpg) Figure 1 Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2.
Figure 1 Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2.
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. During the oxidation of vitamin K in Reaction 1, an amino acid residue is carboxylated and results in the following structure: What was the most likely initial amino acid residue?</strong> A)Aspartic acid B)Asparagine C)Glutamine D)Glutamic acid](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_5ae4_a3bf_119cf7513bca_MD0008_00.jpg) Figure 2 Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K1 and a trans-epoxy vitamin K1, as shown in Figure 3.
Figure 2 Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K1 and a trans-epoxy vitamin K1, as shown in Figure 3.
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. During the oxidation of vitamin K in Reaction 1, an amino acid residue is carboxylated and results in the following structure: What was the most likely initial amino acid residue?</strong> A)Aspartic acid B)Asparagine C)Glutamine D)Glutamic acid](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_5ae5_a3bf_fb8c7e084374_MD0008_00.jpg) Figure 3 Results of HPLC separation
Figure 3 Results of HPLC separation
Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995.
During the oxidation of vitamin K in Reaction 1, an amino acid residue is carboxylated and results in the following structure:![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. During the oxidation of vitamin K in Reaction 1, an amino acid residue is carboxylated and results in the following structure: What was the most likely initial amino acid residue?</strong> A)Aspartic acid B)Asparagine C)Glutamine D)Glutamic acid](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1256_8f3b_a3bf_ab3ccbe33395_MD0008_00.jpg) What was the most likely initial amino acid residue?
What was the most likely initial amino acid residue?
A)Aspartic acid
B)Asparagine
C)Glutamine
D)Glutamic acid
Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1.
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. During the oxidation of vitamin K in Reaction 1, an amino acid residue is carboxylated and results in the following structure: What was the most likely initial amino acid residue?</strong> A)Aspartic acid B)Asparagine C)Glutamine D)Glutamic acid](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_33d3_a3bf_614cec085188_MD0008_00.jpg) Figure 1 Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2.
Figure 1 Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2.![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. During the oxidation of vitamin K in Reaction 1, an amino acid residue is carboxylated and results in the following structure: What was the most likely initial amino acid residue?</strong> A)Aspartic acid B)Asparagine C)Glutamine D)Glutamic acid](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_5ae4_a3bf_119cf7513bca_MD0008_00.jpg) Figure 2 Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K1 and a trans-epoxy vitamin K1, as shown in Figure 3.
Figure 2 Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K1 and a trans-epoxy vitamin K1, as shown in Figure 3.![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. During the oxidation of vitamin K in Reaction 1, an amino acid residue is carboxylated and results in the following structure: What was the most likely initial amino acid residue?</strong> A)Aspartic acid B)Asparagine C)Glutamine D)Glutamic acid](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_5ae5_a3bf_fb8c7e084374_MD0008_00.jpg) Figure 3 Results of HPLC separation
Figure 3 Results of HPLC separationAdapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995.
During the oxidation of vitamin K in Reaction 1, an amino acid residue is carboxylated and results in the following structure:
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. During the oxidation of vitamin K in Reaction 1, an amino acid residue is carboxylated and results in the following structure: What was the most likely initial amino acid residue?</strong> A)Aspartic acid B)Asparagine C)Glutamine D)Glutamic acid](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1256_8f3b_a3bf_ab3ccbe33395_MD0008_00.jpg) What was the most likely initial amino acid residue?
What was the most likely initial amino acid residue?A)Aspartic acid
B)Asparagine
C)Glutamine
D)Glutamic acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
9
Passage
Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1.
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. In the oxidation of vitamin K hydroquinone to vitamin K 2,3-epoxide, at least one carbonyl group is formed. Compared to the original carbon-oxygen bond, the newly formed bond is characterized by:</strong> A)more direct orbital localization. B)longer total bond length. C)overlapping p orbitals. D)having no effect on rotation.](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_33d3_a3bf_614cec085188_MD0008_00.jpg) Figure 1 Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2.
Figure 1 Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2.
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. In the oxidation of vitamin K hydroquinone to vitamin K 2,3-epoxide, at least one carbonyl group is formed. Compared to the original carbon-oxygen bond, the newly formed bond is characterized by:</strong> A)more direct orbital localization. B)longer total bond length. C)overlapping p orbitals. D)having no effect on rotation.](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_5ae4_a3bf_119cf7513bca_MD0008_00.jpg) Figure 2 Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K1 and a trans-epoxy vitamin K1, as shown in Figure 3.
Figure 2 Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K1 and a trans-epoxy vitamin K1, as shown in Figure 3.
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. In the oxidation of vitamin K hydroquinone to vitamin K 2,3-epoxide, at least one carbonyl group is formed. Compared to the original carbon-oxygen bond, the newly formed bond is characterized by:</strong> A)more direct orbital localization. B)longer total bond length. C)overlapping p orbitals. D)having no effect on rotation.](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_5ae5_a3bf_fb8c7e084374_MD0008_00.jpg) Figure 3 Results of HPLC separation
Figure 3 Results of HPLC separation
Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995.
In the oxidation of vitamin K hydroquinone to vitamin K 2,3-epoxide, at least one carbonyl group is formed. Compared to the original carbon-oxygen bond, the newly formed bond is characterized by:
A)more direct orbital localization.
B)longer total bond length.
C)overlapping p orbitals.
D)having no effect on rotation.
Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1.
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. In the oxidation of vitamin K hydroquinone to vitamin K 2,3-epoxide, at least one carbonyl group is formed. Compared to the original carbon-oxygen bond, the newly formed bond is characterized by:</strong> A)more direct orbital localization. B)longer total bond length. C)overlapping p orbitals. D)having no effect on rotation.](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_33d3_a3bf_614cec085188_MD0008_00.jpg) Figure 1 Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2.
Figure 1 Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2.![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. In the oxidation of vitamin K hydroquinone to vitamin K 2,3-epoxide, at least one carbonyl group is formed. Compared to the original carbon-oxygen bond, the newly formed bond is characterized by:</strong> A)more direct orbital localization. B)longer total bond length. C)overlapping p orbitals. D)having no effect on rotation.](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_5ae4_a3bf_119cf7513bca_MD0008_00.jpg) Figure 2 Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K1 and a trans-epoxy vitamin K1, as shown in Figure 3.
Figure 2 Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K1 and a trans-epoxy vitamin K1, as shown in Figure 3.![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. In the oxidation of vitamin K hydroquinone to vitamin K 2,3-epoxide, at least one carbonyl group is formed. Compared to the original carbon-oxygen bond, the newly formed bond is characterized by:</strong> A)more direct orbital localization. B)longer total bond length. C)overlapping p orbitals. D)having no effect on rotation.](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_5ae5_a3bf_fb8c7e084374_MD0008_00.jpg) Figure 3 Results of HPLC separation
Figure 3 Results of HPLC separationAdapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995.
In the oxidation of vitamin K hydroquinone to vitamin K 2,3-epoxide, at least one carbonyl group is formed. Compared to the original carbon-oxygen bond, the newly formed bond is characterized by:
A)more direct orbital localization.
B)longer total bond length.
C)overlapping p orbitals.
D)having no effect on rotation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
10
Passage
The drug paracetamol, also known as acetaminophen, is used to relieve pain and fever, and is a metabolite of the antipyretic drug phenacetin (Figure 1). These two drugs have similar medicinal properties, but phenacetin has been shown to be carcinogenic and cause kidney damage. Acetaminophen is a safer alternative to phenacetin when taken in therapeutic doses.
 Figure 1 Structure of phenacetinA student synthesizes acetaminophen in a laboratory from phenol according to the reactions shown. The synthesis begins with the nitration of phenol by sodium nitrate (NaNO3) to produce the mixture of the isomers 2-nitrophenol and 4-nitrophenol (Reaction 1). These isomers can be easily separated by steam distillation to isolate the desired product, 4-nitrophenol. Reduction of the nitro group with sodium borohydride (NaBH4) (Reaction 2) is followed by acetylation of the amine with acetic anhydride (Reaction 3) to afford the final product, acetaminophen.
Figure 1 Structure of phenacetinA student synthesizes acetaminophen in a laboratory from phenol according to the reactions shown. The synthesis begins with the nitration of phenol by sodium nitrate (NaNO3) to produce the mixture of the isomers 2-nitrophenol and 4-nitrophenol (Reaction 1). These isomers can be easily separated by steam distillation to isolate the desired product, 4-nitrophenol. Reduction of the nitro group with sodium borohydride (NaBH4) (Reaction 2) is followed by acetylation of the amine with acetic anhydride (Reaction 3) to afford the final product, acetaminophen.
 Reaction 1
Reaction 1
 Reaction 2
Reaction 2
 Reaction 3
Reaction 3
During distillation, superheating of the reaction mixture should be avoided. Which of the following would prevent superheating?
A)Adding boiling chips to the distillation flask
B)Increasing the rate at which the distillation flask is heated
C)Using cold water in the condenser
D)Heating the distillation flask under reduced pressure
The drug paracetamol, also known as acetaminophen, is used to relieve pain and fever, and is a metabolite of the antipyretic drug phenacetin (Figure 1). These two drugs have similar medicinal properties, but phenacetin has been shown to be carcinogenic and cause kidney damage. Acetaminophen is a safer alternative to phenacetin when taken in therapeutic doses.
 Figure 1 Structure of phenacetinA student synthesizes acetaminophen in a laboratory from phenol according to the reactions shown. The synthesis begins with the nitration of phenol by sodium nitrate (NaNO3) to produce the mixture of the isomers 2-nitrophenol and 4-nitrophenol (Reaction 1). These isomers can be easily separated by steam distillation to isolate the desired product, 4-nitrophenol. Reduction of the nitro group with sodium borohydride (NaBH4) (Reaction 2) is followed by acetylation of the amine with acetic anhydride (Reaction 3) to afford the final product, acetaminophen.
Figure 1 Structure of phenacetinA student synthesizes acetaminophen in a laboratory from phenol according to the reactions shown. The synthesis begins with the nitration of phenol by sodium nitrate (NaNO3) to produce the mixture of the isomers 2-nitrophenol and 4-nitrophenol (Reaction 1). These isomers can be easily separated by steam distillation to isolate the desired product, 4-nitrophenol. Reduction of the nitro group with sodium borohydride (NaBH4) (Reaction 2) is followed by acetylation of the amine with acetic anhydride (Reaction 3) to afford the final product, acetaminophen. Reaction 1
Reaction 1 Reaction 2
Reaction 2 Reaction 3
Reaction 3During distillation, superheating of the reaction mixture should be avoided. Which of the following would prevent superheating?
A)Adding boiling chips to the distillation flask
B)Increasing the rate at which the distillation flask is heated
C)Using cold water in the condenser
D)Heating the distillation flask under reduced pressure

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
11
Passage
A limited number of cellular functions exist for D-amino acids, such as the structural role of D-alanine and D-glutamate in bacterial cell walls. Because genetically encoded amino acids are synthesized in the L form, production of D-amino acids depends on enzymes called racemases. With the exception of cysteine, conversion of an L-amino acid to a D-amino acid corresponds to the conversion of an S stereoisomer to an R stereoisomer. The mechanism of conversion requires the formation of a high-energy carbanion intermediate. Although pyridoxal phosphate (PLP)-dependent amino acid racemases such as glutamate racemase use PLP as a coenzyme to stabilize this intermediate, the reaction catalyzed by PLP-independent racemases such as alanine racemase proceeds through the enolate intermediate shown in Figure 1.
 Figure 1 PLP-independent amino acid racemase reactionThe first step in most PLP-dependent reactions is the condensation of an aldehyde group on the coenzyme PLP. This forms a Schiff base linking PLP to a lysine side chain on the enzyme's active site. The lysine is then substituted with the amino acid to be converted from L to D configuration (Figure 2).
Figure 1 PLP-independent amino acid racemase reactionThe first step in most PLP-dependent reactions is the condensation of an aldehyde group on the coenzyme PLP. This forms a Schiff base linking PLP to a lysine side chain on the enzyme's active site. The lysine is then substituted with the amino acid to be converted from L to D configuration (Figure 2).
 Figure 2 Formation of an amino acid-PLP adductThe next step in PLP-dependent reactions is the removal of the amino acid's α-hydrogen by a base in the enzyme's active site. A quinonoid intermediate and two additional intermediates are formed, as shown in Figure 3. The final step includes the rebonding of PLP to the enzyme's active site and the release of the D-amino acid.
Figure 2 Formation of an amino acid-PLP adductThe next step in PLP-dependent reactions is the removal of the amino acid's α-hydrogen by a base in the enzyme's active site. A quinonoid intermediate and two additional intermediates are formed, as shown in Figure 3. The final step includes the rebonding of PLP to the enzyme's active site and the release of the D-amino acid.
 Figure 3 Intermediates of a PLP-dependent amino acid racemase-catalyzed reactionOther PLP-dependent enzymes participate in the decarboxylation of amino acids via a similar mechanism. Such enzymes break the bond between the carboxylate carbon and α-carbon in an amino acid by forming a quinonoid intermediate, which helps stabilize the carbanion intermediate of the reaction.
Figure 3 Intermediates of a PLP-dependent amino acid racemase-catalyzed reactionOther PLP-dependent enzymes participate in the decarboxylation of amino acids via a similar mechanism. Such enzymes break the bond between the carboxylate carbon and α-carbon in an amino acid by forming a quinonoid intermediate, which helps stabilize the carbanion intermediate of the reaction.
Adapted from Cava F, Lam H, De pedro MA, Waldor MK. Emerging knowledge of regulatory roles of D-amino acids in bacteria. Cell Mol Life Sci. 2011.
The Schiff base shown in Figure 2 involves the formation of what nitrogen-containing functional group?
A)Amide
B)Enamine
C)Imide
D)Imine
A limited number of cellular functions exist for D-amino acids, such as the structural role of D-alanine and D-glutamate in bacterial cell walls. Because genetically encoded amino acids are synthesized in the L form, production of D-amino acids depends on enzymes called racemases. With the exception of cysteine, conversion of an L-amino acid to a D-amino acid corresponds to the conversion of an S stereoisomer to an R stereoisomer. The mechanism of conversion requires the formation of a high-energy carbanion intermediate. Although pyridoxal phosphate (PLP)-dependent amino acid racemases such as glutamate racemase use PLP as a coenzyme to stabilize this intermediate, the reaction catalyzed by PLP-independent racemases such as alanine racemase proceeds through the enolate intermediate shown in Figure 1.
 Figure 1 PLP-independent amino acid racemase reactionThe first step in most PLP-dependent reactions is the condensation of an aldehyde group on the coenzyme PLP. This forms a Schiff base linking PLP to a lysine side chain on the enzyme's active site. The lysine is then substituted with the amino acid to be converted from L to D configuration (Figure 2).
Figure 1 PLP-independent amino acid racemase reactionThe first step in most PLP-dependent reactions is the condensation of an aldehyde group on the coenzyme PLP. This forms a Schiff base linking PLP to a lysine side chain on the enzyme's active site. The lysine is then substituted with the amino acid to be converted from L to D configuration (Figure 2). Figure 2 Formation of an amino acid-PLP adductThe next step in PLP-dependent reactions is the removal of the amino acid's α-hydrogen by a base in the enzyme's active site. A quinonoid intermediate and two additional intermediates are formed, as shown in Figure 3. The final step includes the rebonding of PLP to the enzyme's active site and the release of the D-amino acid.
Figure 2 Formation of an amino acid-PLP adductThe next step in PLP-dependent reactions is the removal of the amino acid's α-hydrogen by a base in the enzyme's active site. A quinonoid intermediate and two additional intermediates are formed, as shown in Figure 3. The final step includes the rebonding of PLP to the enzyme's active site and the release of the D-amino acid. Figure 3 Intermediates of a PLP-dependent amino acid racemase-catalyzed reactionOther PLP-dependent enzymes participate in the decarboxylation of amino acids via a similar mechanism. Such enzymes break the bond between the carboxylate carbon and α-carbon in an amino acid by forming a quinonoid intermediate, which helps stabilize the carbanion intermediate of the reaction.
Figure 3 Intermediates of a PLP-dependent amino acid racemase-catalyzed reactionOther PLP-dependent enzymes participate in the decarboxylation of amino acids via a similar mechanism. Such enzymes break the bond between the carboxylate carbon and α-carbon in an amino acid by forming a quinonoid intermediate, which helps stabilize the carbanion intermediate of the reaction.Adapted from Cava F, Lam H, De pedro MA, Waldor MK. Emerging knowledge of regulatory roles of D-amino acids in bacteria. Cell Mol Life Sci. 2011.
The Schiff base shown in Figure 2 involves the formation of what nitrogen-containing functional group?
A)Amide
B)Enamine
C)Imide
D)Imine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
12
Passage
The drug paracetamol, also known as acetaminophen, is used to relieve pain and fever, and is a metabolite of the antipyretic drug phenacetin (Figure 1). These two drugs have similar medicinal properties, but phenacetin has been shown to be carcinogenic and cause kidney damage. Acetaminophen is a safer alternative to phenacetin when taken in therapeutic doses.
 Figure 1 Structure of phenacetinA student synthesizes acetaminophen in a laboratory from phenol according to the reactions shown. The synthesis begins with the nitration of phenol by sodium nitrate (NaNO3) to produce the mixture of the isomers 2-nitrophenol and 4-nitrophenol (Reaction 1). These isomers can be easily separated by steam distillation to isolate the desired product, 4-nitrophenol. Reduction of the nitro group with sodium borohydride (NaBH4) (Reaction 2) is followed by acetylation of the amine with acetic anhydride (Reaction 3) to afford the final product, acetaminophen.
Figure 1 Structure of phenacetinA student synthesizes acetaminophen in a laboratory from phenol according to the reactions shown. The synthesis begins with the nitration of phenol by sodium nitrate (NaNO3) to produce the mixture of the isomers 2-nitrophenol and 4-nitrophenol (Reaction 1). These isomers can be easily separated by steam distillation to isolate the desired product, 4-nitrophenol. Reduction of the nitro group with sodium borohydride (NaBH4) (Reaction 2) is followed by acetylation of the amine with acetic anhydride (Reaction 3) to afford the final product, acetaminophen.
 Reaction 1
Reaction 1
 Reaction 2
Reaction 2
 Reaction 3
Reaction 3
Based on the structure of acetaminophen shown in the passage, in which region of the infrared spectrum will it most likely show a strong absorption?
A)1690-1650 cm−1
B)2150-2100 cm−1
C)2260-2240 cm−1
D)2850-2750 cm−1
The drug paracetamol, also known as acetaminophen, is used to relieve pain and fever, and is a metabolite of the antipyretic drug phenacetin (Figure 1). These two drugs have similar medicinal properties, but phenacetin has been shown to be carcinogenic and cause kidney damage. Acetaminophen is a safer alternative to phenacetin when taken in therapeutic doses.
 Figure 1 Structure of phenacetinA student synthesizes acetaminophen in a laboratory from phenol according to the reactions shown. The synthesis begins with the nitration of phenol by sodium nitrate (NaNO3) to produce the mixture of the isomers 2-nitrophenol and 4-nitrophenol (Reaction 1). These isomers can be easily separated by steam distillation to isolate the desired product, 4-nitrophenol. Reduction of the nitro group with sodium borohydride (NaBH4) (Reaction 2) is followed by acetylation of the amine with acetic anhydride (Reaction 3) to afford the final product, acetaminophen.
Figure 1 Structure of phenacetinA student synthesizes acetaminophen in a laboratory from phenol according to the reactions shown. The synthesis begins with the nitration of phenol by sodium nitrate (NaNO3) to produce the mixture of the isomers 2-nitrophenol and 4-nitrophenol (Reaction 1). These isomers can be easily separated by steam distillation to isolate the desired product, 4-nitrophenol. Reduction of the nitro group with sodium borohydride (NaBH4) (Reaction 2) is followed by acetylation of the amine with acetic anhydride (Reaction 3) to afford the final product, acetaminophen. Reaction 1
Reaction 1 Reaction 2
Reaction 2 Reaction 3
Reaction 3Based on the structure of acetaminophen shown in the passage, in which region of the infrared spectrum will it most likely show a strong absorption?
A)1690-1650 cm−1
B)2150-2100 cm−1
C)2260-2240 cm−1
D)2850-2750 cm−1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
13
Passage
High blood pressure, or hypertension, is a prevalent condition in the United States. One in three adults has hypertension, and approximately 20% do not know that they have it. If left untreated, high blood pressure can lead to major health issues, including stroke, heart attack, and kidney disease or failure. A number of drugs can help control hypertension, including those shown in Table 1. These drugs can be classified into different groups based on their mechanism of action.Table 1 Drugs Used to Treat High Blood Pressure
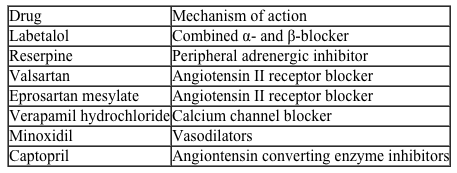 Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R), exhibit biological activity; the other two isomers, (S,S) and (R,S), are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker.
Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R), exhibit biological activity; the other two isomers, (S,S) and (R,S), are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker.
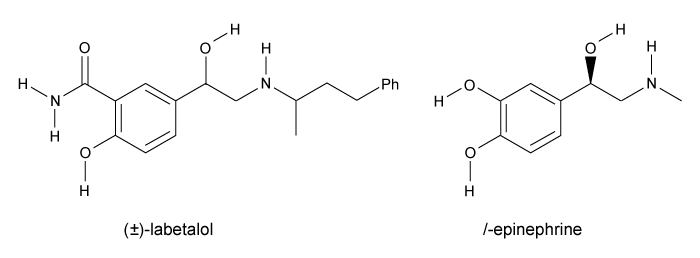 Figure 1 Structure of (±)-labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.
Figure 1 Structure of (±)-labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.
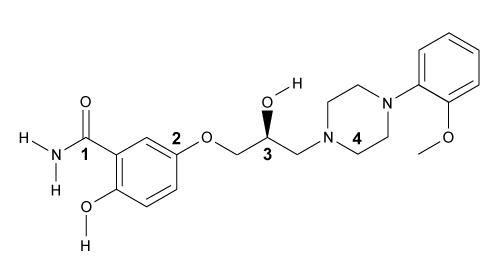 What position(s) on the analog of labetalol would be considered stereocenters?
What position(s) on the analog of labetalol would be considered stereocenters?
A)Positions 1 and 2 only
B)Position 2 only
C)Position 3 only
D)Positions 3 and 4 only
High blood pressure, or hypertension, is a prevalent condition in the United States. One in three adults has hypertension, and approximately 20% do not know that they have it. If left untreated, high blood pressure can lead to major health issues, including stroke, heart attack, and kidney disease or failure. A number of drugs can help control hypertension, including those shown in Table 1. These drugs can be classified into different groups based on their mechanism of action.Table 1 Drugs Used to Treat High Blood Pressure
 Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R), exhibit biological activity; the other two isomers, (S,S) and (R,S), are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker.
Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R), exhibit biological activity; the other two isomers, (S,S) and (R,S), are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker. Figure 1 Structure of (±)-labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.
Figure 1 Structure of (±)-labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors. What position(s) on the analog of labetalol would be considered stereocenters?
What position(s) on the analog of labetalol would be considered stereocenters?A)Positions 1 and 2 only
B)Position 2 only
C)Position 3 only
D)Positions 3 and 4 only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
14
Passage
A limited number of cellular functions exist for D-amino acids, such as the structural role of D-alanine and D-glutamate in bacterial cell walls. Because genetically encoded amino acids are synthesized in the L form, production of D-amino acids depends on enzymes called racemases. With the exception of cysteine, conversion of an L-amino acid to a D-amino acid corresponds to the conversion of an S stereoisomer to an R stereoisomer. The mechanism of conversion requires the formation of a high-energy carbanion intermediate. Although pyridoxal phosphate (PLP)-dependent amino acid racemases such as glutamate racemase use PLP as a coenzyme to stabilize this intermediate, the reaction catalyzed by PLP-independent racemases such as alanine racemase proceeds through the enolate intermediate shown in Figure 1.
 Figure 1 PLP-independent amino acid racemase reactionThe first step in most PLP-dependent reactions is the condensation of an aldehyde group on the coenzyme PLP. This forms a Schiff base linking PLP to a lysine side chain on the enzyme's active site. The lysine is then substituted with the amino acid to be converted from L to D configuration (Figure 2).
Figure 1 PLP-independent amino acid racemase reactionThe first step in most PLP-dependent reactions is the condensation of an aldehyde group on the coenzyme PLP. This forms a Schiff base linking PLP to a lysine side chain on the enzyme's active site. The lysine is then substituted with the amino acid to be converted from L to D configuration (Figure 2).
 Figure 2 Formation of an amino acid-PLP adductThe next step in PLP-dependent reactions is the removal of the amino acid's α-hydrogen by a base in the enzyme's active site. A quinonoid intermediate and two additional intermediates are formed, as shown in Figure 3. The final step includes the rebonding of PLP to the enzyme's active site and the release of the D-amino acid.
Figure 2 Formation of an amino acid-PLP adductThe next step in PLP-dependent reactions is the removal of the amino acid's α-hydrogen by a base in the enzyme's active site. A quinonoid intermediate and two additional intermediates are formed, as shown in Figure 3. The final step includes the rebonding of PLP to the enzyme's active site and the release of the D-amino acid.
 Figure 3 Intermediates of a PLP-dependent amino acid racemase-catalyzed reactionOther PLP-dependent enzymes participate in the decarboxylation of amino acids via a similar mechanism. Such enzymes break the bond between the carboxylate carbon and α-carbon in an amino acid by forming a quinonoid intermediate, which helps stabilize the carbanion intermediate of the reaction.
Figure 3 Intermediates of a PLP-dependent amino acid racemase-catalyzed reactionOther PLP-dependent enzymes participate in the decarboxylation of amino acids via a similar mechanism. Such enzymes break the bond between the carboxylate carbon and α-carbon in an amino acid by forming a quinonoid intermediate, which helps stabilize the carbanion intermediate of the reaction.
Adapted from Cava F, Lam H, De pedro MA, Waldor MK. Emerging knowledge of regulatory roles of D-amino acids in bacteria. Cell Mol Life Sci. 2011.
Which of the following structures is the substrate for the reaction catalyzed by glutamate racemase, whose product is incorporated into bacterial cell walls?
A)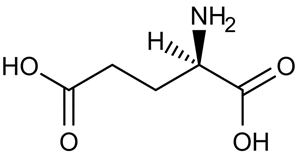
B)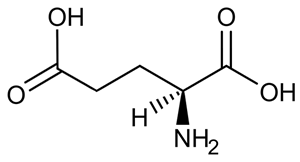
C)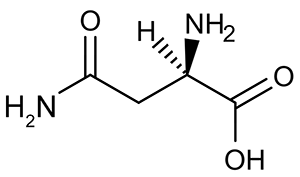
D)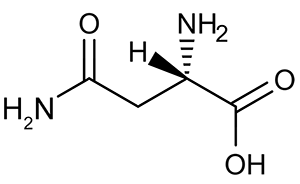
A limited number of cellular functions exist for D-amino acids, such as the structural role of D-alanine and D-glutamate in bacterial cell walls. Because genetically encoded amino acids are synthesized in the L form, production of D-amino acids depends on enzymes called racemases. With the exception of cysteine, conversion of an L-amino acid to a D-amino acid corresponds to the conversion of an S stereoisomer to an R stereoisomer. The mechanism of conversion requires the formation of a high-energy carbanion intermediate. Although pyridoxal phosphate (PLP)-dependent amino acid racemases such as glutamate racemase use PLP as a coenzyme to stabilize this intermediate, the reaction catalyzed by PLP-independent racemases such as alanine racemase proceeds through the enolate intermediate shown in Figure 1.
 Figure 1 PLP-independent amino acid racemase reactionThe first step in most PLP-dependent reactions is the condensation of an aldehyde group on the coenzyme PLP. This forms a Schiff base linking PLP to a lysine side chain on the enzyme's active site. The lysine is then substituted with the amino acid to be converted from L to D configuration (Figure 2).
Figure 1 PLP-independent amino acid racemase reactionThe first step in most PLP-dependent reactions is the condensation of an aldehyde group on the coenzyme PLP. This forms a Schiff base linking PLP to a lysine side chain on the enzyme's active site. The lysine is then substituted with the amino acid to be converted from L to D configuration (Figure 2). Figure 2 Formation of an amino acid-PLP adductThe next step in PLP-dependent reactions is the removal of the amino acid's α-hydrogen by a base in the enzyme's active site. A quinonoid intermediate and two additional intermediates are formed, as shown in Figure 3. The final step includes the rebonding of PLP to the enzyme's active site and the release of the D-amino acid.
Figure 2 Formation of an amino acid-PLP adductThe next step in PLP-dependent reactions is the removal of the amino acid's α-hydrogen by a base in the enzyme's active site. A quinonoid intermediate and two additional intermediates are formed, as shown in Figure 3. The final step includes the rebonding of PLP to the enzyme's active site and the release of the D-amino acid. Figure 3 Intermediates of a PLP-dependent amino acid racemase-catalyzed reactionOther PLP-dependent enzymes participate in the decarboxylation of amino acids via a similar mechanism. Such enzymes break the bond between the carboxylate carbon and α-carbon in an amino acid by forming a quinonoid intermediate, which helps stabilize the carbanion intermediate of the reaction.
Figure 3 Intermediates of a PLP-dependent amino acid racemase-catalyzed reactionOther PLP-dependent enzymes participate in the decarboxylation of amino acids via a similar mechanism. Such enzymes break the bond between the carboxylate carbon and α-carbon in an amino acid by forming a quinonoid intermediate, which helps stabilize the carbanion intermediate of the reaction.Adapted from Cava F, Lam H, De pedro MA, Waldor MK. Emerging knowledge of regulatory roles of D-amino acids in bacteria. Cell Mol Life Sci. 2011.
Which of the following structures is the substrate for the reaction catalyzed by glutamate racemase, whose product is incorporated into bacterial cell walls?
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
15
Passage
A limited number of cellular functions exist for D-amino acids, such as the structural role of D-alanine and D-glutamate in bacterial cell walls. Because genetically encoded amino acids are synthesized in the L form, production of D-amino acids depends on enzymes called racemases. With the exception of cysteine, conversion of an L-amino acid to a D-amino acid corresponds to the conversion of an S stereoisomer to an R stereoisomer. The mechanism of conversion requires the formation of a high-energy carbanion intermediate. Although pyridoxal phosphate (PLP)-dependent amino acid racemases such as glutamate racemase use PLP as a coenzyme to stabilize this intermediate, the reaction catalyzed by PLP-independent racemases such as alanine racemase proceeds through the enolate intermediate shown in Figure 1.
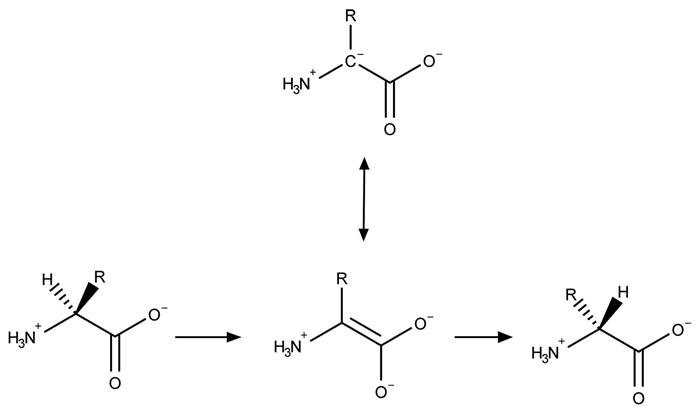 Figure 1 PLP-independent amino acid racemase reactionThe first step in most PLP-dependent reactions is the condensation of an aldehyde group on the coenzyme PLP. This forms a Schiff base linking PLP to a lysine side chain on the enzyme's active site. The lysine is then substituted with the amino acid to be converted from L to D configuration (Figure 2).
Figure 1 PLP-independent amino acid racemase reactionThe first step in most PLP-dependent reactions is the condensation of an aldehyde group on the coenzyme PLP. This forms a Schiff base linking PLP to a lysine side chain on the enzyme's active site. The lysine is then substituted with the amino acid to be converted from L to D configuration (Figure 2).
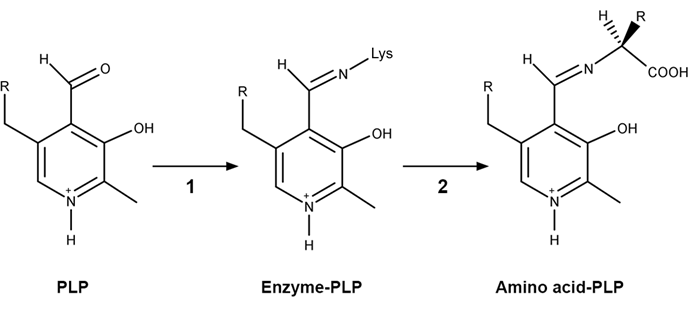 Figure 2 Formation of an amino acid-PLP adductThe next step in PLP-dependent reactions is the removal of the amino acid's α-hydrogen by a base in the enzyme's active site. A quinonoid intermediate and two additional intermediates are formed, as shown in Figure 3. The final step includes the rebonding of PLP to the enzyme's active site and the release of the D-amino acid.
Figure 2 Formation of an amino acid-PLP adductThe next step in PLP-dependent reactions is the removal of the amino acid's α-hydrogen by a base in the enzyme's active site. A quinonoid intermediate and two additional intermediates are formed, as shown in Figure 3. The final step includes the rebonding of PLP to the enzyme's active site and the release of the D-amino acid.
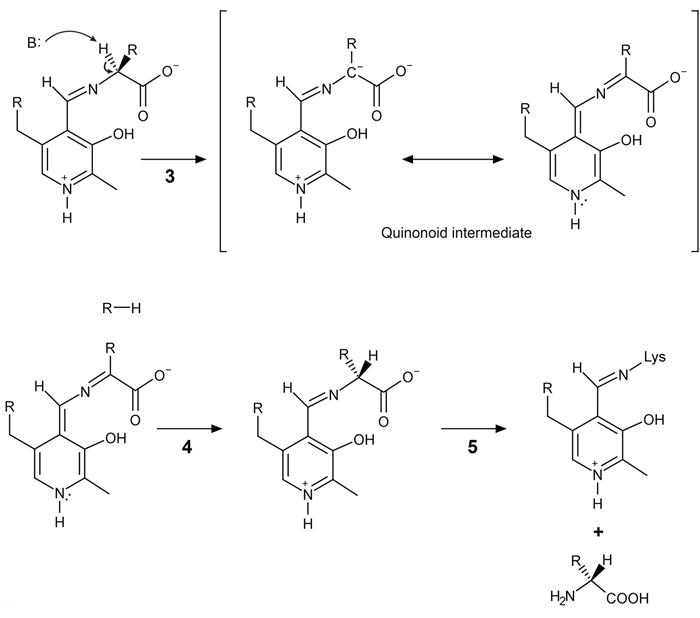 Figure 3 Intermediates of a PLP-dependent amino acid racemase-catalyzed reactionOther PLP-dependent enzymes participate in the decarboxylation of amino acids via a similar mechanism. Such enzymes break the bond between the carboxylate carbon and α-carbon in an amino acid by forming a quinonoid intermediate, which helps stabilize the carbanion intermediate of the reaction.
Figure 3 Intermediates of a PLP-dependent amino acid racemase-catalyzed reactionOther PLP-dependent enzymes participate in the decarboxylation of amino acids via a similar mechanism. Such enzymes break the bond between the carboxylate carbon and α-carbon in an amino acid by forming a quinonoid intermediate, which helps stabilize the carbanion intermediate of the reaction.
Adapted from Cava F, Lam H, De pedro MA, Waldor MK. Emerging knowledge of regulatory roles of D-amino acids in bacteria. Cell Mol Life Sci. 2011.
Which of the following is true regarding the relative reactivity of carbanion and carbocation species?
A)A primary carbanion is more stable than a tertiary carbanion.
B)A primary carbocation is more stable than a tertiary carbocation.
C)A tertiary carbanion is more stable than a primary carbanion.
D)Reactivity is independent of surrounding alkyl groups.
A limited number of cellular functions exist for D-amino acids, such as the structural role of D-alanine and D-glutamate in bacterial cell walls. Because genetically encoded amino acids are synthesized in the L form, production of D-amino acids depends on enzymes called racemases. With the exception of cysteine, conversion of an L-amino acid to a D-amino acid corresponds to the conversion of an S stereoisomer to an R stereoisomer. The mechanism of conversion requires the formation of a high-energy carbanion intermediate. Although pyridoxal phosphate (PLP)-dependent amino acid racemases such as glutamate racemase use PLP as a coenzyme to stabilize this intermediate, the reaction catalyzed by PLP-independent racemases such as alanine racemase proceeds through the enolate intermediate shown in Figure 1.
 Figure 1 PLP-independent amino acid racemase reactionThe first step in most PLP-dependent reactions is the condensation of an aldehyde group on the coenzyme PLP. This forms a Schiff base linking PLP to a lysine side chain on the enzyme's active site. The lysine is then substituted with the amino acid to be converted from L to D configuration (Figure 2).
Figure 1 PLP-independent amino acid racemase reactionThe first step in most PLP-dependent reactions is the condensation of an aldehyde group on the coenzyme PLP. This forms a Schiff base linking PLP to a lysine side chain on the enzyme's active site. The lysine is then substituted with the amino acid to be converted from L to D configuration (Figure 2). Figure 2 Formation of an amino acid-PLP adductThe next step in PLP-dependent reactions is the removal of the amino acid's α-hydrogen by a base in the enzyme's active site. A quinonoid intermediate and two additional intermediates are formed, as shown in Figure 3. The final step includes the rebonding of PLP to the enzyme's active site and the release of the D-amino acid.
Figure 2 Formation of an amino acid-PLP adductThe next step in PLP-dependent reactions is the removal of the amino acid's α-hydrogen by a base in the enzyme's active site. A quinonoid intermediate and two additional intermediates are formed, as shown in Figure 3. The final step includes the rebonding of PLP to the enzyme's active site and the release of the D-amino acid. Figure 3 Intermediates of a PLP-dependent amino acid racemase-catalyzed reactionOther PLP-dependent enzymes participate in the decarboxylation of amino acids via a similar mechanism. Such enzymes break the bond between the carboxylate carbon and α-carbon in an amino acid by forming a quinonoid intermediate, which helps stabilize the carbanion intermediate of the reaction.
Figure 3 Intermediates of a PLP-dependent amino acid racemase-catalyzed reactionOther PLP-dependent enzymes participate in the decarboxylation of amino acids via a similar mechanism. Such enzymes break the bond between the carboxylate carbon and α-carbon in an amino acid by forming a quinonoid intermediate, which helps stabilize the carbanion intermediate of the reaction.Adapted from Cava F, Lam H, De pedro MA, Waldor MK. Emerging knowledge of regulatory roles of D-amino acids in bacteria. Cell Mol Life Sci. 2011.
Which of the following is true regarding the relative reactivity of carbanion and carbocation species?
A)A primary carbanion is more stable than a tertiary carbanion.
B)A primary carbocation is more stable than a tertiary carbocation.
C)A tertiary carbanion is more stable than a primary carbanion.
D)Reactivity is independent of surrounding alkyl groups.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
16
Passage
The drug paracetamol, also known as acetaminophen, is used to relieve pain and fever, and is a metabolite of the antipyretic drug phenacetin (Figure 1). These two drugs have similar medicinal properties, but phenacetin has been shown to be carcinogenic and cause kidney damage. Acetaminophen is a safer alternative to phenacetin when taken in therapeutic doses.
 Figure 1 Structure of phenacetinA student synthesizes acetaminophen in a laboratory from phenol according to the reactions shown. The synthesis begins with the nitration of phenol by sodium nitrate (NaNO3) to produce the mixture of the isomers 2-nitrophenol and 4-nitrophenol (Reaction 1). These isomers can be easily separated by steam distillation to isolate the desired product, 4-nitrophenol. Reduction of the nitro group with sodium borohydride (NaBH4) (Reaction 2) is followed by acetylation of the amine with acetic anhydride (Reaction 3) to afford the final product, acetaminophen.
Figure 1 Structure of phenacetinA student synthesizes acetaminophen in a laboratory from phenol according to the reactions shown. The synthesis begins with the nitration of phenol by sodium nitrate (NaNO3) to produce the mixture of the isomers 2-nitrophenol and 4-nitrophenol (Reaction 1). These isomers can be easily separated by steam distillation to isolate the desired product, 4-nitrophenol. Reduction of the nitro group with sodium borohydride (NaBH4) (Reaction 2) is followed by acetylation of the amine with acetic anhydride (Reaction 3) to afford the final product, acetaminophen.
 Reaction 1
Reaction 1
 Reaction 2
Reaction 2
 Reaction 3
Reaction 3
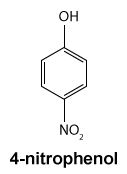 The compound 4-nitrophenol shown contains all of the following signals in its infrared spectrum EXCEPT:
The compound 4-nitrophenol shown contains all of the following signals in its infrared spectrum EXCEPT:
A)O-H stretch
B)C=C stretch
C)N-O stretch
D)N-H stretch
The drug paracetamol, also known as acetaminophen, is used to relieve pain and fever, and is a metabolite of the antipyretic drug phenacetin (Figure 1). These two drugs have similar medicinal properties, but phenacetin has been shown to be carcinogenic and cause kidney damage. Acetaminophen is a safer alternative to phenacetin when taken in therapeutic doses.
 Figure 1 Structure of phenacetinA student synthesizes acetaminophen in a laboratory from phenol according to the reactions shown. The synthesis begins with the nitration of phenol by sodium nitrate (NaNO3) to produce the mixture of the isomers 2-nitrophenol and 4-nitrophenol (Reaction 1). These isomers can be easily separated by steam distillation to isolate the desired product, 4-nitrophenol. Reduction of the nitro group with sodium borohydride (NaBH4) (Reaction 2) is followed by acetylation of the amine with acetic anhydride (Reaction 3) to afford the final product, acetaminophen.
Figure 1 Structure of phenacetinA student synthesizes acetaminophen in a laboratory from phenol according to the reactions shown. The synthesis begins with the nitration of phenol by sodium nitrate (NaNO3) to produce the mixture of the isomers 2-nitrophenol and 4-nitrophenol (Reaction 1). These isomers can be easily separated by steam distillation to isolate the desired product, 4-nitrophenol. Reduction of the nitro group with sodium borohydride (NaBH4) (Reaction 2) is followed by acetylation of the amine with acetic anhydride (Reaction 3) to afford the final product, acetaminophen. Reaction 1
Reaction 1 Reaction 2
Reaction 2 Reaction 3
Reaction 3 The compound 4-nitrophenol shown contains all of the following signals in its infrared spectrum EXCEPT:
The compound 4-nitrophenol shown contains all of the following signals in its infrared spectrum EXCEPT:A)O-H stretch
B)C=C stretch
C)N-O stretch
D)N-H stretch

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
17
Passage
Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1.
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. In Reaction 1, what hybridization state changes do the methylated carbon atom in the ringed structure of vitamin K hydroquinone and the oxygen atom involved in epoxide formation undergo?</strong> A)sp<sup>2</sup> to sp<sup>3</sup> for the methylated carbon and sp<sup>2</sup> to sp<sup>3</sup> for the oxygen atom B)sp<sup>2</sup> to sp<sup>3</sup> for the methylated carbon and sp to sp<sup>2</sup> for the oxygen atom C)sp<sup>2</sup> to sp<sup>3</sup> for the methylated carbon and sp<sup>2</sup> to sp<sup>2</sup> for the oxygen atom D)sp to sp<sup>2</sup> for the methylated carbon and sp<sup>2</sup> to sp<sup>3</sup> for the oxygen atom](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_33d3_a3bf_614cec085188_MD0008_00.jpg) Figure 1 Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2.
Figure 1 Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2.
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. In Reaction 1, what hybridization state changes do the methylated carbon atom in the ringed structure of vitamin K hydroquinone and the oxygen atom involved in epoxide formation undergo?</strong> A)sp<sup>2</sup> to sp<sup>3</sup> for the methylated carbon and sp<sup>2</sup> to sp<sup>3</sup> for the oxygen atom B)sp<sup>2</sup> to sp<sup>3</sup> for the methylated carbon and sp to sp<sup>2</sup> for the oxygen atom C)sp<sup>2</sup> to sp<sup>3</sup> for the methylated carbon and sp<sup>2</sup> to sp<sup>2</sup> for the oxygen atom D)sp to sp<sup>2</sup> for the methylated carbon and sp<sup>2</sup> to sp<sup>3</sup> for the oxygen atom](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_5ae4_a3bf_119cf7513bca_MD0008_00.jpg) Figure 2 Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K1 and a trans-epoxy vitamin K1, as shown in Figure 3.
Figure 2 Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K1 and a trans-epoxy vitamin K1, as shown in Figure 3.
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. In Reaction 1, what hybridization state changes do the methylated carbon atom in the ringed structure of vitamin K hydroquinone and the oxygen atom involved in epoxide formation undergo?</strong> A)sp<sup>2</sup> to sp<sup>3</sup> for the methylated carbon and sp<sup>2</sup> to sp<sup>3</sup> for the oxygen atom B)sp<sup>2</sup> to sp<sup>3</sup> for the methylated carbon and sp to sp<sup>2</sup> for the oxygen atom C)sp<sup>2</sup> to sp<sup>3</sup> for the methylated carbon and sp<sup>2</sup> to sp<sup>2</sup> for the oxygen atom D)sp to sp<sup>2</sup> for the methylated carbon and sp<sup>2</sup> to sp<sup>3</sup> for the oxygen atom](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_5ae5_a3bf_fb8c7e084374_MD0008_00.jpg) Figure 3 Results of HPLC separation
Figure 3 Results of HPLC separation
Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995.
In Reaction 1, what hybridization state changes do the methylated carbon atom in the ringed structure of vitamin K hydroquinone and the oxygen atom involved in epoxide formation undergo?
A)sp2 to sp3 for the methylated carbon and sp2 to sp3 for the oxygen atom
B)sp2 to sp3 for the methylated carbon and sp to sp2 for the oxygen atom
C)sp2 to sp3 for the methylated carbon and sp2 to sp2 for the oxygen atom
D)sp to sp2 for the methylated carbon and sp2 to sp3 for the oxygen atom
Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1.
![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. In Reaction 1, what hybridization state changes do the methylated carbon atom in the ringed structure of vitamin K hydroquinone and the oxygen atom involved in epoxide formation undergo?</strong> A)sp<sup>2</sup> to sp<sup>3</sup> for the methylated carbon and sp<sup>2</sup> to sp<sup>3</sup> for the oxygen atom B)sp<sup>2</sup> to sp<sup>3</sup> for the methylated carbon and sp to sp<sup>2</sup> for the oxygen atom C)sp<sup>2</sup> to sp<sup>3</sup> for the methylated carbon and sp<sup>2</sup> to sp<sup>2</sup> for the oxygen atom D)sp to sp<sup>2</sup> for the methylated carbon and sp<sup>2</sup> to sp<sup>3</sup> for the oxygen atom](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_33d3_a3bf_614cec085188_MD0008_00.jpg) Figure 1 Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2.
Figure 1 Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2.![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. In Reaction 1, what hybridization state changes do the methylated carbon atom in the ringed structure of vitamin K hydroquinone and the oxygen atom involved in epoxide formation undergo?</strong> A)sp<sup>2</sup> to sp<sup>3</sup> for the methylated carbon and sp<sup>2</sup> to sp<sup>3</sup> for the oxygen atom B)sp<sup>2</sup> to sp<sup>3</sup> for the methylated carbon and sp to sp<sup>2</sup> for the oxygen atom C)sp<sup>2</sup> to sp<sup>3</sup> for the methylated carbon and sp<sup>2</sup> to sp<sup>2</sup> for the oxygen atom D)sp to sp<sup>2</sup> for the methylated carbon and sp<sup>2</sup> to sp<sup>3</sup> for the oxygen atom](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_5ae4_a3bf_119cf7513bca_MD0008_00.jpg) Figure 2 Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K1 and a trans-epoxy vitamin K1, as shown in Figure 3.
Figure 2 Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K1 and a trans-epoxy vitamin K1, as shown in Figure 3.![<strong>Passage Hemophilia B is a blood clotting disorder caused by a factor IX (FIX) deficiency. FIX is a 57-kDa, vitamin K-dependent protease that activates factor X, leading to the conversion of prothrombin to thrombin for propagation of the clotting cascade. Activated FIX has two major domains: a γ-carboxyglutamic acid domain and a serine protease domain. The γ-carboxyglutamic acid domain participates in the oxidation of vitamin K using metallic cofactors, as shown in Figure 1. <strong>Figure 1</strong> Oxidation of vitamin KTo further analyze the γ-carboxyglutamic acid-rich domain of FIX, an analogous synthetic peptide composed of matching residues 1 through 49 on FIX was evaluated by proton nuclear magnetic resonance (NMR) spectroscopy. Analysis of the proton chemical shift before the addition of metal ions suggested that the synthetic peptide contained normal structural elements. Large chemical shifts were observed after the addition of calcium and beryllium, as shown in Figure 2. <strong>Figure 2</strong> Results of NMR spectroscopy (tetramethylsilane [TMS] peak has been removed)The synthetic analog was then placed in solution with vitamin K hydroquinone and cofactors required for vitamin K oxidation. The oxidation products of vitamin K in Reactions 1 and 2 were collected and evaluated under high-performance liquid chromatography (HPLC) using hexane as the mobile phase. Analysis demonstrated cis and trans isomers of vitamin K<sub>1</sub> and a trans-epoxy vitamin K<sub>1</sub>, as shown in Figure 3. <strong>Figure 3</strong> Results of HPLC separation Adapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995. In Reaction 1, what hybridization state changes do the methylated carbon atom in the ringed structure of vitamin K hydroquinone and the oxygen atom involved in epoxide formation undergo?</strong> A)sp<sup>2</sup> to sp<sup>3</sup> for the methylated carbon and sp<sup>2</sup> to sp<sup>3</sup> for the oxygen atom B)sp<sup>2</sup> to sp<sup>3</sup> for the methylated carbon and sp to sp<sup>2</sup> for the oxygen atom C)sp<sup>2</sup> to sp<sup>3</sup> for the methylated carbon and sp<sup>2</sup> to sp<sup>2</sup> for the oxygen atom D)sp to sp<sup>2</sup> for the methylated carbon and sp<sup>2</sup> to sp<sup>3</sup> for the oxygen atom](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1253_5ae5_a3bf_fb8c7e084374_MD0008_00.jpg) Figure 3 Results of HPLC separation
Figure 3 Results of HPLC separationAdapted from Freedman SJ, Furie BC, Furie B, Baleja JD. Structure of the metal-free gamma-carboxyglutamic acid-rich membrane binding region of factor IX by two-dimensional NMR spectroscopy. J Biol Chem. 1995.
In Reaction 1, what hybridization state changes do the methylated carbon atom in the ringed structure of vitamin K hydroquinone and the oxygen atom involved in epoxide formation undergo?
A)sp2 to sp3 for the methylated carbon and sp2 to sp3 for the oxygen atom
B)sp2 to sp3 for the methylated carbon and sp to sp2 for the oxygen atom
C)sp2 to sp3 for the methylated carbon and sp2 to sp2 for the oxygen atom
D)sp to sp2 for the methylated carbon and sp2 to sp3 for the oxygen atom

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
18
Passage
High blood pressure, or hypertension, is a prevalent condition in the United States. One in three adults has hypertension, and approximately 20% do not know that they have it. If left untreated, high blood pressure can lead to major health issues, including stroke, heart attack, and kidney disease or failure. A number of drugs can help control hypertension, including those shown in Table 1. These drugs can be classified into different groups based on their mechanism of action.Table 1 Drugs Used to Treat High Blood Pressure
 Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R), exhibit biological activity; the other two isomers, (S,S) and (R,S), are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker.
Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R), exhibit biological activity; the other two isomers, (S,S) and (R,S), are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker.
 Figure 1 Structure of (±)-labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.
Figure 1 Structure of (±)-labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.
The structure of eprosartan mesylate is shown below. Double bonds 1 and 2, respectively, can be classified as: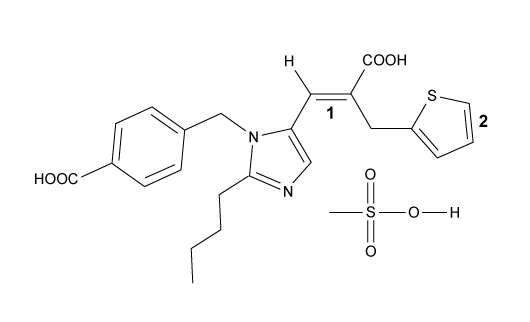
A)E for double bond 1 and cis for double bond 2
B)E for double bond 1 and trans for double bond 2
C)Z for double bond 1 and cis for double bond 2
D)Z for double bond 1 and trans for double bond 2
High blood pressure, or hypertension, is a prevalent condition in the United States. One in three adults has hypertension, and approximately 20% do not know that they have it. If left untreated, high blood pressure can lead to major health issues, including stroke, heart attack, and kidney disease or failure. A number of drugs can help control hypertension, including those shown in Table 1. These drugs can be classified into different groups based on their mechanism of action.Table 1 Drugs Used to Treat High Blood Pressure
 Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R), exhibit biological activity; the other two isomers, (S,S) and (R,S), are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker.
Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R), exhibit biological activity; the other two isomers, (S,S) and (R,S), are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker. Figure 1 Structure of (±)-labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.
Figure 1 Structure of (±)-labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.The structure of eprosartan mesylate is shown below. Double bonds 1 and 2, respectively, can be classified as:

A)E for double bond 1 and cis for double bond 2
B)E for double bond 1 and trans for double bond 2
C)Z for double bond 1 and cis for double bond 2
D)Z for double bond 1 and trans for double bond 2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
19
Passage
The drug paracetamol, also known as acetaminophen, is used to relieve pain and fever, and is a metabolite of the antipyretic drug phenacetin (Figure 1). These two drugs have similar medicinal properties, but phenacetin has been shown to be carcinogenic and cause kidney damage. Acetaminophen is a safer alternative to phenacetin when taken in therapeutic doses.
 Figure 1 Structure of phenacetinA student synthesizes acetaminophen in a laboratory from phenol according to the reactions shown. The synthesis begins with the nitration of phenol by sodium nitrate (NaNO3) to produce the mixture of the isomers 2-nitrophenol and 4-nitrophenol (Reaction 1). These isomers can be easily separated by steam distillation to isolate the desired product, 4-nitrophenol. Reduction of the nitro group with sodium borohydride (NaBH4) (Reaction 2) is followed by acetylation of the amine with acetic anhydride (Reaction 3) to afford the final product, acetaminophen.
Figure 1 Structure of phenacetinA student synthesizes acetaminophen in a laboratory from phenol according to the reactions shown. The synthesis begins with the nitration of phenol by sodium nitrate (NaNO3) to produce the mixture of the isomers 2-nitrophenol and 4-nitrophenol (Reaction 1). These isomers can be easily separated by steam distillation to isolate the desired product, 4-nitrophenol. Reduction of the nitro group with sodium borohydride (NaBH4) (Reaction 2) is followed by acetylation of the amine with acetic anhydride (Reaction 3) to afford the final product, acetaminophen.
 Reaction 1
Reaction 1
 Reaction 2
Reaction 2
 Reaction 3
Reaction 3
Which technique could monitor the formation of 4-aminophenol by excitation of electrons with high-energy photons?
A)Mass spectrometry
B)1H NMR spectroscopy
C)TLC with ultraviolet (UV) light
D)Gas chromatography
The drug paracetamol, also known as acetaminophen, is used to relieve pain and fever, and is a metabolite of the antipyretic drug phenacetin (Figure 1). These two drugs have similar medicinal properties, but phenacetin has been shown to be carcinogenic and cause kidney damage. Acetaminophen is a safer alternative to phenacetin when taken in therapeutic doses.
 Figure 1 Structure of phenacetinA student synthesizes acetaminophen in a laboratory from phenol according to the reactions shown. The synthesis begins with the nitration of phenol by sodium nitrate (NaNO3) to produce the mixture of the isomers 2-nitrophenol and 4-nitrophenol (Reaction 1). These isomers can be easily separated by steam distillation to isolate the desired product, 4-nitrophenol. Reduction of the nitro group with sodium borohydride (NaBH4) (Reaction 2) is followed by acetylation of the amine with acetic anhydride (Reaction 3) to afford the final product, acetaminophen.
Figure 1 Structure of phenacetinA student synthesizes acetaminophen in a laboratory from phenol according to the reactions shown. The synthesis begins with the nitration of phenol by sodium nitrate (NaNO3) to produce the mixture of the isomers 2-nitrophenol and 4-nitrophenol (Reaction 1). These isomers can be easily separated by steam distillation to isolate the desired product, 4-nitrophenol. Reduction of the nitro group with sodium borohydride (NaBH4) (Reaction 2) is followed by acetylation of the amine with acetic anhydride (Reaction 3) to afford the final product, acetaminophen. Reaction 1
Reaction 1 Reaction 2
Reaction 2 Reaction 3
Reaction 3Which technique could monitor the formation of 4-aminophenol by excitation of electrons with high-energy photons?
A)Mass spectrometry
B)1H NMR spectroscopy
C)TLC with ultraviolet (UV) light
D)Gas chromatography

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
20
Passage
A limited number of cellular functions exist for D-amino acids, such as the structural role of D-alanine and D-glutamate in bacterial cell walls. Because genetically encoded amino acids are synthesized in the L form, production of D-amino acids depends on enzymes called racemases. With the exception of cysteine, conversion of an L-amino acid to a D-amino acid corresponds to the conversion of an S stereoisomer to an R stereoisomer. The mechanism of conversion requires the formation of a high-energy carbanion intermediate. Although pyridoxal phosphate (PLP)-dependent amino acid racemases such as glutamate racemase use PLP as a coenzyme to stabilize this intermediate, the reaction catalyzed by PLP-independent racemases such as alanine racemase proceeds through the enolate intermediate shown in Figure 1.
 Figure 1 PLP-independent amino acid racemase reactionThe first step in most PLP-dependent reactions is the condensation of an aldehyde group on the coenzyme PLP. This forms a Schiff base linking PLP to a lysine side chain on the enzyme's active site. The lysine is then substituted with the amino acid to be converted from L to D configuration (Figure 2).
Figure 1 PLP-independent amino acid racemase reactionThe first step in most PLP-dependent reactions is the condensation of an aldehyde group on the coenzyme PLP. This forms a Schiff base linking PLP to a lysine side chain on the enzyme's active site. The lysine is then substituted with the amino acid to be converted from L to D configuration (Figure 2).
 Figure 2 Formation of an amino acid-PLP adductThe next step in PLP-dependent reactions is the removal of the amino acid's α-hydrogen by a base in the enzyme's active site. A quinonoid intermediate and two additional intermediates are formed, as shown in Figure 3. The final step includes the rebonding of PLP to the enzyme's active site and the release of the D-amino acid.
Figure 2 Formation of an amino acid-PLP adductThe next step in PLP-dependent reactions is the removal of the amino acid's α-hydrogen by a base in the enzyme's active site. A quinonoid intermediate and two additional intermediates are formed, as shown in Figure 3. The final step includes the rebonding of PLP to the enzyme's active site and the release of the D-amino acid.
 Figure 3 Intermediates of a PLP-dependent amino acid racemase-catalyzed reactionOther PLP-dependent enzymes participate in the decarboxylation of amino acids via a similar mechanism. Such enzymes break the bond between the carboxylate carbon and α-carbon in an amino acid by forming a quinonoid intermediate, which helps stabilize the carbanion intermediate of the reaction.
Figure 3 Intermediates of a PLP-dependent amino acid racemase-catalyzed reactionOther PLP-dependent enzymes participate in the decarboxylation of amino acids via a similar mechanism. Such enzymes break the bond between the carboxylate carbon and α-carbon in an amino acid by forming a quinonoid intermediate, which helps stabilize the carbanion intermediate of the reaction.
Adapted from Cava F, Lam H, De pedro MA, Waldor MK. Emerging knowledge of regulatory roles of D-amino acids in bacteria. Cell Mol Life Sci. 2011.
Compared to the enolate intermediate shown in Figure 1, the quinonoid intermediate in Figure 3 is:
A)less stable because of its greater number of resonance forms.
B)less stable because of its increased delocalization of electron density.
C)more stable because of its expanded network of conjugated pi bonds.
D)more stable because of its fewer number of resonance forms.
A limited number of cellular functions exist for D-amino acids, such as the structural role of D-alanine and D-glutamate in bacterial cell walls. Because genetically encoded amino acids are synthesized in the L form, production of D-amino acids depends on enzymes called racemases. With the exception of cysteine, conversion of an L-amino acid to a D-amino acid corresponds to the conversion of an S stereoisomer to an R stereoisomer. The mechanism of conversion requires the formation of a high-energy carbanion intermediate. Although pyridoxal phosphate (PLP)-dependent amino acid racemases such as glutamate racemase use PLP as a coenzyme to stabilize this intermediate, the reaction catalyzed by PLP-independent racemases such as alanine racemase proceeds through the enolate intermediate shown in Figure 1.
 Figure 1 PLP-independent amino acid racemase reactionThe first step in most PLP-dependent reactions is the condensation of an aldehyde group on the coenzyme PLP. This forms a Schiff base linking PLP to a lysine side chain on the enzyme's active site. The lysine is then substituted with the amino acid to be converted from L to D configuration (Figure 2).
Figure 1 PLP-independent amino acid racemase reactionThe first step in most PLP-dependent reactions is the condensation of an aldehyde group on the coenzyme PLP. This forms a Schiff base linking PLP to a lysine side chain on the enzyme's active site. The lysine is then substituted with the amino acid to be converted from L to D configuration (Figure 2). Figure 2 Formation of an amino acid-PLP adductThe next step in PLP-dependent reactions is the removal of the amino acid's α-hydrogen by a base in the enzyme's active site. A quinonoid intermediate and two additional intermediates are formed, as shown in Figure 3. The final step includes the rebonding of PLP to the enzyme's active site and the release of the D-amino acid.
Figure 2 Formation of an amino acid-PLP adductThe next step in PLP-dependent reactions is the removal of the amino acid's α-hydrogen by a base in the enzyme's active site. A quinonoid intermediate and two additional intermediates are formed, as shown in Figure 3. The final step includes the rebonding of PLP to the enzyme's active site and the release of the D-amino acid. Figure 3 Intermediates of a PLP-dependent amino acid racemase-catalyzed reactionOther PLP-dependent enzymes participate in the decarboxylation of amino acids via a similar mechanism. Such enzymes break the bond between the carboxylate carbon and α-carbon in an amino acid by forming a quinonoid intermediate, which helps stabilize the carbanion intermediate of the reaction.
Figure 3 Intermediates of a PLP-dependent amino acid racemase-catalyzed reactionOther PLP-dependent enzymes participate in the decarboxylation of amino acids via a similar mechanism. Such enzymes break the bond between the carboxylate carbon and α-carbon in an amino acid by forming a quinonoid intermediate, which helps stabilize the carbanion intermediate of the reaction.Adapted from Cava F, Lam H, De pedro MA, Waldor MK. Emerging knowledge of regulatory roles of D-amino acids in bacteria. Cell Mol Life Sci. 2011.
Compared to the enolate intermediate shown in Figure 1, the quinonoid intermediate in Figure 3 is:
A)less stable because of its greater number of resonance forms.
B)less stable because of its increased delocalization of electron density.
C)more stable because of its expanded network of conjugated pi bonds.
D)more stable because of its fewer number of resonance forms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
21
Passage
High blood pressure, or hypertension, is a prevalent condition in the United States. One in three adults has hypertension, and approximately 20% do not know that they have it. If left untreated, high blood pressure can lead to major health issues, including stroke, heart attack, and kidney disease or failure. A number of drugs can help control hypertension, including those shown in Table 1. These drugs can be classified into different groups based on their mechanism of action.Table 1 Drugs Used to Treat High Blood Pressure
 Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R), exhibit biological activity; the other two isomers, (S,S) and (R,S), are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker.
Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R), exhibit biological activity; the other two isomers, (S,S) and (R,S), are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker.
 Figure 1 Structure of (±)-labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.
Figure 1 Structure of (±)-labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.
Would (R,R)- and (S,S)-labetalol be expected to rotate plane-polarized light in the same direction?
A)Yes, because they are diastereomers.
B)Yes, because the rotations would have the same magnitude.
C)No, because they are enantiomers.
D)No, because the rotations would cancel out each other.
High blood pressure, or hypertension, is a prevalent condition in the United States. One in three adults has hypertension, and approximately 20% do not know that they have it. If left untreated, high blood pressure can lead to major health issues, including stroke, heart attack, and kidney disease or failure. A number of drugs can help control hypertension, including those shown in Table 1. These drugs can be classified into different groups based on their mechanism of action.Table 1 Drugs Used to Treat High Blood Pressure
 Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R), exhibit biological activity; the other two isomers, (S,S) and (R,S), are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker.
Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R), exhibit biological activity; the other two isomers, (S,S) and (R,S), are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker. Figure 1 Structure of (±)-labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.
Figure 1 Structure of (±)-labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.Would (R,R)- and (S,S)-labetalol be expected to rotate plane-polarized light in the same direction?
A)Yes, because they are diastereomers.
B)Yes, because the rotations would have the same magnitude.
C)No, because they are enantiomers.
D)No, because the rotations would cancel out each other.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
22
Passage
The cryptophycins, isolated from cyanobacteria, are a family of macrocyclic molecules. Researchers have studied the biological activity of these compounds and found that many of them are active against multi-drug-resistant tumor cells.Cryptophycin-52, an analogue of the natural cryptophycins, has been tested in clinical trials for its efficacy against cancer cells, but the studies were suspended due to neurotoxic side effects. Nevertheless, the synthesis of similar analogues continues to be of interest in cancer research. A key step in the synthesis of cryptophycin-52 is the stereospecific formation of an epoxide from an alkene and oxone (Figure 1), which can be facilitated by the Shi catalyst. The Shi catalyst (Figure 2) is derived from the carbohydrate D-fructose and is known among organic chemists for its ability to form epoxides in hydrocarbons in a stereospecific manner.
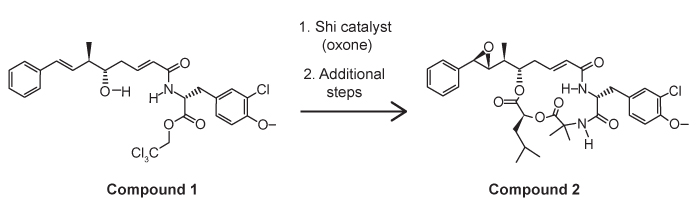 Figure 1 Shi epoxidation of compound 1
Figure 1 Shi epoxidation of compound 1
 Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC). When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3.
Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC). When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3.
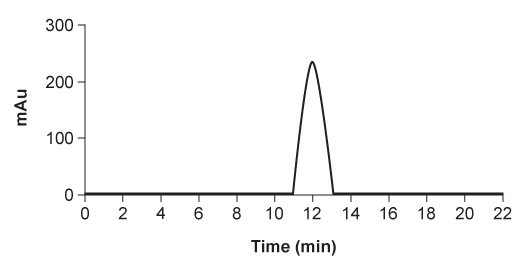 Figure 3 HPLC of epoxide standard
Figure 3 HPLC of epoxide standard
Adapted from Weiß C, Bogner T, Sammet B, Sewald N. Total synthesis and biological evaluation of fluorinated cryptophycins. Beilstein J Org Chem. 2012.
After isolation of compound 1, an infrared spectrum was obtained. The spectrum gives all of the following information EXCEPT:
A)the spin-spin splitting of atoms in a compound.
B)the signals corresponding to stretching vibrations and rotations.
C)the amount of light absorbed at a certain frequency.
D)the relative amount of energy needed to stretch a bond.
The cryptophycins, isolated from cyanobacteria, are a family of macrocyclic molecules. Researchers have studied the biological activity of these compounds and found that many of them are active against multi-drug-resistant tumor cells.Cryptophycin-52, an analogue of the natural cryptophycins, has been tested in clinical trials for its efficacy against cancer cells, but the studies were suspended due to neurotoxic side effects. Nevertheless, the synthesis of similar analogues continues to be of interest in cancer research. A key step in the synthesis of cryptophycin-52 is the stereospecific formation of an epoxide from an alkene and oxone (Figure 1), which can be facilitated by the Shi catalyst. The Shi catalyst (Figure 2) is derived from the carbohydrate D-fructose and is known among organic chemists for its ability to form epoxides in hydrocarbons in a stereospecific manner.
 Figure 1 Shi epoxidation of compound 1
Figure 1 Shi epoxidation of compound 1 Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC). When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3.
Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC). When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3. Figure 3 HPLC of epoxide standard
Figure 3 HPLC of epoxide standardAdapted from Weiß C, Bogner T, Sammet B, Sewald N. Total synthesis and biological evaluation of fluorinated cryptophycins. Beilstein J Org Chem. 2012.
After isolation of compound 1, an infrared spectrum was obtained. The spectrum gives all of the following information EXCEPT:
A)the spin-spin splitting of atoms in a compound.
B)the signals corresponding to stretching vibrations and rotations.
C)the amount of light absorbed at a certain frequency.
D)the relative amount of energy needed to stretch a bond.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
23
Passage
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1).
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
 Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
 Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Researchers produced ethylhexyl glycerin using a partially deuterated ethylhexyl halide and generated the product shown below.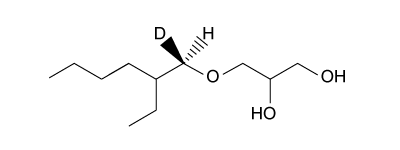 What was the configuration of the deuterated carbon prior to the reaction?
What was the configuration of the deuterated carbon prior to the reaction?
A)S, because the configuration is inverted by the reaction
B)R, because the configuration is inverted by the reaction
C)S, because the configuration is preserved by the reaction
D)R, because the configuration is preserved by the reaction
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1).
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2. Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.Researchers produced ethylhexyl glycerin using a partially deuterated ethylhexyl halide and generated the product shown below.
 What was the configuration of the deuterated carbon prior to the reaction?
What was the configuration of the deuterated carbon prior to the reaction?A)S, because the configuration is inverted by the reaction
B)R, because the configuration is inverted by the reaction
C)S, because the configuration is preserved by the reaction
D)R, because the configuration is preserved by the reaction

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
24
Passage
High blood pressure, or hypertension, is a prevalent condition in the United States. One in three adults has hypertension, and approximately 20% do not know that they have it. If left untreated, high blood pressure can lead to major health issues, including stroke, heart attack, and kidney disease or failure. A number of drugs can help control hypertension, including those shown in Table 1. These drugs can be classified into different groups based on their mechanism of action.Table 1 Drugs Used to Treat High Blood Pressure
 Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R), exhibit biological activity; the other two isomers, (S,S) and (R,S), are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker.
Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R), exhibit biological activity; the other two isomers, (S,S) and (R,S), are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker.
 Figure 1 Structure of (±)-labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.
Figure 1 Structure of (±)-labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.
The number of stereoisomers possible for a given molecule is 2n. The variable n denotes the number of:
A)enantiomers.
B)stereocenters.
C)diastereomers.
D)epimers.
High blood pressure, or hypertension, is a prevalent condition in the United States. One in three adults has hypertension, and approximately 20% do not know that they have it. If left untreated, high blood pressure can lead to major health issues, including stroke, heart attack, and kidney disease or failure. A number of drugs can help control hypertension, including those shown in Table 1. These drugs can be classified into different groups based on their mechanism of action.Table 1 Drugs Used to Treat High Blood Pressure
 Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R), exhibit biological activity; the other two isomers, (S,S) and (R,S), are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker.
Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R), exhibit biological activity; the other two isomers, (S,S) and (R,S), are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker. Figure 1 Structure of (±)-labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.
Figure 1 Structure of (±)-labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.The number of stereoisomers possible for a given molecule is 2n. The variable n denotes the number of:
A)enantiomers.
B)stereocenters.
C)diastereomers.
D)epimers.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
25
Passage
The cryptophycins, isolated from cyanobacteria, are a family of macrocyclic molecules. Researchers have studied the biological activity of these compounds and found that many of them are active against multi-drug-resistant tumor cells.Cryptophycin-52, an analogue of the natural cryptophycins, has been tested in clinical trials for its efficacy against cancer cells, but the studies were suspended due to neurotoxic side effects. Nevertheless, the synthesis of similar analogues continues to be of interest in cancer research. A key step in the synthesis of cryptophycin-52 is the stereospecific formation of an epoxide from an alkene and oxone (Figure 1), which can be facilitated by the Shi catalyst. The Shi catalyst (Figure 2) is derived from the carbohydrate D-fructose and is known among organic chemists for its ability to form epoxides in hydrocarbons in a stereospecific manner.
 Figure 1 Shi epoxidation of compound 1
Figure 1 Shi epoxidation of compound 1
 Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC). When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3.
Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC). When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3.
 Figure 3 HPLC of epoxide standard
Figure 3 HPLC of epoxide standard
Adapted from Weiß C, Bogner T, Sammet B, Sewald N. Total synthesis and biological evaluation of fluorinated cryptophycins. Beilstein J Org Chem. 2012.
Fructose for the synthesis of the Shi catalyst can be obtained by cleaving sucrose. What type of reaction occurs when sucrose is cleaved?
A)Condensation of a glycosidic bond
B)Condensation of a peptide bond
C)Hydrolysis of a peptide bond
D)Hydrolysis of a glycosidic bond
The cryptophycins, isolated from cyanobacteria, are a family of macrocyclic molecules. Researchers have studied the biological activity of these compounds and found that many of them are active against multi-drug-resistant tumor cells.Cryptophycin-52, an analogue of the natural cryptophycins, has been tested in clinical trials for its efficacy against cancer cells, but the studies were suspended due to neurotoxic side effects. Nevertheless, the synthesis of similar analogues continues to be of interest in cancer research. A key step in the synthesis of cryptophycin-52 is the stereospecific formation of an epoxide from an alkene and oxone (Figure 1), which can be facilitated by the Shi catalyst. The Shi catalyst (Figure 2) is derived from the carbohydrate D-fructose and is known among organic chemists for its ability to form epoxides in hydrocarbons in a stereospecific manner.
 Figure 1 Shi epoxidation of compound 1
Figure 1 Shi epoxidation of compound 1 Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC). When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3.
Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC). When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3. Figure 3 HPLC of epoxide standard
Figure 3 HPLC of epoxide standardAdapted from Weiß C, Bogner T, Sammet B, Sewald N. Total synthesis and biological evaluation of fluorinated cryptophycins. Beilstein J Org Chem. 2012.
Fructose for the synthesis of the Shi catalyst can be obtained by cleaving sucrose. What type of reaction occurs when sucrose is cleaved?
A)Condensation of a glycosidic bond
B)Condensation of a peptide bond
C)Hydrolysis of a peptide bond
D)Hydrolysis of a glycosidic bond

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
26
Passage
Aldehydes are organic compounds encountered in everyday life and used as intermediates in metabolism. Common aldehydes include formaldehyde, which is used in the fixation of tissues, and cinnamaldehyde, which provides the flavor and smell of cinnamon (Figure 1).
 Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde.
Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde.
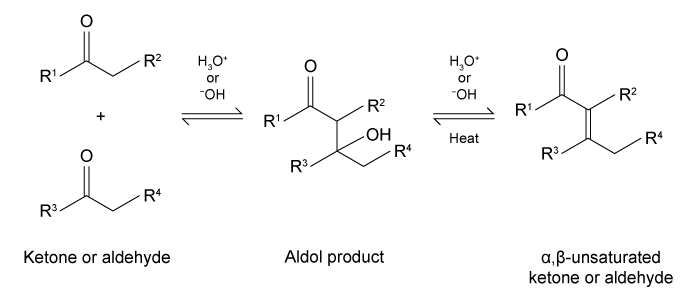 Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), respectively (Figure 3).
Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), respectively (Figure 3).
 Figure 3 Retro-aldol in glycolysis
Figure 3 Retro-aldol in glycolysis
Which of the following reagents CANNOT be used to make benzoic acid from benzaldehyde?
A)CrO3
B)KMnO4
C)H2CrO4
D)PCC
Aldehydes are organic compounds encountered in everyday life and used as intermediates in metabolism. Common aldehydes include formaldehyde, which is used in the fixation of tissues, and cinnamaldehyde, which provides the flavor and smell of cinnamon (Figure 1).
 Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde.
Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde. Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), respectively (Figure 3).
Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), respectively (Figure 3). Figure 3 Retro-aldol in glycolysis
Figure 3 Retro-aldol in glycolysisWhich of the following reagents CANNOT be used to make benzoic acid from benzaldehyde?
A)CrO3
B)KMnO4
C)H2CrO4
D)PCC

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
27
Passage
Aldehydes are organic compounds encountered in everyday life and used as intermediates in metabolism. Common aldehydes include formaldehyde, which is used in the fixation of tissues, and cinnamaldehyde, which provides the flavor and smell of cinnamon (Figure 1).
 Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde.
Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde.
 Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), respectively (Figure 3).
Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), respectively (Figure 3).
 Figure 3 Retro-aldol in glycolysis
Figure 3 Retro-aldol in glycolysis
Consider the reaction below.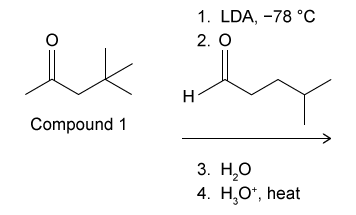 Which of the following would be the kinetic product if compound 1 undergoes an aldol condensation as described in Figure 2?
Which of the following would be the kinetic product if compound 1 undergoes an aldol condensation as described in Figure 2?
A)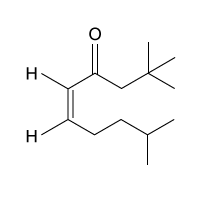
B)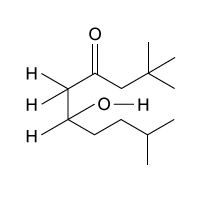
C)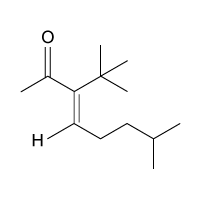
D)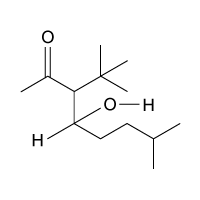
Aldehydes are organic compounds encountered in everyday life and used as intermediates in metabolism. Common aldehydes include formaldehyde, which is used in the fixation of tissues, and cinnamaldehyde, which provides the flavor and smell of cinnamon (Figure 1).
 Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde.
Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde. Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), respectively (Figure 3).
Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), respectively (Figure 3). Figure 3 Retro-aldol in glycolysis
Figure 3 Retro-aldol in glycolysisConsider the reaction below.
 Which of the following would be the kinetic product if compound 1 undergoes an aldol condensation as described in Figure 2?
Which of the following would be the kinetic product if compound 1 undergoes an aldol condensation as described in Figure 2?A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
28
Passage
Aldehydes are organic compounds encountered in everyday life and used as intermediates in metabolism. Common aldehydes include formaldehyde, which is used in the fixation of tissues, and cinnamaldehyde, which provides the flavor and smell of cinnamon (Figure 1).
 Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde.
Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde.
 Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), respectively (Figure 3).
Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), respectively (Figure 3).
 Figure 3 Retro-aldol in glycolysis
Figure 3 Retro-aldol in glycolysis
What is the product if the compound shown below undergoes a retro-aldol reaction?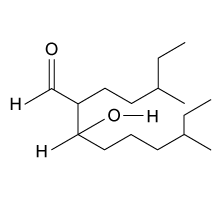
A)5-ethylhexanal
B)5-methylheptanal
C)5-ethylhexanone
D)5-methylheptanone
Aldehydes are organic compounds encountered in everyday life and used as intermediates in metabolism. Common aldehydes include formaldehyde, which is used in the fixation of tissues, and cinnamaldehyde, which provides the flavor and smell of cinnamon (Figure 1).
 Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde.
Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde. Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), respectively (Figure 3).
Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), respectively (Figure 3). Figure 3 Retro-aldol in glycolysis
Figure 3 Retro-aldol in glycolysisWhat is the product if the compound shown below undergoes a retro-aldol reaction?

A)5-ethylhexanal
B)5-methylheptanal
C)5-ethylhexanone
D)5-methylheptanone

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
29
Passage
The cryptophycins, isolated from cyanobacteria, are a family of macrocyclic molecules. Researchers have studied the biological activity of these compounds and found that many of them are active against multi-drug-resistant tumor cells.Cryptophycin-52, an analogue of the natural cryptophycins, has been tested in clinical trials for its efficacy against cancer cells, but the studies were suspended due to neurotoxic side effects. Nevertheless, the synthesis of similar analogues continues to be of interest in cancer research. A key step in the synthesis of cryptophycin-52 is the stereospecific formation of an epoxide from an alkene and oxone (Figure 1), which can be facilitated by the Shi catalyst. The Shi catalyst (Figure 2) is derived from the carbohydrate D-fructose and is known among organic chemists for its ability to form epoxides in hydrocarbons in a stereospecific manner.
 Figure 1 Shi epoxidation of compound 1
Figure 1 Shi epoxidation of compound 1
 Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC). When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3.
Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC). When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3.
 Figure 3 HPLC of epoxide standard
Figure 3 HPLC of epoxide standard
Adapted from Weiß C, Bogner T, Sammet B, Sewald N. Total synthesis and biological evaluation of fluorinated cryptophycins. Beilstein J Org Chem. 2012.
Ketoses such as fructose are expected to give a positive Tollens test because:
A)ketoses cannot mutarotate.
B)ketoses are not reducing sugars.
C)ketoses tautomerize to aldoses.
D)ketoses are not hemiacetals.
The cryptophycins, isolated from cyanobacteria, are a family of macrocyclic molecules. Researchers have studied the biological activity of these compounds and found that many of them are active against multi-drug-resistant tumor cells.Cryptophycin-52, an analogue of the natural cryptophycins, has been tested in clinical trials for its efficacy against cancer cells, but the studies were suspended due to neurotoxic side effects. Nevertheless, the synthesis of similar analogues continues to be of interest in cancer research. A key step in the synthesis of cryptophycin-52 is the stereospecific formation of an epoxide from an alkene and oxone (Figure 1), which can be facilitated by the Shi catalyst. The Shi catalyst (Figure 2) is derived from the carbohydrate D-fructose and is known among organic chemists for its ability to form epoxides in hydrocarbons in a stereospecific manner.
 Figure 1 Shi epoxidation of compound 1
Figure 1 Shi epoxidation of compound 1 Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC). When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3.
Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC). When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3. Figure 3 HPLC of epoxide standard
Figure 3 HPLC of epoxide standardAdapted from Weiß C, Bogner T, Sammet B, Sewald N. Total synthesis and biological evaluation of fluorinated cryptophycins. Beilstein J Org Chem. 2012.
Ketoses such as fructose are expected to give a positive Tollens test because:
A)ketoses cannot mutarotate.
B)ketoses are not reducing sugars.
C)ketoses tautomerize to aldoses.
D)ketoses are not hemiacetals.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
30
Passage
High blood pressure, or hypertension, is a prevalent condition in the United States. One in three adults has hypertension, and approximately 20% do not know that they have it. If left untreated, high blood pressure can lead to major health issues, including stroke, heart attack, and kidney disease or failure. A number of drugs can help control hypertension, including those shown in Table 1. These drugs can be classified into different groups based on their mechanism of action.Table 1 Drugs Used to Treat High Blood Pressure
 Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R), exhibit biological activity; the other two isomers, (S,S) and (R,S), are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker.
Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R), exhibit biological activity; the other two isomers, (S,S) and (R,S), are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker.
 Figure 1 Structure of (±)-labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.
Figure 1 Structure of (±)-labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.
(S,R)- and (R,R)-labetalol, the active forms of the drug, can be described as which of the following?DiastereomersEnantiomersConformational isomers
A)I only
B)I and II only
C)II and III only
D)I, II, and III
High blood pressure, or hypertension, is a prevalent condition in the United States. One in three adults has hypertension, and approximately 20% do not know that they have it. If left untreated, high blood pressure can lead to major health issues, including stroke, heart attack, and kidney disease or failure. A number of drugs can help control hypertension, including those shown in Table 1. These drugs can be classified into different groups based on their mechanism of action.Table 1 Drugs Used to Treat High Blood Pressure
 Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R), exhibit biological activity; the other two isomers, (S,S) and (R,S), are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker.
Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R), exhibit biological activity; the other two isomers, (S,S) and (R,S), are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker. Figure 1 Structure of (±)-labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.
Figure 1 Structure of (±)-labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.(S,R)- and (R,R)-labetalol, the active forms of the drug, can be described as which of the following?DiastereomersEnantiomersConformational isomers
A)I only
B)I and II only
C)II and III only
D)I, II, and III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
31
Passage
Aldehydes are organic compounds encountered in everyday life and used as intermediates in metabolism. Common aldehydes include formaldehyde, which is used in the fixation of tissues, and cinnamaldehyde, which provides the flavor and smell of cinnamon (Figure 1).
 Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde.
Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde.
 Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), respectively (Figure 3).
Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), respectively (Figure 3).
 Figure 3 Retro-aldol in glycolysis
Figure 3 Retro-aldol in glycolysis
Do aldehydes and ketones tend to have higher or lower boiling points than their corresponding alcohols?
A)Higher, because they are at a higher oxidation state
B)Higher, because they have a dipole moment
C)Lower, because they cannot form hydrogen bonds with each other
D)Lower, because they have increased intermolecular forces
Aldehydes are organic compounds encountered in everyday life and used as intermediates in metabolism. Common aldehydes include formaldehyde, which is used in the fixation of tissues, and cinnamaldehyde, which provides the flavor and smell of cinnamon (Figure 1).
 Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde.
Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde. Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), respectively (Figure 3).
Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), respectively (Figure 3). Figure 3 Retro-aldol in glycolysis
Figure 3 Retro-aldol in glycolysisDo aldehydes and ketones tend to have higher or lower boiling points than their corresponding alcohols?
A)Higher, because they are at a higher oxidation state
B)Higher, because they have a dipole moment
C)Lower, because they cannot form hydrogen bonds with each other
D)Lower, because they have increased intermolecular forces

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
32
Passage
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1).
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
 Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
 Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Acid helps catalyze the Fischer esterification reaction by doing all of the following EXCEPT:
A)enhancing the nucleophilicity of alcohols.
B)forming a stable carbocation intermediate.
C)creating a good leaving group.
D)increasing carbonyl electrophilicity.
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1).
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2. Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.Acid helps catalyze the Fischer esterification reaction by doing all of the following EXCEPT:
A)enhancing the nucleophilicity of alcohols.
B)forming a stable carbocation intermediate.
C)creating a good leaving group.
D)increasing carbonyl electrophilicity.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
33
Passage
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1).
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
 Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
 Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Why does the bond between glycerol and the ethylhexyl group tend to form at glycerol's C1 hydroxyl group instead of the C2 hydroxyl group?
A)Secondary alcohols are more likely to undergo SN1 reactions than primary alcohols.
B)Secondary alcohols are more sterically hindered than primary alcohols.
C)Primary alcohols are less acidic than secondary alcohols.
D)Primary alcohols are more electrophilic than secondary alcohols.
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1).
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2. Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.Why does the bond between glycerol and the ethylhexyl group tend to form at glycerol's C1 hydroxyl group instead of the C2 hydroxyl group?
A)Secondary alcohols are more likely to undergo SN1 reactions than primary alcohols.
B)Secondary alcohols are more sterically hindered than primary alcohols.
C)Primary alcohols are less acidic than secondary alcohols.
D)Primary alcohols are more electrophilic than secondary alcohols.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
34
Passage
The cryptophycins, isolated from cyanobacteria, are a family of macrocyclic molecules. Researchers have studied the biological activity of these compounds and found that many of them are active against multi-drug-resistant tumor cells.Cryptophycin-52, an analogue of the natural cryptophycins, has been tested in clinical trials for its efficacy against cancer cells, but the studies were suspended due to neurotoxic side effects. Nevertheless, the synthesis of similar analogues continues to be of interest in cancer research. A key step in the synthesis of cryptophycin-52 is the stereospecific formation of an epoxide from an alkene and oxone (Figure 1), which can be facilitated by the Shi catalyst. The Shi catalyst (Figure 2) is derived from the carbohydrate D-fructose and is known among organic chemists for its ability to form epoxides in hydrocarbons in a stereospecific manner.
 Figure 1 Shi epoxidation of compound 1
Figure 1 Shi epoxidation of compound 1
 Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC). When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3.
Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC). When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3.
 Figure 3 HPLC of epoxide standard
Figure 3 HPLC of epoxide standard
Adapted from Weiß C, Bogner T, Sammet B, Sewald N. Total synthesis and biological evaluation of fluorinated cryptophycins. Beilstein J Org Chem. 2012.
The HPLC analysis of the Shi epoxide revealed a 10:1 mixture of isomers in favor of the desired isomer. Based on the chromatogram shown in Figure 3, which of the following most likely depicts the chromatogram of the mixture?
A)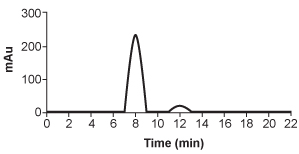
B)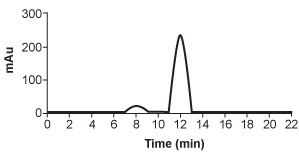
C)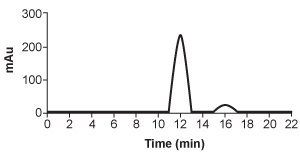
D)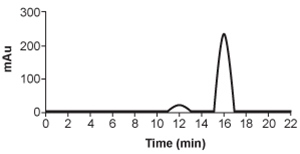
The cryptophycins, isolated from cyanobacteria, are a family of macrocyclic molecules. Researchers have studied the biological activity of these compounds and found that many of them are active against multi-drug-resistant tumor cells.Cryptophycin-52, an analogue of the natural cryptophycins, has been tested in clinical trials for its efficacy against cancer cells, but the studies were suspended due to neurotoxic side effects. Nevertheless, the synthesis of similar analogues continues to be of interest in cancer research. A key step in the synthesis of cryptophycin-52 is the stereospecific formation of an epoxide from an alkene and oxone (Figure 1), which can be facilitated by the Shi catalyst. The Shi catalyst (Figure 2) is derived from the carbohydrate D-fructose and is known among organic chemists for its ability to form epoxides in hydrocarbons in a stereospecific manner.
 Figure 1 Shi epoxidation of compound 1
Figure 1 Shi epoxidation of compound 1 Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC). When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3.
Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC). When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3. Figure 3 HPLC of epoxide standard
Figure 3 HPLC of epoxide standardAdapted from Weiß C, Bogner T, Sammet B, Sewald N. Total synthesis and biological evaluation of fluorinated cryptophycins. Beilstein J Org Chem. 2012.
The HPLC analysis of the Shi epoxide revealed a 10:1 mixture of isomers in favor of the desired isomer. Based on the chromatogram shown in Figure 3, which of the following most likely depicts the chromatogram of the mixture?
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
35
Passage
Aldehydes are organic compounds encountered in everyday life and used as intermediates in metabolism. Common aldehydes include formaldehyde, which is used in the fixation of tissues, and cinnamaldehyde, which provides the flavor and smell of cinnamon (Figure 1).
 Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde.
Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde.
 Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), respectively (Figure 3).
Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), respectively (Figure 3).
 Figure 3 Retro-aldol in glycolysis
Figure 3 Retro-aldol in glycolysis
In some synthesis reactions, the carbonyl functional group needs to be protected from nucleophilic attack. The reaction below of cinnamaldehyde with ethylene glycol shows the formation of which carbonyl-protecting group?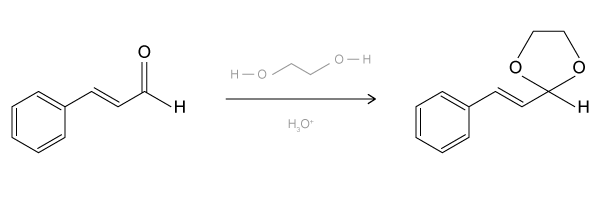
A)Acetal
B)Hemiacetal
C)Ketal
D)Hemiketal
Aldehydes are organic compounds encountered in everyday life and used as intermediates in metabolism. Common aldehydes include formaldehyde, which is used in the fixation of tissues, and cinnamaldehyde, which provides the flavor and smell of cinnamon (Figure 1).
 Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde.
Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde. Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), respectively (Figure 3).
Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), respectively (Figure 3). Figure 3 Retro-aldol in glycolysis
Figure 3 Retro-aldol in glycolysisIn some synthesis reactions, the carbonyl functional group needs to be protected from nucleophilic attack. The reaction below of cinnamaldehyde with ethylene glycol shows the formation of which carbonyl-protecting group?

A)Acetal
B)Hemiacetal
C)Ketal
D)Hemiketal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
36
Passage
The cryptophycins, isolated from cyanobacteria, are a family of macrocyclic molecules. Researchers have studied the biological activity of these compounds and found that many of them are active against multi-drug-resistant tumor cells.Cryptophycin-52, an analogue of the natural cryptophycins, has been tested in clinical trials for its efficacy against cancer cells, but the studies were suspended due to neurotoxic side effects. Nevertheless, the synthesis of similar analogues continues to be of interest in cancer research. A key step in the synthesis of cryptophycin-52 is the stereospecific formation of an epoxide from an alkene and oxone (Figure 1), which can be facilitated by the Shi catalyst. The Shi catalyst (Figure 2) is derived from the carbohydrate D-fructose and is known among organic chemists for its ability to form epoxides in hydrocarbons in a stereospecific manner.
 Figure 1 Shi epoxidation of compound 1
Figure 1 Shi epoxidation of compound 1
 Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC). When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3.
Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC). When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3.
 Figure 3 HPLC of epoxide standard
Figure 3 HPLC of epoxide standard
Adapted from Weiß C, Bogner T, Sammet B, Sewald N. Total synthesis and biological evaluation of fluorinated cryptophycins. Beilstein J Org Chem. 2012.
Which of the following functional groups will be reduced when LiAlH4 is added to Compound 1, the substrate for the Shi epoxidation described in the passage?
A)Carbon-carbon double bond
B)Ester
C)Alcohol
D)Aromatic ring
The cryptophycins, isolated from cyanobacteria, are a family of macrocyclic molecules. Researchers have studied the biological activity of these compounds and found that many of them are active against multi-drug-resistant tumor cells.Cryptophycin-52, an analogue of the natural cryptophycins, has been tested in clinical trials for its efficacy against cancer cells, but the studies were suspended due to neurotoxic side effects. Nevertheless, the synthesis of similar analogues continues to be of interest in cancer research. A key step in the synthesis of cryptophycin-52 is the stereospecific formation of an epoxide from an alkene and oxone (Figure 1), which can be facilitated by the Shi catalyst. The Shi catalyst (Figure 2) is derived from the carbohydrate D-fructose and is known among organic chemists for its ability to form epoxides in hydrocarbons in a stereospecific manner.
 Figure 1 Shi epoxidation of compound 1
Figure 1 Shi epoxidation of compound 1 Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC). When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3.
Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC). When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3. Figure 3 HPLC of epoxide standard
Figure 3 HPLC of epoxide standardAdapted from Weiß C, Bogner T, Sammet B, Sewald N. Total synthesis and biological evaluation of fluorinated cryptophycins. Beilstein J Org Chem. 2012.
Which of the following functional groups will be reduced when LiAlH4 is added to Compound 1, the substrate for the Shi epoxidation described in the passage?
A)Carbon-carbon double bond
B)Ester
C)Alcohol
D)Aromatic ring

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
37
Passage
Aldehydes are organic compounds encountered in everyday life and used as intermediates in metabolism. Common aldehydes include formaldehyde, which is used in the fixation of tissues, and cinnamaldehyde, which provides the flavor and smell of cinnamon (Figure 1).
 Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde.
Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde.
 Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), respectively (Figure 3).
Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), respectively (Figure 3).
 Figure 3 Retro-aldol in glycolysis
Figure 3 Retro-aldol in glycolysis
Which of the following compounds would be a plausible intermediate in the base-catalyzed aldol reaction of the first step of the citric acid cycle shown below?
A)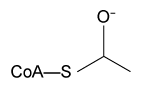
B)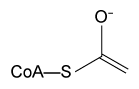
C)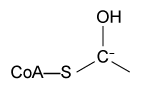
D)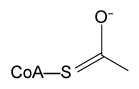
Aldehydes are organic compounds encountered in everyday life and used as intermediates in metabolism. Common aldehydes include formaldehyde, which is used in the fixation of tissues, and cinnamaldehyde, which provides the flavor and smell of cinnamon (Figure 1).
 Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde.
Figure 1 Common aldehydesThe aldol condensation shown in Figure 2 is an important carbon-carbon bond-forming reaction that has many applications in the synthesis of bioactive molecules. It also takes place in some metabolic processes, such as the citric acid cycle and gluconeogenesis. The aldol condensation begins with the aldol reaction, which can be acid- or base-catalyzed and requires nucleophilic addition to a carbonyl to provide the aldol product, which can be a β-hydroxy ketone or aldehyde. The final step of the aldol condensation consists of an acid- or base-catalyzed dehydration of the aldol product to yield an α,β-unsaturated ketone or aldehyde. Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), respectively (Figure 3).
Figure 2 General aldol reaction and condensationThe reverse of an aldol reaction or condensation is known as a retro-aldol, which is important in metabolism, most notably in glycolysis. This reaction breaks a carbon-carbon bond to form aldehydes and/or ketones. In glycolysis, fructose 1,6-bisphosphate is broken down into an aldehyde and a ketone, glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), respectively (Figure 3). Figure 3 Retro-aldol in glycolysis
Figure 3 Retro-aldol in glycolysisWhich of the following compounds would be a plausible intermediate in the base-catalyzed aldol reaction of the first step of the citric acid cycle shown below?

A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
38
Passage
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1).
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
 Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
 Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Ethylhexyl glycerin could be most efficiently synthesized from glycerol and which of the following?
A)
B)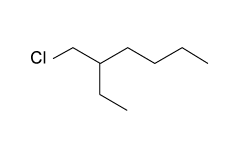
C)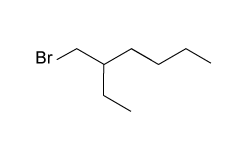
D)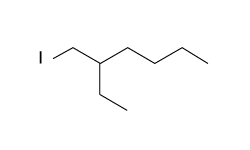
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1).
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2. Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.Ethylhexyl glycerin could be most efficiently synthesized from glycerol and which of the following?
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
39
Passage
The cryptophycins, isolated from cyanobacteria, are a family of macrocyclic molecules. Researchers have studied the biological activity of these compounds and found that many of them are active against multi-drug-resistant tumor cells.Cryptophycin-52, an analogue of the natural cryptophycins, has been tested in clinical trials for its efficacy against cancer cells, but the studies were suspended due to neurotoxic side effects. Nevertheless, the synthesis of similar analogues continues to be of interest in cancer research. A key step in the synthesis of cryptophycin-52 is the stereospecific formation of an epoxide from an alkene and oxone (Figure 1), which can be facilitated by the Shi catalyst. The Shi catalyst (Figure 2) is derived from the carbohydrate D-fructose and is known among organic chemists for its ability to form epoxides in hydrocarbons in a stereospecific manner.
 Figure 1 Shi epoxidation of compound 1
Figure 1 Shi epoxidation of compound 1
 Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC). When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3.
Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC). When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3.
 Figure 3 HPLC of epoxide standard
Figure 3 HPLC of epoxide standard
Adapted from Weiß C, Bogner T, Sammet B, Sewald N. Total synthesis and biological evaluation of fluorinated cryptophycins. Beilstein J Org Chem. 2012.
The Shi catalyst is derived from D-fructose based on the reaction below. The structure of D-fructose in the form shown below can be classified as: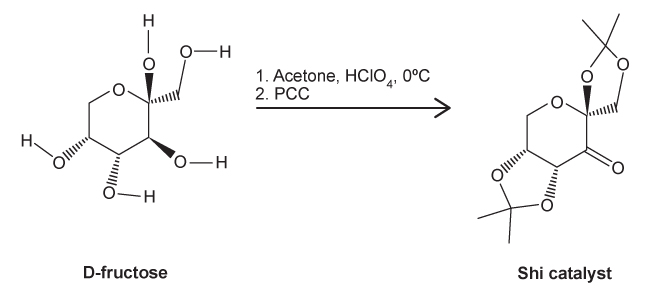 pyranose.furanose.ketohexose.aldohexose.
pyranose.furanose.ketohexose.aldohexose.
A)I and III only
B)I and IV only
C)II and III only
D)II and IV only
The cryptophycins, isolated from cyanobacteria, are a family of macrocyclic molecules. Researchers have studied the biological activity of these compounds and found that many of them are active against multi-drug-resistant tumor cells.Cryptophycin-52, an analogue of the natural cryptophycins, has been tested in clinical trials for its efficacy against cancer cells, but the studies were suspended due to neurotoxic side effects. Nevertheless, the synthesis of similar analogues continues to be of interest in cancer research. A key step in the synthesis of cryptophycin-52 is the stereospecific formation of an epoxide from an alkene and oxone (Figure 1), which can be facilitated by the Shi catalyst. The Shi catalyst (Figure 2) is derived from the carbohydrate D-fructose and is known among organic chemists for its ability to form epoxides in hydrocarbons in a stereospecific manner.
 Figure 1 Shi epoxidation of compound 1
Figure 1 Shi epoxidation of compound 1 Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC). When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3.
Figure 2 Structure of the Shi catalystThe epoxidation of compound 1 was monitored by thin-layer chromatography (TLC). When the reaction was completed, high-performance liquid chromatography (HPLC) was used to analyze the epoxide product. A chiral column that had an affinity for the desired epoxide was used. A standard of the desired epoxide was obtained to optimize the conditions and determine the retention time of the epoxide. The HPLC chromatogram of the epoxide standard is shown in Figure 3. Figure 3 HPLC of epoxide standard
Figure 3 HPLC of epoxide standardAdapted from Weiß C, Bogner T, Sammet B, Sewald N. Total synthesis and biological evaluation of fluorinated cryptophycins. Beilstein J Org Chem. 2012.
The Shi catalyst is derived from D-fructose based on the reaction below. The structure of D-fructose in the form shown below can be classified as:
 pyranose.furanose.ketohexose.aldohexose.
pyranose.furanose.ketohexose.aldohexose.A)I and III only
B)I and IV only
C)II and III only
D)II and IV only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
40
Passage
High blood pressure, or hypertension, is a prevalent condition in the United States. One in three adults has hypertension, and approximately 20% do not know that they have it. If left untreated, high blood pressure can lead to major health issues, including stroke, heart attack, and kidney disease or failure. A number of drugs can help control hypertension, including those shown in Table 1. These drugs can be classified into different groups based on their mechanism of action.Table 1 Drugs Used to Treat High Blood Pressure
 Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R), exhibit biological activity; the other two isomers, (S,S) and (R,S), are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker.
Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R), exhibit biological activity; the other two isomers, (S,S) and (R,S), are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker.
 Figure 1 Structure of (±)-labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.
Figure 1 Structure of (±)-labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.
Below is the structure of valsartan. All of the following are structural isomers of valsartan EXCEPT: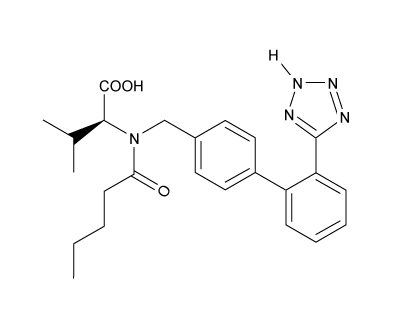
A)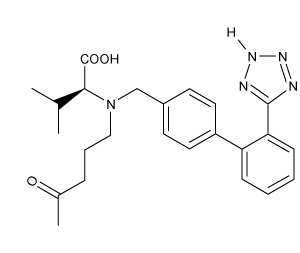
B)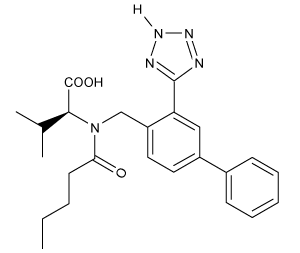
C)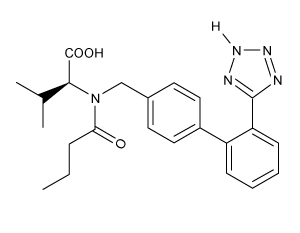
D)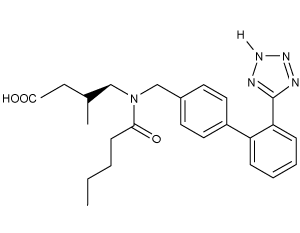
High blood pressure, or hypertension, is a prevalent condition in the United States. One in three adults has hypertension, and approximately 20% do not know that they have it. If left untreated, high blood pressure can lead to major health issues, including stroke, heart attack, and kidney disease or failure. A number of drugs can help control hypertension, including those shown in Table 1. These drugs can be classified into different groups based on their mechanism of action.Table 1 Drugs Used to Treat High Blood Pressure
 Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R), exhibit biological activity; the other two isomers, (S,S) and (R,S), are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker.
Labetalol (Figure 1) exists as four stereoisomers and is administered as a mixture of all four. This drug can be classified as a pseudohybrid drug because two of the isomers, (R,R) and (S,R), exhibit biological activity; the other two isomers, (S,S) and (R,S), are not active. The (R,R) isomer acts as a nonselective β-adrenergic receptor blocker, and the (S,R) isomer acts as a selective α-adrenergic receptor blocker. Figure 1 Structure of (±)-labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.
Figure 1 Structure of (±)-labetalol and l-epinephrinel-epinephrine (Figure 1) is the agonist of the α- and β-adrenergic receptors. The structural motifs that allow l-epinephrine to bind to the adrenergic receptors include an amine separated from an aromatic ring by two carbon units, a hydroxyl group at a chiral center beta to the amine, and two hydroxyl groups on the aromatic ring in the meta and para positions. With a few structural modifications, the agonist l-epinephrine can be converted into the antagonist labetalol. Substitution of a hydroxyl on the aromatic ring with an amide group and extension of the methyl group on the amine transforms l-epinephrine into labetalol. These substitutions keep a majority of the structural motifs necessary for labetalol to mimic l-epinephrine and bind to the adrenergic receptors.Below is the structure of valsartan. All of the following are structural isomers of valsartan EXCEPT:

A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
41
Passage
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1).
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
 Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
 Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Counting the number of bacteria recovered from which of the following would NOT help researchers verify that soap reduces the number of bacteria transferred to objects?
A)Unwashed hands directly
B)Spheres after washing hands with water only
C)Untouched spheres
D)Spheres touched with unwashed hands
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1).
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2. Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.Counting the number of bacteria recovered from which of the following would NOT help researchers verify that soap reduces the number of bacteria transferred to objects?
A)Unwashed hands directly
B)Spheres after washing hands with water only
C)Untouched spheres
D)Spheres touched with unwashed hands

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
42
Passage
Ethanol is an important source of energy that is frequently used as a supplement to gasoline. It can be obtained from renewable resources such as the cellulose found in plant matter. When subjected to a process called gasification, the plant mass is converted to a mixture called syngas, composed predominantly of carbon monoxide and molecular hydrogen. Syngas can be passed over a rhodium catalyst to generate ethanol and other products. After using this method, a group of researchers analyzed the resulting products by gas chromatography using helium as a carrier gas. The results are shown in Figure 1.
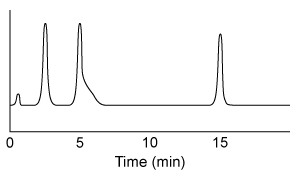 Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1.
Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1.
 Scheme 1
Scheme 1
Adapted from Lopez L, Velasco J, Montes V, Marinas A, Cabrera S, Boutonnet M, Järås S. Catalysts 2015.
Ethyl acetate could be better separated from ethanol by doing which of the following?
A)Replacing helium with nitrogen as the carrier gas
B)Running the mixture through a longer column
C)Starting the chromatograph at a higher temperature
D)Increasing the carrier gas flow rate
Ethanol is an important source of energy that is frequently used as a supplement to gasoline. It can be obtained from renewable resources such as the cellulose found in plant matter. When subjected to a process called gasification, the plant mass is converted to a mixture called syngas, composed predominantly of carbon monoxide and molecular hydrogen. Syngas can be passed over a rhodium catalyst to generate ethanol and other products. After using this method, a group of researchers analyzed the resulting products by gas chromatography using helium as a carrier gas. The results are shown in Figure 1.
 Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1.
Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1. Scheme 1
Scheme 1Adapted from Lopez L, Velasco J, Montes V, Marinas A, Cabrera S, Boutonnet M, Järås S. Catalysts 2015.
Ethyl acetate could be better separated from ethanol by doing which of the following?
A)Replacing helium with nitrogen as the carrier gas
B)Running the mixture through a longer column
C)Starting the chromatograph at a higher temperature
D)Increasing the carrier gas flow rate

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
43
Passage
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1).
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
 Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
 Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Which of the following peaks in Figure 3 most likely corresponds to ethyl acetate?
A)The singlet peak at 0 ppm in the spectrum from the GC extraction
B)The quartet peak at 4.1 ppm in the spectrum from the GC extraction
C)The singlet peak at 7.3 ppm in the spectrum from the B&D extraction
D)The multiplet peak at 4.3 ppm in the spectrum from the B&D extraction
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1).
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2. Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.Which of the following peaks in Figure 3 most likely corresponds to ethyl acetate?
A)The singlet peak at 0 ppm in the spectrum from the GC extraction
B)The quartet peak at 4.1 ppm in the spectrum from the GC extraction
C)The singlet peak at 7.3 ppm in the spectrum from the B&D extraction
D)The multiplet peak at 4.3 ppm in the spectrum from the B&D extraction

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
44
Passage
The β-lactam scaffold is an important feature in a class of broad-spectrum antibiotics that includes the penicillins, cephalosporins, and monobactams. These antibiotics are used to treat a variety of diseases caused by bacteria. The penicillins work by inhibiting a step in the synthesis of peptidoglycan. However, the development of antibiotic resistance in the form of enzymes such as β-lactamase is an ongoing problem.An aromatic ring linked to the β-lactam ring has been reported to participate in hydrophobic interactions with β-lactamase active sites, and an aromatic ring bonded to the nitrogen of the β-lactam has shown to be beneficial to biological activity. With this in mind, researchers synthesized a group of penicillanic acid analogues with substituted aromatic rings bonded to the β-lactam ring in an effort to overcome the challenge of antibiotic resistance. The substituents on the aromatic rings were varied, including -OCH3 and -NO2, to make several analogues. A key step in the synthesis of these analogues was the coupling of Compounds 1 and 2 to form a compound with two β-lactam rings (Reaction 1).
 Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC)
Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC)
 The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1.
The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1.
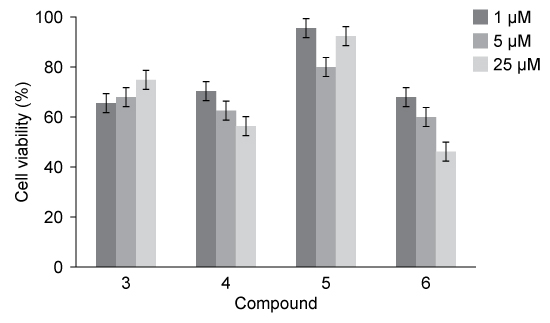 Figure 1 Cell viability assay results
Figure 1 Cell viability assay results
Adapted from De rosa M, Vigliotta G, Palma G, Saturnino C, Soriente A. Novel Penicillin-Type Analogues Bearing a Variable Substituted 2-Azetidinone Ring at Position 6: Synthesis and Biological Evaluation. Molecules. 2015.
The preparation of Compound 1 begins with a carboxylic acid (CA) derivative undergoing nucleophilic acyl substitution with methanol under reflux conditions to form butanoic acid with a methyl ester substituent on carbon 4. Which structure was most likely the starting CA derivative?
A)
B)
C)
D)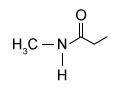
The β-lactam scaffold is an important feature in a class of broad-spectrum antibiotics that includes the penicillins, cephalosporins, and monobactams. These antibiotics are used to treat a variety of diseases caused by bacteria. The penicillins work by inhibiting a step in the synthesis of peptidoglycan. However, the development of antibiotic resistance in the form of enzymes such as β-lactamase is an ongoing problem.An aromatic ring linked to the β-lactam ring has been reported to participate in hydrophobic interactions with β-lactamase active sites, and an aromatic ring bonded to the nitrogen of the β-lactam has shown to be beneficial to biological activity. With this in mind, researchers synthesized a group of penicillanic acid analogues with substituted aromatic rings bonded to the β-lactam ring in an effort to overcome the challenge of antibiotic resistance. The substituents on the aromatic rings were varied, including -OCH3 and -NO2, to make several analogues. A key step in the synthesis of these analogues was the coupling of Compounds 1 and 2 to form a compound with two β-lactam rings (Reaction 1).
 Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC)
Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC) The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1.
The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1. Figure 1 Cell viability assay results
Figure 1 Cell viability assay resultsAdapted from De rosa M, Vigliotta G, Palma G, Saturnino C, Soriente A. Novel Penicillin-Type Analogues Bearing a Variable Substituted 2-Azetidinone Ring at Position 6: Synthesis and Biological Evaluation. Molecules. 2015.
The preparation of Compound 1 begins with a carboxylic acid (CA) derivative undergoing nucleophilic acyl substitution with methanol under reflux conditions to form butanoic acid with a methyl ester substituent on carbon 4. Which structure was most likely the starting CA derivative?
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
45
Passage
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1).
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
 Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
 Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Which additional extraction steps would cause phosphatidylethanolamine and 2,6-dimethoxyphenol to enter the aqueous layer consecutively?
A)Add 0.05 M NaOH(aq) to the organic layer followed by 0.01 M H2SO4(aq).
B)Add 0.01 M NaHCO3(aq) to the organic layer followed by 0.05 M HCl(aq).
C)Add 0.01 M H2CO3(aq) to the organic layer followed by 0.05 M NaHCO3(aq).
D)Add 0.05 M H2SO4(aq) to the organic layer followed by 0.01 M NaOH(aq).
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1).
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2. Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.Which additional extraction steps would cause phosphatidylethanolamine and 2,6-dimethoxyphenol to enter the aqueous layer consecutively?
A)Add 0.05 M NaOH(aq) to the organic layer followed by 0.01 M H2SO4(aq).
B)Add 0.01 M NaHCO3(aq) to the organic layer followed by 0.05 M HCl(aq).
C)Add 0.01 M H2CO3(aq) to the organic layer followed by 0.05 M NaHCO3(aq).
D)Add 0.05 M H2SO4(aq) to the organic layer followed by 0.01 M NaOH(aq).

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
46
Passage
Ethanol is an important source of energy that is frequently used as a supplement to gasoline. It can be obtained from renewable resources such as the cellulose found in plant matter. When subjected to a process called gasification, the plant mass is converted to a mixture called syngas, composed predominantly of carbon monoxide and molecular hydrogen. Syngas can be passed over a rhodium catalyst to generate ethanol and other products. After using this method, a group of researchers analyzed the resulting products by gas chromatography using helium as a carrier gas. The results are shown in Figure 1.
 Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1.
Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1.
 Scheme 1
Scheme 1
Adapted from Lopez L, Velasco J, Montes V, Marinas A, Cabrera S, Boutonnet M, Järås S. Catalysts 2015.
Acetic acid most likely eluted from the gas chromatograph at what time point?
A)1 min
B)2 min
C)5 min
D)15 min
Ethanol is an important source of energy that is frequently used as a supplement to gasoline. It can be obtained from renewable resources such as the cellulose found in plant matter. When subjected to a process called gasification, the plant mass is converted to a mixture called syngas, composed predominantly of carbon monoxide and molecular hydrogen. Syngas can be passed over a rhodium catalyst to generate ethanol and other products. After using this method, a group of researchers analyzed the resulting products by gas chromatography using helium as a carrier gas. The results are shown in Figure 1.
 Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1.
Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1. Scheme 1
Scheme 1Adapted from Lopez L, Velasco J, Montes V, Marinas A, Cabrera S, Boutonnet M, Järås S. Catalysts 2015.
Acetic acid most likely eluted from the gas chromatograph at what time point?
A)1 min
B)2 min
C)5 min
D)15 min

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
47
Passage
The β-lactam scaffold is an important feature in a class of broad-spectrum antibiotics that includes the penicillins, cephalosporins, and monobactams. These antibiotics are used to treat a variety of diseases caused by bacteria. The penicillins work by inhibiting a step in the synthesis of peptidoglycan. However, the development of antibiotic resistance in the form of enzymes such as β-lactamase is an ongoing problem.An aromatic ring linked to the β-lactam ring has been reported to participate in hydrophobic interactions with β-lactamase active sites, and an aromatic ring bonded to the nitrogen of the β-lactam has shown to be beneficial to biological activity. With this in mind, researchers synthesized a group of penicillanic acid analogues with substituted aromatic rings bonded to the β-lactam ring in an effort to overcome the challenge of antibiotic resistance. The substituents on the aromatic rings were varied, including -OCH3 and -NO2, to make several analogues. A key step in the synthesis of these analogues was the coupling of Compounds 1 and 2 to form a compound with two β-lactam rings (Reaction 1).
 Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC)
Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC)
 The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1.
The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1.
 Figure 1 Cell viability assay results
Figure 1 Cell viability assay results
Adapted from De rosa M, Vigliotta G, Palma G, Saturnino C, Soriente A. Novel Penicillin-Type Analogues Bearing a Variable Substituted 2-Azetidinone Ring at Position 6: Synthesis and Biological Evaluation. Molecules. 2015.
The synthesis of Compound 1 requires hydrolysis of an ester with LiOH and produces a carboxylate ion. The reaction is quenched in the next step by the addition of HCl. What is the expected result if blue litmus paper is immersed in the fully quenched solution? The blue litmus paper will:
A)turn red as the carboxylate becomes protonated.
B)turn red as the carboxylate becomes deprotonated.
C)remain blue as the solution becomes acidic.
D)remain blue as the solution becomes basic.
The β-lactam scaffold is an important feature in a class of broad-spectrum antibiotics that includes the penicillins, cephalosporins, and monobactams. These antibiotics are used to treat a variety of diseases caused by bacteria. The penicillins work by inhibiting a step in the synthesis of peptidoglycan. However, the development of antibiotic resistance in the form of enzymes such as β-lactamase is an ongoing problem.An aromatic ring linked to the β-lactam ring has been reported to participate in hydrophobic interactions with β-lactamase active sites, and an aromatic ring bonded to the nitrogen of the β-lactam has shown to be beneficial to biological activity. With this in mind, researchers synthesized a group of penicillanic acid analogues with substituted aromatic rings bonded to the β-lactam ring in an effort to overcome the challenge of antibiotic resistance. The substituents on the aromatic rings were varied, including -OCH3 and -NO2, to make several analogues. A key step in the synthesis of these analogues was the coupling of Compounds 1 and 2 to form a compound with two β-lactam rings (Reaction 1).
 Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC)
Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC) The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1.
The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1. Figure 1 Cell viability assay results
Figure 1 Cell viability assay resultsAdapted from De rosa M, Vigliotta G, Palma G, Saturnino C, Soriente A. Novel Penicillin-Type Analogues Bearing a Variable Substituted 2-Azetidinone Ring at Position 6: Synthesis and Biological Evaluation. Molecules. 2015.
The synthesis of Compound 1 requires hydrolysis of an ester with LiOH and produces a carboxylate ion. The reaction is quenched in the next step by the addition of HCl. What is the expected result if blue litmus paper is immersed in the fully quenched solution? The blue litmus paper will:
A)turn red as the carboxylate becomes protonated.
B)turn red as the carboxylate becomes deprotonated.
C)remain blue as the solution becomes acidic.
D)remain blue as the solution becomes basic.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
48
Passage
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1).
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
 Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
 Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
According to Experiment 2, the samples from the lipid extract were diluted by what factor to generate the NMR samples?
A)2
B)20
C)40
D)80
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1).
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2. Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.According to Experiment 2, the samples from the lipid extract were diluted by what factor to generate the NMR samples?
A)2
B)20
C)40
D)80

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
49
Passage
The β-lactam scaffold is an important feature in a class of broad-spectrum antibiotics that includes the penicillins, cephalosporins, and monobactams. These antibiotics are used to treat a variety of diseases caused by bacteria. The penicillins work by inhibiting a step in the synthesis of peptidoglycan. However, the development of antibiotic resistance in the form of enzymes such as β-lactamase is an ongoing problem.An aromatic ring linked to the β-lactam ring has been reported to participate in hydrophobic interactions with β-lactamase active sites, and an aromatic ring bonded to the nitrogen of the β-lactam has shown to be beneficial to biological activity. With this in mind, researchers synthesized a group of penicillanic acid analogues with substituted aromatic rings bonded to the β-lactam ring in an effort to overcome the challenge of antibiotic resistance. The substituents on the aromatic rings were varied, including -OCH3 and -NO2, to make several analogues. A key step in the synthesis of these analogues was the coupling of Compounds 1 and 2 to form a compound with two β-lactam rings (Reaction 1).
 Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC)
Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC)
 The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1.
The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1.
 Figure 1 Cell viability assay results
Figure 1 Cell viability assay results
Adapted from De rosa M, Vigliotta G, Palma G, Saturnino C, Soriente A. Novel Penicillin-Type Analogues Bearing a Variable Substituted 2-Azetidinone Ring at Position 6: Synthesis and Biological Evaluation. Molecules. 2015.
The key step in the synthesis of the penicillin analogues shown in Reaction 1 is the coupling of Compounds 1 and 2, driven by dicyclohexylcarbodiimide. Which functional group forms in this reaction?
A)Ester
B)Anhydride
C)Amide
D)Enamine
The β-lactam scaffold is an important feature in a class of broad-spectrum antibiotics that includes the penicillins, cephalosporins, and monobactams. These antibiotics are used to treat a variety of diseases caused by bacteria. The penicillins work by inhibiting a step in the synthesis of peptidoglycan. However, the development of antibiotic resistance in the form of enzymes such as β-lactamase is an ongoing problem.An aromatic ring linked to the β-lactam ring has been reported to participate in hydrophobic interactions with β-lactamase active sites, and an aromatic ring bonded to the nitrogen of the β-lactam has shown to be beneficial to biological activity. With this in mind, researchers synthesized a group of penicillanic acid analogues with substituted aromatic rings bonded to the β-lactam ring in an effort to overcome the challenge of antibiotic resistance. The substituents on the aromatic rings were varied, including -OCH3 and -NO2, to make several analogues. A key step in the synthesis of these analogues was the coupling of Compounds 1 and 2 to form a compound with two β-lactam rings (Reaction 1).
 Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC)
Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC) The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1.
The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1. Figure 1 Cell viability assay results
Figure 1 Cell viability assay resultsAdapted from De rosa M, Vigliotta G, Palma G, Saturnino C, Soriente A. Novel Penicillin-Type Analogues Bearing a Variable Substituted 2-Azetidinone Ring at Position 6: Synthesis and Biological Evaluation. Molecules. 2015.
The key step in the synthesis of the penicillin analogues shown in Reaction 1 is the coupling of Compounds 1 and 2, driven by dicyclohexylcarbodiimide. Which functional group forms in this reaction?
A)Ester
B)Anhydride
C)Amide
D)Enamine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
50
Passage
The β-lactam scaffold is an important feature in a class of broad-spectrum antibiotics that includes the penicillins, cephalosporins, and monobactams. These antibiotics are used to treat a variety of diseases caused by bacteria. The penicillins work by inhibiting a step in the synthesis of peptidoglycan. However, the development of antibiotic resistance in the form of enzymes such as β-lactamase is an ongoing problem.An aromatic ring linked to the β-lactam ring has been reported to participate in hydrophobic interactions with β-lactamase active sites, and an aromatic ring bonded to the nitrogen of the β-lactam has shown to be beneficial to biological activity. With this in mind, researchers synthesized a group of penicillanic acid analogues with substituted aromatic rings bonded to the β-lactam ring in an effort to overcome the challenge of antibiotic resistance. The substituents on the aromatic rings were varied, including -OCH3 and -NO2, to make several analogues. A key step in the synthesis of these analogues was the coupling of Compounds 1 and 2 to form a compound with two β-lactam rings (Reaction 1).
 Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC)
Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC)
 The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1.
The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1.
 Figure 1 Cell viability assay results
Figure 1 Cell viability assay results
Adapted from De rosa M, Vigliotta G, Palma G, Saturnino C, Soriente A. Novel Penicillin-Type Analogues Bearing a Variable Substituted 2-Azetidinone Ring at Position 6: Synthesis and Biological Evaluation. Molecules. 2015.
Which of the following properties of β-lactams like those described in the passage best explains their reactivity?
A)sp2 hybridization of nitrogen in β-lactam
B)Angles less than 109.5° in the β-lactam ring
C)Trigonal planar geometry of the nitrogen atom
D)Increased resonance
The β-lactam scaffold is an important feature in a class of broad-spectrum antibiotics that includes the penicillins, cephalosporins, and monobactams. These antibiotics are used to treat a variety of diseases caused by bacteria. The penicillins work by inhibiting a step in the synthesis of peptidoglycan. However, the development of antibiotic resistance in the form of enzymes such as β-lactamase is an ongoing problem.An aromatic ring linked to the β-lactam ring has been reported to participate in hydrophobic interactions with β-lactamase active sites, and an aromatic ring bonded to the nitrogen of the β-lactam has shown to be beneficial to biological activity. With this in mind, researchers synthesized a group of penicillanic acid analogues with substituted aromatic rings bonded to the β-lactam ring in an effort to overcome the challenge of antibiotic resistance. The substituents on the aromatic rings were varied, including -OCH3 and -NO2, to make several analogues. A key step in the synthesis of these analogues was the coupling of Compounds 1 and 2 to form a compound with two β-lactam rings (Reaction 1).
 Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC)
Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC) The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1.
The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1. Figure 1 Cell viability assay results
Figure 1 Cell viability assay resultsAdapted from De rosa M, Vigliotta G, Palma G, Saturnino C, Soriente A. Novel Penicillin-Type Analogues Bearing a Variable Substituted 2-Azetidinone Ring at Position 6: Synthesis and Biological Evaluation. Molecules. 2015.
Which of the following properties of β-lactams like those described in the passage best explains their reactivity?
A)sp2 hybridization of nitrogen in β-lactam
B)Angles less than 109.5° in the β-lactam ring
C)Trigonal planar geometry of the nitrogen atom
D)Increased resonance

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
51
Passage
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1).
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
 Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
 Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Which layer in the B&D and GC methods in Figure 2 is expected to contain sterols isolated from the Y. lipolytica extract?
A)L1 in the B&D method and L2 in the GC method
B)L3 in the B&D method and L1 in the GC method
C)L3 in the GC method and L2 in the B&D method
D)L3 in the GC method and L1 in the B&D method
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1).
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2. Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.Which layer in the B&D and GC methods in Figure 2 is expected to contain sterols isolated from the Y. lipolytica extract?
A)L1 in the B&D method and L2 in the GC method
B)L3 in the B&D method and L1 in the GC method
C)L3 in the GC method and L2 in the B&D method
D)L3 in the GC method and L1 in the B&D method

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
52
Passage
Ethanol is an important source of energy that is frequently used as a supplement to gasoline. It can be obtained from renewable resources such as the cellulose found in plant matter. When subjected to a process called gasification, the plant mass is converted to a mixture called syngas, composed predominantly of carbon monoxide and molecular hydrogen. Syngas can be passed over a rhodium catalyst to generate ethanol and other products. After using this method, a group of researchers analyzed the resulting products by gas chromatography using helium as a carrier gas. The results are shown in Figure 1.
 Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1.
Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1.
 Scheme 1
Scheme 1
Adapted from Lopez L, Velasco J, Montes V, Marinas A, Cabrera S, Boutonnet M, Järås S. Catalysts 2015.
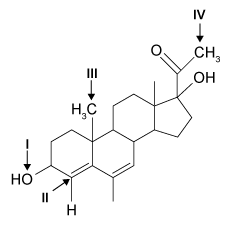 What atom indicated would be most readily deprotonated if Compound 2 reacts with NaOH?
What atom indicated would be most readily deprotonated if Compound 2 reacts with NaOH?
A)I
B)II
C)III
D)IV
Ethanol is an important source of energy that is frequently used as a supplement to gasoline. It can be obtained from renewable resources such as the cellulose found in plant matter. When subjected to a process called gasification, the plant mass is converted to a mixture called syngas, composed predominantly of carbon monoxide and molecular hydrogen. Syngas can be passed over a rhodium catalyst to generate ethanol and other products. After using this method, a group of researchers analyzed the resulting products by gas chromatography using helium as a carrier gas. The results are shown in Figure 1.
 Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1.
Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1. Scheme 1
Scheme 1Adapted from Lopez L, Velasco J, Montes V, Marinas A, Cabrera S, Boutonnet M, Järås S. Catalysts 2015.
 What atom indicated would be most readily deprotonated if Compound 2 reacts with NaOH?
What atom indicated would be most readily deprotonated if Compound 2 reacts with NaOH?A)I
B)II
C)III
D)IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
53
Passage
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1).
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
 Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
 Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
A reaction between a long-chain anhydride and phenylethylamine is done to produce a long-chain amide and a carboxylic acid. Which of the following aqueous solutions can separate the products of this reaction in an extraction?
A)NaHSO4
B)HNO3
C)LiOH
D)HClO4
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1).
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2. Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.A reaction between a long-chain anhydride and phenylethylamine is done to produce a long-chain amide and a carboxylic acid. Which of the following aqueous solutions can separate the products of this reaction in an extraction?
A)NaHSO4
B)HNO3
C)LiOH
D)HClO4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
54
Passage
Ethanol is an important source of energy that is frequently used as a supplement to gasoline. It can be obtained from renewable resources such as the cellulose found in plant matter. When subjected to a process called gasification, the plant mass is converted to a mixture called syngas, composed predominantly of carbon monoxide and molecular hydrogen. Syngas can be passed over a rhodium catalyst to generate ethanol and other products. After using this method, a group of researchers analyzed the resulting products by gas chromatography using helium as a carrier gas. The results are shown in Figure 1.
 Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1.
Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1.
 Scheme 1
Scheme 1
Adapted from Lopez L, Velasco J, Montes V, Marinas A, Cabrera S, Boutonnet M, Järås S. Catalysts 2015.
The conversion of megestrol to megestrol acetate shown in Scheme 1 represents which kind of reaction?
A)Transesterification
B)Saponification
C)Aldol condensation
D)Oxidation
Ethanol is an important source of energy that is frequently used as a supplement to gasoline. It can be obtained from renewable resources such as the cellulose found in plant matter. When subjected to a process called gasification, the plant mass is converted to a mixture called syngas, composed predominantly of carbon monoxide and molecular hydrogen. Syngas can be passed over a rhodium catalyst to generate ethanol and other products. After using this method, a group of researchers analyzed the resulting products by gas chromatography using helium as a carrier gas. The results are shown in Figure 1.
 Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1.
Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1. Scheme 1
Scheme 1Adapted from Lopez L, Velasco J, Montes V, Marinas A, Cabrera S, Boutonnet M, Järås S. Catalysts 2015.
The conversion of megestrol to megestrol acetate shown in Scheme 1 represents which kind of reaction?
A)Transesterification
B)Saponification
C)Aldol condensation
D)Oxidation

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
55
Passage
Ethanol is an important source of energy that is frequently used as a supplement to gasoline. It can be obtained from renewable resources such as the cellulose found in plant matter. When subjected to a process called gasification, the plant mass is converted to a mixture called syngas, composed predominantly of carbon monoxide and molecular hydrogen. Syngas can be passed over a rhodium catalyst to generate ethanol and other products. After using this method, a group of researchers analyzed the resulting products by gas chromatography using helium as a carrier gas. The results are shown in Figure 1.
 Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1.
Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1.
 Scheme 1
Scheme 1
Adapted from Lopez L, Velasco J, Montes V, Marinas A, Cabrera S, Boutonnet M, Järås S. Catalysts 2015.
In Scheme 1, conversion of Compound 1 to Compound 2 most likely begins with which of the following steps?
A)Nucleophilic attack by water, causing iodide to leave
B)Deprotonation of water to enhance its nucleophilicity
C)Formation of a carbocation as iodide leaves
D)Protonation of iodide to form a better leaving group
Ethanol is an important source of energy that is frequently used as a supplement to gasoline. It can be obtained from renewable resources such as the cellulose found in plant matter. When subjected to a process called gasification, the plant mass is converted to a mixture called syngas, composed predominantly of carbon monoxide and molecular hydrogen. Syngas can be passed over a rhodium catalyst to generate ethanol and other products. After using this method, a group of researchers analyzed the resulting products by gas chromatography using helium as a carrier gas. The results are shown in Figure 1.
 Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1.
Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1. Scheme 1
Scheme 1Adapted from Lopez L, Velasco J, Montes V, Marinas A, Cabrera S, Boutonnet M, Järås S. Catalysts 2015.
In Scheme 1, conversion of Compound 1 to Compound 2 most likely begins with which of the following steps?
A)Nucleophilic attack by water, causing iodide to leave
B)Deprotonation of water to enhance its nucleophilicity
C)Formation of a carbocation as iodide leaves
D)Protonation of iodide to form a better leaving group

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
56
Passage
Ethanol is an important source of energy that is frequently used as a supplement to gasoline. It can be obtained from renewable resources such as the cellulose found in plant matter. When subjected to a process called gasification, the plant mass is converted to a mixture called syngas, composed predominantly of carbon monoxide and molecular hydrogen. Syngas can be passed over a rhodium catalyst to generate ethanol and other products. After using this method, a group of researchers analyzed the resulting products by gas chromatography using helium as a carrier gas. The results are shown in Figure 1.
 Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1.
Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1.
 Scheme 1
Scheme 1
Adapted from Lopez L, Velasco J, Montes V, Marinas A, Cabrera S, Boutonnet M, Järås S. Catalysts 2015.
During the conversion of Compound 2 to megestrol, why was the secondary alcohol affected, whereas the tertiary alcohol was not?
A)Tertiary alcohols cannot be readily oxidized.
B)The secondary alcohol is stabilized by resonance.
C)The tertiary alcohol is less acidic than the secondary alcohol.
D)Secondary alcohols are better nucleophiles.
Ethanol is an important source of energy that is frequently used as a supplement to gasoline. It can be obtained from renewable resources such as the cellulose found in plant matter. When subjected to a process called gasification, the plant mass is converted to a mixture called syngas, composed predominantly of carbon monoxide and molecular hydrogen. Syngas can be passed over a rhodium catalyst to generate ethanol and other products. After using this method, a group of researchers analyzed the resulting products by gas chromatography using helium as a carrier gas. The results are shown in Figure 1.
 Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1.
Figure 1 Gas chromatograph of syngas catalysis productsMass spectrometry revealed that the process yielded similar amounts of ethanol, acetic acid, and acetaldehyde, with trace amounts of ethane. In addition, the ethanol peak was found to be contaminated with ethyl acetate. Another group of researchers in the same laboratory had recently produced Compound 1 as a by-product in a separate reaction. They combined Compound 1 with purified ethyl acetate to form megestrol acetate, a drug used in the treatment of certain cancers. This reaction also produced ethanol, as shown in Scheme 1. Scheme 1
Scheme 1Adapted from Lopez L, Velasco J, Montes V, Marinas A, Cabrera S, Boutonnet M, Järås S. Catalysts 2015.
During the conversion of Compound 2 to megestrol, why was the secondary alcohol affected, whereas the tertiary alcohol was not?
A)Tertiary alcohols cannot be readily oxidized.
B)The secondary alcohol is stabilized by resonance.
C)The tertiary alcohol is less acidic than the secondary alcohol.
D)Secondary alcohols are better nucleophiles.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
57
Passage
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1).
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
 Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
 Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Chemically equivalent protons are present in each of the following compounds EXCEPT:
A)ethyl acetate.
B)chloroform.
C)ethanol.
D)methanol.
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1).
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2. Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.Chemically equivalent protons are present in each of the following compounds EXCEPT:
A)ethyl acetate.
B)chloroform.
C)ethanol.
D)methanol.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
58
Which structure is the product of the reaction of 4-tert-butyl-2-octanone and NaCN?
A)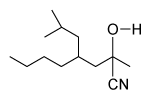
B)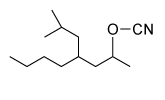
C)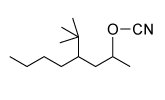
D)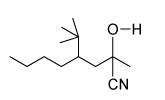
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
59
Passage
The β-lactam scaffold is an important feature in a class of broad-spectrum antibiotics that includes the penicillins, cephalosporins, and monobactams. These antibiotics are used to treat a variety of diseases caused by bacteria. The penicillins work by inhibiting a step in the synthesis of peptidoglycan. However, the development of antibiotic resistance in the form of enzymes such as β-lactamase is an ongoing problem.An aromatic ring linked to the β-lactam ring has been reported to participate in hydrophobic interactions with β-lactamase active sites, and an aromatic ring bonded to the nitrogen of the β-lactam has shown to be beneficial to biological activity. With this in mind, researchers synthesized a group of penicillanic acid analogues with substituted aromatic rings bonded to the β-lactam ring in an effort to overcome the challenge of antibiotic resistance. The substituents on the aromatic rings were varied, including -OCH3 and -NO2, to make several analogues. A key step in the synthesis of these analogues was the coupling of Compounds 1 and 2 to form a compound with two β-lactam rings (Reaction 1).
 Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC)
Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC)
 The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1.
The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1.
 Figure 1 Cell viability assay results
Figure 1 Cell viability assay results
Adapted from De rosa M, Vigliotta G, Palma G, Saturnino C, Soriente A. Novel Penicillin-Type Analogues Bearing a Variable Substituted 2-Azetidinone Ring at Position 6: Synthesis and Biological Evaluation. Molecules. 2015.
The precursor to Compounds 3-6 is shown below with carbonyl carbon atoms labeled. Which of the carbonyl carbon atoms is most likely to undergo hydrolysis when treated with a weak acid at room temperature?
Which of the carbonyl carbon atoms is most likely to undergo hydrolysis when treated with a weak acid at room temperature?
A)Carbon 1 only
B)Carbons 1, 2, and 3 only
C)Carbons 1 and 4 only
D)Carbon 4 only
The β-lactam scaffold is an important feature in a class of broad-spectrum antibiotics that includes the penicillins, cephalosporins, and monobactams. These antibiotics are used to treat a variety of diseases caused by bacteria. The penicillins work by inhibiting a step in the synthesis of peptidoglycan. However, the development of antibiotic resistance in the form of enzymes such as β-lactamase is an ongoing problem.An aromatic ring linked to the β-lactam ring has been reported to participate in hydrophobic interactions with β-lactamase active sites, and an aromatic ring bonded to the nitrogen of the β-lactam has shown to be beneficial to biological activity. With this in mind, researchers synthesized a group of penicillanic acid analogues with substituted aromatic rings bonded to the β-lactam ring in an effort to overcome the challenge of antibiotic resistance. The substituents on the aromatic rings were varied, including -OCH3 and -NO2, to make several analogues. A key step in the synthesis of these analogues was the coupling of Compounds 1 and 2 to form a compound with two β-lactam rings (Reaction 1).
 Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC)
Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC) The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1.
The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1. Figure 1 Cell viability assay results
Figure 1 Cell viability assay resultsAdapted from De rosa M, Vigliotta G, Palma G, Saturnino C, Soriente A. Novel Penicillin-Type Analogues Bearing a Variable Substituted 2-Azetidinone Ring at Position 6: Synthesis and Biological Evaluation. Molecules. 2015.
The precursor to Compounds 3-6 is shown below with carbonyl carbon atoms labeled.
 Which of the carbonyl carbon atoms is most likely to undergo hydrolysis when treated with a weak acid at room temperature?
Which of the carbonyl carbon atoms is most likely to undergo hydrolysis when treated with a weak acid at room temperature?A)Carbon 1 only
B)Carbons 1, 2, and 3 only
C)Carbons 1 and 4 only
D)Carbon 4 only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
60
Passage
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1).
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
 Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
 Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
By approximately what factor were the bacteria recovered from hands reduced when hands were washed with 3 mL of soap versus no soap?
A)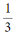
B)2
C)15
D)80
Two key ingredients found in many soaps are PEG 150 distearate and ethylhexyl glycerin. Both can be synthesized from glyceryl tristearate (Figure 1).
 Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2.
Figure 1 Formation of PEG 150 distearate and ethylhexyl glycerin from glyceryl tristearateThe first step in this process requires the saponification of glyceryl tristearate. The resulting stearate molecules can then react with PEG 150 in an acid-catalyzed Fischer esterification to form PEG 150 distearate. The glycerol produced during saponification is used as a nucleophile to form ethylhexyl glycerin through a base-catalyzed SN2 reaction with an ethylhexyl halide.Studies have shown that hand washing with soap can substantially reduce the number of germs present on hands and that washing hands before preparing food is particularly effective in reducing illness. Researchers examined the effect of washing for 20 seconds with different amounts of soap containing PEG 150 distearate and ethylhexyl glycerin and found an inverse correlation between the volume of soap used and the amount of bacteria recovered from the hands. The data are shown in Figure 2. Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects
Figure 2 Bacteria recovered from hands after washing with varying amounts of soapThe researchers then investigated the effect of hand washing with different kinds of soap on the transfer of bacteria from hands to objects. Volunteers washed with a fixed volume of soap for 30 seconds, then handled sterilized plastic spheres for 30 seconds. Bacteria were then recovered from the spheres. The results are shown in Table 1.Table 1 Bacteria Recovered from Objects Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.
Adapted from Fuls JL, Rodgers ND, Fischler GE, et al. Alternative hand contamination technique to compare the activities of antimicrobial and nonantimicrobial soaps under different test conditions. Appl Environ Microbiol. 2008.By approximately what factor were the bacteria recovered from hands reduced when hands were washed with 3 mL of soap versus no soap?
A)
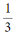
B)2
C)15
D)80

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
61
If an alcohol were to undergo a substitution reaction, which of the following modifications would improve the alcohol's leaving group ability?
A)Protection of the alcohol
B)Deprotonation of the alcohol
C)Conversion of the alcohol to a mesylate
D)Oxidation of the alcohol
A)Protection of the alcohol
B)Deprotonation of the alcohol
C)Conversion of the alcohol to a mesylate
D)Oxidation of the alcohol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
62
What is the correct systematic name for the compound shown below? 
A)(2R, 3S, 5S)-2-amino-5-hydroxy-3-methyl-4-oxohexanal
B)(2S, 4S, 5R)-5-amino-2-hydroxy-4-methyl-6-oxohexan-3-one
C)(1S, 3S, 4R)-4-amino-1,3-dimethyl-2,5-dioxopentan-1-ol
D)(2R, 3S, 5S)-5-hydroxy-3,5-dimethyl-1,4-dioxopentan-2-amine

A)(2R, 3S, 5S)-2-amino-5-hydroxy-3-methyl-4-oxohexanal
B)(2S, 4S, 5R)-5-amino-2-hydroxy-4-methyl-6-oxohexan-3-one
C)(1S, 3S, 4R)-4-amino-1,3-dimethyl-2,5-dioxopentan-1-ol
D)(2R, 3S, 5S)-5-hydroxy-3,5-dimethyl-1,4-dioxopentan-2-amine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
63
Passage
Fluorescent amino acids are useful for labeling proteins because they can be visualized spectroscopically, facilitating the study of protein structure and interactions. The noncanonical amino acid dansylalanine, a fluorescent amino acid that is sensitive to the hydrophobicity of its environment, is a good candidate to be incorporated into proteins because of its small size and fluorescent capabilities. Dansyl chloride and Compound 1 were used to synthesize dansylalanine, as shown in Reaction 1. Dansylalanine absorbs maximally at 340 nm and emits at approximately 540 nm.
 Reaction 1 Synthesis of dansylalanineA leucyl-tRNA synthetase from Escherichia coli was engineered to aminoacylate bacterial tRNAs containing a CUA anticodon with dansylalanine. After verifying that the tRNA/synthetase pair does not interact with endogenous amino acids or tRNAs, it was transfected into the yeast Saccharomyces cerevisiae along with a plasmid encoding a mutated human superoxide dismutase (hSOD). Using this system, dansylalanine was genetically incorporated into hSOD in place of either Gln-16 or Trp-33, and expressed in S. cerevisiae.Position 16 is located on the surface of hSOD at the N-terminus of a β-strand, and position 33 lies in the center of a strand within a β-barrel. To probe changes in the structure of hSOD, researchers exposed the purified protein to guanidinium chloride (GdmCl) to denature the protein at concentrations ranging 0-4.5 M. The experiment was conducted in a 35 mM sodium phosphate buffer at pH 7.2. The fluorescence of hSOD with dansylalanine at either position 16 or 33 was monitored at each GdmCl concentration, and the results of the unfolding experiment are shown in Figure 1.
Reaction 1 Synthesis of dansylalanineA leucyl-tRNA synthetase from Escherichia coli was engineered to aminoacylate bacterial tRNAs containing a CUA anticodon with dansylalanine. After verifying that the tRNA/synthetase pair does not interact with endogenous amino acids or tRNAs, it was transfected into the yeast Saccharomyces cerevisiae along with a plasmid encoding a mutated human superoxide dismutase (hSOD). Using this system, dansylalanine was genetically incorporated into hSOD in place of either Gln-16 or Trp-33, and expressed in S. cerevisiae.Position 16 is located on the surface of hSOD at the N-terminus of a β-strand, and position 33 lies in the center of a strand within a β-barrel. To probe changes in the structure of hSOD, researchers exposed the purified protein to guanidinium chloride (GdmCl) to denature the protein at concentrations ranging 0-4.5 M. The experiment was conducted in a 35 mM sodium phosphate buffer at pH 7.2. The fluorescence of hSOD with dansylalanine at either position 16 or 33 was monitored at each GdmCl concentration, and the results of the unfolding experiment are shown in Figure 1.
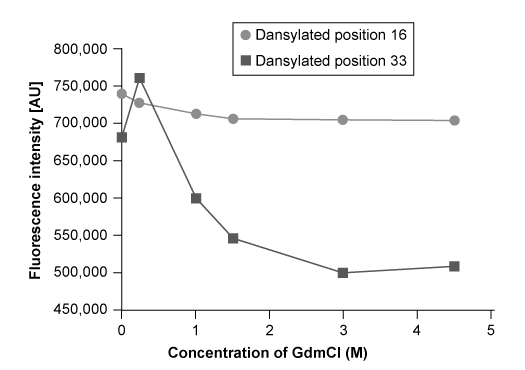 Figure 1 Plot of dansylalanine fluorescence at different concentrations of GdmCl
Figure 1 Plot of dansylalanine fluorescence at different concentrations of GdmCl
Adapted from Summerer D, Chen S, Wu N, Deiters A, Chin JW, Schultz PG. A genetically encoded fluorescent amino acid. Proc Natl Acad Sci USA. 2006.
What is the charge of the N-terminus during the protein unfolding experiment?
A)+1, because the amine group is not protonated
B)+1, because the amine group is in the conjugate acid form
C)0, because the amine group is protonated
D)0, because the amine group is in the conjugate base form
Fluorescent amino acids are useful for labeling proteins because they can be visualized spectroscopically, facilitating the study of protein structure and interactions. The noncanonical amino acid dansylalanine, a fluorescent amino acid that is sensitive to the hydrophobicity of its environment, is a good candidate to be incorporated into proteins because of its small size and fluorescent capabilities. Dansyl chloride and Compound 1 were used to synthesize dansylalanine, as shown in Reaction 1. Dansylalanine absorbs maximally at 340 nm and emits at approximately 540 nm.
 Reaction 1 Synthesis of dansylalanineA leucyl-tRNA synthetase from Escherichia coli was engineered to aminoacylate bacterial tRNAs containing a CUA anticodon with dansylalanine. After verifying that the tRNA/synthetase pair does not interact with endogenous amino acids or tRNAs, it was transfected into the yeast Saccharomyces cerevisiae along with a plasmid encoding a mutated human superoxide dismutase (hSOD). Using this system, dansylalanine was genetically incorporated into hSOD in place of either Gln-16 or Trp-33, and expressed in S. cerevisiae.Position 16 is located on the surface of hSOD at the N-terminus of a β-strand, and position 33 lies in the center of a strand within a β-barrel. To probe changes in the structure of hSOD, researchers exposed the purified protein to guanidinium chloride (GdmCl) to denature the protein at concentrations ranging 0-4.5 M. The experiment was conducted in a 35 mM sodium phosphate buffer at pH 7.2. The fluorescence of hSOD with dansylalanine at either position 16 or 33 was monitored at each GdmCl concentration, and the results of the unfolding experiment are shown in Figure 1.
Reaction 1 Synthesis of dansylalanineA leucyl-tRNA synthetase from Escherichia coli was engineered to aminoacylate bacterial tRNAs containing a CUA anticodon with dansylalanine. After verifying that the tRNA/synthetase pair does not interact with endogenous amino acids or tRNAs, it was transfected into the yeast Saccharomyces cerevisiae along with a plasmid encoding a mutated human superoxide dismutase (hSOD). Using this system, dansylalanine was genetically incorporated into hSOD in place of either Gln-16 or Trp-33, and expressed in S. cerevisiae.Position 16 is located on the surface of hSOD at the N-terminus of a β-strand, and position 33 lies in the center of a strand within a β-barrel. To probe changes in the structure of hSOD, researchers exposed the purified protein to guanidinium chloride (GdmCl) to denature the protein at concentrations ranging 0-4.5 M. The experiment was conducted in a 35 mM sodium phosphate buffer at pH 7.2. The fluorescence of hSOD with dansylalanine at either position 16 or 33 was monitored at each GdmCl concentration, and the results of the unfolding experiment are shown in Figure 1. Figure 1 Plot of dansylalanine fluorescence at different concentrations of GdmCl
Figure 1 Plot of dansylalanine fluorescence at different concentrations of GdmClAdapted from Summerer D, Chen S, Wu N, Deiters A, Chin JW, Schultz PG. A genetically encoded fluorescent amino acid. Proc Natl Acad Sci USA. 2006.
What is the charge of the N-terminus during the protein unfolding experiment?
A)+1, because the amine group is not protonated
B)+1, because the amine group is in the conjugate acid form
C)0, because the amine group is protonated
D)0, because the amine group is in the conjugate base form

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
64
Passage
Fluorescent amino acids are useful for labeling proteins because they can be visualized spectroscopically, facilitating the study of protein structure and interactions. The noncanonical amino acid dansylalanine, a fluorescent amino acid that is sensitive to the hydrophobicity of its environment, is a good candidate to be incorporated into proteins because of its small size and fluorescent capabilities. Dansyl chloride and Compound 1 were used to synthesize dansylalanine, as shown in Reaction 1. Dansylalanine absorbs maximally at 340 nm and emits at approximately 540 nm.
 Reaction 1 Synthesis of dansylalanineA leucyl-tRNA synthetase from Escherichia coli was engineered to aminoacylate bacterial tRNAs containing a CUA anticodon with dansylalanine. After verifying that the tRNA/synthetase pair does not interact with endogenous amino acids or tRNAs, it was transfected into the yeast Saccharomyces cerevisiae along with a plasmid encoding a mutated human superoxide dismutase (hSOD). Using this system, dansylalanine was genetically incorporated into hSOD in place of either Gln-16 or Trp-33, and expressed in S. cerevisiae.Position 16 is located on the surface of hSOD at the N-terminus of a β-strand, and position 33 lies in the center of a strand within a β-barrel. To probe changes in the structure of hSOD, researchers exposed the purified protein to guanidinium chloride (GdmCl) to denature the protein at concentrations ranging 0-4.5 M. The experiment was conducted in a 35 mM sodium phosphate buffer at pH 7.2. The fluorescence of hSOD with dansylalanine at either position 16 or 33 was monitored at each GdmCl concentration, and the results of the unfolding experiment are shown in Figure 1.
Reaction 1 Synthesis of dansylalanineA leucyl-tRNA synthetase from Escherichia coli was engineered to aminoacylate bacterial tRNAs containing a CUA anticodon with dansylalanine. After verifying that the tRNA/synthetase pair does not interact with endogenous amino acids or tRNAs, it was transfected into the yeast Saccharomyces cerevisiae along with a plasmid encoding a mutated human superoxide dismutase (hSOD). Using this system, dansylalanine was genetically incorporated into hSOD in place of either Gln-16 or Trp-33, and expressed in S. cerevisiae.Position 16 is located on the surface of hSOD at the N-terminus of a β-strand, and position 33 lies in the center of a strand within a β-barrel. To probe changes in the structure of hSOD, researchers exposed the purified protein to guanidinium chloride (GdmCl) to denature the protein at concentrations ranging 0-4.5 M. The experiment was conducted in a 35 mM sodium phosphate buffer at pH 7.2. The fluorescence of hSOD with dansylalanine at either position 16 or 33 was monitored at each GdmCl concentration, and the results of the unfolding experiment are shown in Figure 1.
 Figure 1 Plot of dansylalanine fluorescence at different concentrations of GdmCl
Figure 1 Plot of dansylalanine fluorescence at different concentrations of GdmCl
Adapted from Summerer D, Chen S, Wu N, Deiters A, Chin JW, Schultz PG. A genetically encoded fluorescent amino acid. Proc Natl Acad Sci USA. 2006.
If researchers used the Gabriel synthesis to make the dansylalanine backbone, which of the following statements is true?
A)Potassium phthalimide is a starting material.
B)Diethyl malonate is a starting material.
C)Potassium cyanide is a starting material.
D)An aldehyde is a starting material.
Fluorescent amino acids are useful for labeling proteins because they can be visualized spectroscopically, facilitating the study of protein structure and interactions. The noncanonical amino acid dansylalanine, a fluorescent amino acid that is sensitive to the hydrophobicity of its environment, is a good candidate to be incorporated into proteins because of its small size and fluorescent capabilities. Dansyl chloride and Compound 1 were used to synthesize dansylalanine, as shown in Reaction 1. Dansylalanine absorbs maximally at 340 nm and emits at approximately 540 nm.
 Reaction 1 Synthesis of dansylalanineA leucyl-tRNA synthetase from Escherichia coli was engineered to aminoacylate bacterial tRNAs containing a CUA anticodon with dansylalanine. After verifying that the tRNA/synthetase pair does not interact with endogenous amino acids or tRNAs, it was transfected into the yeast Saccharomyces cerevisiae along with a plasmid encoding a mutated human superoxide dismutase (hSOD). Using this system, dansylalanine was genetically incorporated into hSOD in place of either Gln-16 or Trp-33, and expressed in S. cerevisiae.Position 16 is located on the surface of hSOD at the N-terminus of a β-strand, and position 33 lies in the center of a strand within a β-barrel. To probe changes in the structure of hSOD, researchers exposed the purified protein to guanidinium chloride (GdmCl) to denature the protein at concentrations ranging 0-4.5 M. The experiment was conducted in a 35 mM sodium phosphate buffer at pH 7.2. The fluorescence of hSOD with dansylalanine at either position 16 or 33 was monitored at each GdmCl concentration, and the results of the unfolding experiment are shown in Figure 1.
Reaction 1 Synthesis of dansylalanineA leucyl-tRNA synthetase from Escherichia coli was engineered to aminoacylate bacterial tRNAs containing a CUA anticodon with dansylalanine. After verifying that the tRNA/synthetase pair does not interact with endogenous amino acids or tRNAs, it was transfected into the yeast Saccharomyces cerevisiae along with a plasmid encoding a mutated human superoxide dismutase (hSOD). Using this system, dansylalanine was genetically incorporated into hSOD in place of either Gln-16 or Trp-33, and expressed in S. cerevisiae.Position 16 is located on the surface of hSOD at the N-terminus of a β-strand, and position 33 lies in the center of a strand within a β-barrel. To probe changes in the structure of hSOD, researchers exposed the purified protein to guanidinium chloride (GdmCl) to denature the protein at concentrations ranging 0-4.5 M. The experiment was conducted in a 35 mM sodium phosphate buffer at pH 7.2. The fluorescence of hSOD with dansylalanine at either position 16 or 33 was monitored at each GdmCl concentration, and the results of the unfolding experiment are shown in Figure 1. Figure 1 Plot of dansylalanine fluorescence at different concentrations of GdmCl
Figure 1 Plot of dansylalanine fluorescence at different concentrations of GdmClAdapted from Summerer D, Chen S, Wu N, Deiters A, Chin JW, Schultz PG. A genetically encoded fluorescent amino acid. Proc Natl Acad Sci USA. 2006.
If researchers used the Gabriel synthesis to make the dansylalanine backbone, which of the following statements is true?
A)Potassium phthalimide is a starting material.
B)Diethyl malonate is a starting material.
C)Potassium cyanide is a starting material.
D)An aldehyde is a starting material.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
65
Which of the following does NOT describe why a nucleophile will react more quickly with acetic anhydride than with N,N-diisopropylisobutyramide?
A)N,N-diisopropylisobutyramide has a greater inductive effect than acetic anhydride.
B)Acetic anhydride is a stronger electrophile than N,N-diisopropylisobutyramide.
C)Acetic anhydride is less sterically hindered than N,N-diisopropylisobutyramide.
D)N,N-diisopropylisobutyramide has a less stable leaving group than acetic anhydride.
A)N,N-diisopropylisobutyramide has a greater inductive effect than acetic anhydride.
B)Acetic anhydride is a stronger electrophile than N,N-diisopropylisobutyramide.
C)Acetic anhydride is less sterically hindered than N,N-diisopropylisobutyramide.
D)N,N-diisopropylisobutyramide has a less stable leaving group than acetic anhydride.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
66
A student needs to separate a mixture of chloroform (bp 61°C) and benzene (bp 80°C). What type(s) of distillation would be expected to give the best separation of the two compounds?Simple distillationFractional distillationVacuum distillation
A)I only
B)II only
C)I and III only
D)II and III only
A)I only
B)II only
C)I and III only
D)II and III only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
67
When the compound shown below undergoes an SN2 reaction with hydroxide, which of the following compounds will most likely form as the major product? 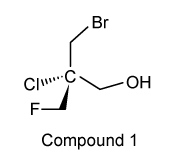
A)2-chloro-2-(fluoromethyl)propane-1,3-diol
B)2-(bromomethyl)-2-chloropropane-1,3-diol
C)(2R)-3-bromo-2-(fluoromethyl)propane-1,2-diol
D)(2S)-3-bromo-2-(fluoromethyl)propane-1,2-diol

A)2-chloro-2-(fluoromethyl)propane-1,3-diol
B)2-(bromomethyl)-2-chloropropane-1,3-diol
C)(2R)-3-bromo-2-(fluoromethyl)propane-1,2-diol
D)(2S)-3-bromo-2-(fluoromethyl)propane-1,2-diol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
68
Two separate reactions are conducted in which a compound containing a ketone, an ester, and a carboxylic acid is reacted with borane (BH3) in THF in one reaction and with NaBH4 in methanol in the other. Which of the following explains why different products are observed?
A)BH3 will selectively reduce ketones, and NaBH4 will only reduce carboxylic acids.
B)BH3 does not reduce ketones, and NaBH4 will selectively reduce esters.
C)BH3 reduces carboxylic acids and esters, and NaBH4 will only reduce esters.
D)BH3 will selectively reduce carboxylic acids, and NaBH4 will selectively reduce ketones.
A)BH3 will selectively reduce ketones, and NaBH4 will only reduce carboxylic acids.
B)BH3 does not reduce ketones, and NaBH4 will selectively reduce esters.
C)BH3 reduces carboxylic acids and esters, and NaBH4 will only reduce esters.
D)BH3 will selectively reduce carboxylic acids, and NaBH4 will selectively reduce ketones.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
69
Which structure is a tautomer of guanine?
A)
B)
C)
D)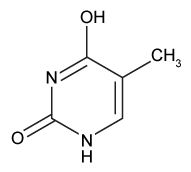
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
70
Passage
Fluorescent amino acids are useful for labeling proteins because they can be visualized spectroscopically, facilitating the study of protein structure and interactions. The noncanonical amino acid dansylalanine, a fluorescent amino acid that is sensitive to the hydrophobicity of its environment, is a good candidate to be incorporated into proteins because of its small size and fluorescent capabilities. Dansyl chloride and Compound 1 were used to synthesize dansylalanine, as shown in Reaction 1. Dansylalanine absorbs maximally at 340 nm and emits at approximately 540 nm.
 Reaction 1 Synthesis of dansylalanineA leucyl-tRNA synthetase from Escherichia coli was engineered to aminoacylate bacterial tRNAs containing a CUA anticodon with dansylalanine. After verifying that the tRNA/synthetase pair does not interact with endogenous amino acids or tRNAs, it was transfected into the yeast Saccharomyces cerevisiae along with a plasmid encoding a mutated human superoxide dismutase (hSOD). Using this system, dansylalanine was genetically incorporated into hSOD in place of either Gln-16 or Trp-33, and expressed in S. cerevisiae.Position 16 is located on the surface of hSOD at the N-terminus of a β-strand, and position 33 lies in the center of a strand within a β-barrel. To probe changes in the structure of hSOD, researchers exposed the purified protein to guanidinium chloride (GdmCl) to denature the protein at concentrations ranging 0-4.5 M. The experiment was conducted in a 35 mM sodium phosphate buffer at pH 7.2. The fluorescence of hSOD with dansylalanine at either position 16 or 33 was monitored at each GdmCl concentration, and the results of the unfolding experiment are shown in Figure 1.
Reaction 1 Synthesis of dansylalanineA leucyl-tRNA synthetase from Escherichia coli was engineered to aminoacylate bacterial tRNAs containing a CUA anticodon with dansylalanine. After verifying that the tRNA/synthetase pair does not interact with endogenous amino acids or tRNAs, it was transfected into the yeast Saccharomyces cerevisiae along with a plasmid encoding a mutated human superoxide dismutase (hSOD). Using this system, dansylalanine was genetically incorporated into hSOD in place of either Gln-16 or Trp-33, and expressed in S. cerevisiae.Position 16 is located on the surface of hSOD at the N-terminus of a β-strand, and position 33 lies in the center of a strand within a β-barrel. To probe changes in the structure of hSOD, researchers exposed the purified protein to guanidinium chloride (GdmCl) to denature the protein at concentrations ranging 0-4.5 M. The experiment was conducted in a 35 mM sodium phosphate buffer at pH 7.2. The fluorescence of hSOD with dansylalanine at either position 16 or 33 was monitored at each GdmCl concentration, and the results of the unfolding experiment are shown in Figure 1.
 Figure 1 Plot of dansylalanine fluorescence at different concentrations of GdmCl
Figure 1 Plot of dansylalanine fluorescence at different concentrations of GdmCl
Adapted from Summerer D, Chen S, Wu N, Deiters A, Chin JW, Schultz PG. A genetically encoded fluorescent amino acid. Proc Natl Acad Sci USA. 2006.
What feature of dansylalanine allows for monitoring protein unfolding?
A)Isomerization of double bonds
B)Conjugated pi bonds
C)Resonance structure at the C-terminus
D)Chirality of the amino acid
Fluorescent amino acids are useful for labeling proteins because they can be visualized spectroscopically, facilitating the study of protein structure and interactions. The noncanonical amino acid dansylalanine, a fluorescent amino acid that is sensitive to the hydrophobicity of its environment, is a good candidate to be incorporated into proteins because of its small size and fluorescent capabilities. Dansyl chloride and Compound 1 were used to synthesize dansylalanine, as shown in Reaction 1. Dansylalanine absorbs maximally at 340 nm and emits at approximately 540 nm.
 Reaction 1 Synthesis of dansylalanineA leucyl-tRNA synthetase from Escherichia coli was engineered to aminoacylate bacterial tRNAs containing a CUA anticodon with dansylalanine. After verifying that the tRNA/synthetase pair does not interact with endogenous amino acids or tRNAs, it was transfected into the yeast Saccharomyces cerevisiae along with a plasmid encoding a mutated human superoxide dismutase (hSOD). Using this system, dansylalanine was genetically incorporated into hSOD in place of either Gln-16 or Trp-33, and expressed in S. cerevisiae.Position 16 is located on the surface of hSOD at the N-terminus of a β-strand, and position 33 lies in the center of a strand within a β-barrel. To probe changes in the structure of hSOD, researchers exposed the purified protein to guanidinium chloride (GdmCl) to denature the protein at concentrations ranging 0-4.5 M. The experiment was conducted in a 35 mM sodium phosphate buffer at pH 7.2. The fluorescence of hSOD with dansylalanine at either position 16 or 33 was monitored at each GdmCl concentration, and the results of the unfolding experiment are shown in Figure 1.
Reaction 1 Synthesis of dansylalanineA leucyl-tRNA synthetase from Escherichia coli was engineered to aminoacylate bacterial tRNAs containing a CUA anticodon with dansylalanine. After verifying that the tRNA/synthetase pair does not interact with endogenous amino acids or tRNAs, it was transfected into the yeast Saccharomyces cerevisiae along with a plasmid encoding a mutated human superoxide dismutase (hSOD). Using this system, dansylalanine was genetically incorporated into hSOD in place of either Gln-16 or Trp-33, and expressed in S. cerevisiae.Position 16 is located on the surface of hSOD at the N-terminus of a β-strand, and position 33 lies in the center of a strand within a β-barrel. To probe changes in the structure of hSOD, researchers exposed the purified protein to guanidinium chloride (GdmCl) to denature the protein at concentrations ranging 0-4.5 M. The experiment was conducted in a 35 mM sodium phosphate buffer at pH 7.2. The fluorescence of hSOD with dansylalanine at either position 16 or 33 was monitored at each GdmCl concentration, and the results of the unfolding experiment are shown in Figure 1. Figure 1 Plot of dansylalanine fluorescence at different concentrations of GdmCl
Figure 1 Plot of dansylalanine fluorescence at different concentrations of GdmClAdapted from Summerer D, Chen S, Wu N, Deiters A, Chin JW, Schultz PG. A genetically encoded fluorescent amino acid. Proc Natl Acad Sci USA. 2006.
What feature of dansylalanine allows for monitoring protein unfolding?
A)Isomerization of double bonds
B)Conjugated pi bonds
C)Resonance structure at the C-terminus
D)Chirality of the amino acid

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
71
Passage
Fluorescent amino acids are useful for labeling proteins because they can be visualized spectroscopically, facilitating the study of protein structure and interactions. The noncanonical amino acid dansylalanine, a fluorescent amino acid that is sensitive to the hydrophobicity of its environment, is a good candidate to be incorporated into proteins because of its small size and fluorescent capabilities. Dansyl chloride and Compound 1 were used to synthesize dansylalanine, as shown in Reaction 1. Dansylalanine absorbs maximally at 340 nm and emits at approximately 540 nm.
 Reaction 1 Synthesis of dansylalanineA leucyl-tRNA synthetase from Escherichia coli was engineered to aminoacylate bacterial tRNAs containing a CUA anticodon with dansylalanine. After verifying that the tRNA/synthetase pair does not interact with endogenous amino acids or tRNAs, it was transfected into the yeast Saccharomyces cerevisiae along with a plasmid encoding a mutated human superoxide dismutase (hSOD). Using this system, dansylalanine was genetically incorporated into hSOD in place of either Gln-16 or Trp-33, and expressed in S. cerevisiae.Position 16 is located on the surface of hSOD at the N-terminus of a β-strand, and position 33 lies in the center of a strand within a β-barrel. To probe changes in the structure of hSOD, researchers exposed the purified protein to guanidinium chloride (GdmCl) to denature the protein at concentrations ranging 0-4.5 M. The experiment was conducted in a 35 mM sodium phosphate buffer at pH 7.2. The fluorescence of hSOD with dansylalanine at either position 16 or 33 was monitored at each GdmCl concentration, and the results of the unfolding experiment are shown in Figure 1.
Reaction 1 Synthesis of dansylalanineA leucyl-tRNA synthetase from Escherichia coli was engineered to aminoacylate bacterial tRNAs containing a CUA anticodon with dansylalanine. After verifying that the tRNA/synthetase pair does not interact with endogenous amino acids or tRNAs, it was transfected into the yeast Saccharomyces cerevisiae along with a plasmid encoding a mutated human superoxide dismutase (hSOD). Using this system, dansylalanine was genetically incorporated into hSOD in place of either Gln-16 or Trp-33, and expressed in S. cerevisiae.Position 16 is located on the surface of hSOD at the N-terminus of a β-strand, and position 33 lies in the center of a strand within a β-barrel. To probe changes in the structure of hSOD, researchers exposed the purified protein to guanidinium chloride (GdmCl) to denature the protein at concentrations ranging 0-4.5 M. The experiment was conducted in a 35 mM sodium phosphate buffer at pH 7.2. The fluorescence of hSOD with dansylalanine at either position 16 or 33 was monitored at each GdmCl concentration, and the results of the unfolding experiment are shown in Figure 1.
 Figure 1 Plot of dansylalanine fluorescence at different concentrations of GdmCl
Figure 1 Plot of dansylalanine fluorescence at different concentrations of GdmCl
Adapted from Summerer D, Chen S, Wu N, Deiters A, Chin JW, Schultz PG. A genetically encoded fluorescent amino acid. Proc Natl Acad Sci USA. 2006.
Is the absolute configuration of dansylalanine shown in Reaction 1 different from most naturally occurring amino acids?
A)Yes, because dansylalanine is an L-amino acid, and all natural chiral amino acids are D configuration
B)Yes, because dansylalanine is a D-amino acid, and all natural chiral amino acids are L configuration
C)No, because dansylalanine has an R configuration, as do all natural chiral amino acids except methionine
D)No, because dansylalanine has an S configuration, as do all natural chiral amino acids except cysteine
Fluorescent amino acids are useful for labeling proteins because they can be visualized spectroscopically, facilitating the study of protein structure and interactions. The noncanonical amino acid dansylalanine, a fluorescent amino acid that is sensitive to the hydrophobicity of its environment, is a good candidate to be incorporated into proteins because of its small size and fluorescent capabilities. Dansyl chloride and Compound 1 were used to synthesize dansylalanine, as shown in Reaction 1. Dansylalanine absorbs maximally at 340 nm and emits at approximately 540 nm.
 Reaction 1 Synthesis of dansylalanineA leucyl-tRNA synthetase from Escherichia coli was engineered to aminoacylate bacterial tRNAs containing a CUA anticodon with dansylalanine. After verifying that the tRNA/synthetase pair does not interact with endogenous amino acids or tRNAs, it was transfected into the yeast Saccharomyces cerevisiae along with a plasmid encoding a mutated human superoxide dismutase (hSOD). Using this system, dansylalanine was genetically incorporated into hSOD in place of either Gln-16 or Trp-33, and expressed in S. cerevisiae.Position 16 is located on the surface of hSOD at the N-terminus of a β-strand, and position 33 lies in the center of a strand within a β-barrel. To probe changes in the structure of hSOD, researchers exposed the purified protein to guanidinium chloride (GdmCl) to denature the protein at concentrations ranging 0-4.5 M. The experiment was conducted in a 35 mM sodium phosphate buffer at pH 7.2. The fluorescence of hSOD with dansylalanine at either position 16 or 33 was monitored at each GdmCl concentration, and the results of the unfolding experiment are shown in Figure 1.
Reaction 1 Synthesis of dansylalanineA leucyl-tRNA synthetase from Escherichia coli was engineered to aminoacylate bacterial tRNAs containing a CUA anticodon with dansylalanine. After verifying that the tRNA/synthetase pair does not interact with endogenous amino acids or tRNAs, it was transfected into the yeast Saccharomyces cerevisiae along with a plasmid encoding a mutated human superoxide dismutase (hSOD). Using this system, dansylalanine was genetically incorporated into hSOD in place of either Gln-16 or Trp-33, and expressed in S. cerevisiae.Position 16 is located on the surface of hSOD at the N-terminus of a β-strand, and position 33 lies in the center of a strand within a β-barrel. To probe changes in the structure of hSOD, researchers exposed the purified protein to guanidinium chloride (GdmCl) to denature the protein at concentrations ranging 0-4.5 M. The experiment was conducted in a 35 mM sodium phosphate buffer at pH 7.2. The fluorescence of hSOD with dansylalanine at either position 16 or 33 was monitored at each GdmCl concentration, and the results of the unfolding experiment are shown in Figure 1. Figure 1 Plot of dansylalanine fluorescence at different concentrations of GdmCl
Figure 1 Plot of dansylalanine fluorescence at different concentrations of GdmClAdapted from Summerer D, Chen S, Wu N, Deiters A, Chin JW, Schultz PG. A genetically encoded fluorescent amino acid. Proc Natl Acad Sci USA. 2006.
Is the absolute configuration of dansylalanine shown in Reaction 1 different from most naturally occurring amino acids?
A)Yes, because dansylalanine is an L-amino acid, and all natural chiral amino acids are D configuration
B)Yes, because dansylalanine is a D-amino acid, and all natural chiral amino acids are L configuration
C)No, because dansylalanine has an R configuration, as do all natural chiral amino acids except methionine
D)No, because dansylalanine has an S configuration, as do all natural chiral amino acids except cysteine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
72
Ubiquinone (shown below) is an important molecule in the electron transport chain, where it receives electrons in complex I originating from NADH.  What is the structure of the product formed when electrons are added to ubiquinone?
What is the structure of the product formed when electrons are added to ubiquinone?
A)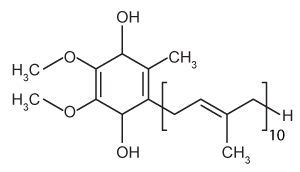
B)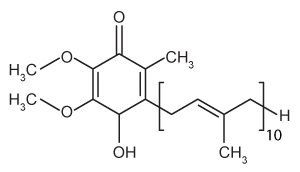
C)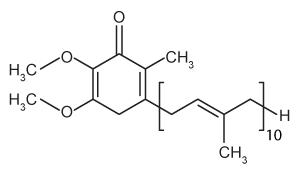
D)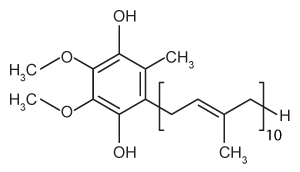
 What is the structure of the product formed when electrons are added to ubiquinone?
What is the structure of the product formed when electrons are added to ubiquinone?A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
73
The molecule (3R,4S)-3-Bromo-4-chlorocyclohexanone is shown below:  Which of the following structures depicts the same molecule?
Which of the following structures depicts the same molecule?
A)
B)
C)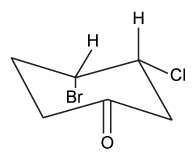
D)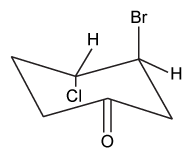
 Which of the following structures depicts the same molecule?
Which of the following structures depicts the same molecule?A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
74
Passage
The β-lactam scaffold is an important feature in a class of broad-spectrum antibiotics that includes the penicillins, cephalosporins, and monobactams. These antibiotics are used to treat a variety of diseases caused by bacteria. The penicillins work by inhibiting a step in the synthesis of peptidoglycan. However, the development of antibiotic resistance in the form of enzymes such as β-lactamase is an ongoing problem.An aromatic ring linked to the β-lactam ring has been reported to participate in hydrophobic interactions with β-lactamase active sites, and an aromatic ring bonded to the nitrogen of the β-lactam has shown to be beneficial to biological activity. With this in mind, researchers synthesized a group of penicillanic acid analogues with substituted aromatic rings bonded to the β-lactam ring in an effort to overcome the challenge of antibiotic resistance. The substituents on the aromatic rings were varied, including -OCH3 and -NO2, to make several analogues. A key step in the synthesis of these analogues was the coupling of Compounds 1 and 2 to form a compound with two β-lactam rings (Reaction 1).
 Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC)
Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC)
 The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1.
The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1.
 Figure 1 Cell viability assay results
Figure 1 Cell viability assay results
Adapted from De rosa M, Vigliotta G, Palma G, Saturnino C, Soriente A. Novel Penicillin-Type Analogues Bearing a Variable Substituted 2-Azetidinone Ring at Position 6: Synthesis and Biological Evaluation. Molecules. 2015.
Which conclusion about the bioactivity of Compounds 3-6 is best supported by the data in Table 1 and Figure 1?
A)The addition of substituted aromatic rings to the penicillin core is detrimental to cell viability.
B)Compound 5 has the greatest effect on cell viability.
C)Compound 3 demonstrates the greatest antimicrobial activity against S. aureus.
D)Compared to ampicillin, Compounds 3-6 display an increased antimicrobial activity against gram-negative bacteria.
The β-lactam scaffold is an important feature in a class of broad-spectrum antibiotics that includes the penicillins, cephalosporins, and monobactams. These antibiotics are used to treat a variety of diseases caused by bacteria. The penicillins work by inhibiting a step in the synthesis of peptidoglycan. However, the development of antibiotic resistance in the form of enzymes such as β-lactamase is an ongoing problem.An aromatic ring linked to the β-lactam ring has been reported to participate in hydrophobic interactions with β-lactamase active sites, and an aromatic ring bonded to the nitrogen of the β-lactam has shown to be beneficial to biological activity. With this in mind, researchers synthesized a group of penicillanic acid analogues with substituted aromatic rings bonded to the β-lactam ring in an effort to overcome the challenge of antibiotic resistance. The substituents on the aromatic rings were varied, including -OCH3 and -NO2, to make several analogues. A key step in the synthesis of these analogues was the coupling of Compounds 1 and 2 to form a compound with two β-lactam rings (Reaction 1).
 Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC)
Reaction 1The researchers studied the analogues' structure-activity relationship and antimicrobial activity against the gram-negative bacteria Escherichia coli and the gram-positive bacteria Staphylococcus aureus. Ampicillin, a penicillin derivative used to treat illnesses brought about by gram-positive and gram-negative bacteria, was used as a control. The minimal inhibitory concentrations are shown in Table 1.Table 1 Minimal Inhibitory Concentration (MIC) The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1.
The penicillanic acid analogues were tested for their effect on mammalian cell viability because toxicity to cells is also an important factor to consider for potential drug candidates. The general acceptable level of cell viability for a drug candidate is 70%. These results are shown in Figure 1. Figure 1 Cell viability assay results
Figure 1 Cell viability assay resultsAdapted from De rosa M, Vigliotta G, Palma G, Saturnino C, Soriente A. Novel Penicillin-Type Analogues Bearing a Variable Substituted 2-Azetidinone Ring at Position 6: Synthesis and Biological Evaluation. Molecules. 2015.
Which conclusion about the bioactivity of Compounds 3-6 is best supported by the data in Table 1 and Figure 1?
A)The addition of substituted aromatic rings to the penicillin core is detrimental to cell viability.
B)Compound 5 has the greatest effect on cell viability.
C)Compound 3 demonstrates the greatest antimicrobial activity against S. aureus.
D)Compared to ampicillin, Compounds 3-6 display an increased antimicrobial activity against gram-negative bacteria.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
75
Researchers want to synthesize valine from isovaleric acid. The enol shown below, with certain carbon atoms labeled, is formed from the reaction of isovaleric acid with PBr3. 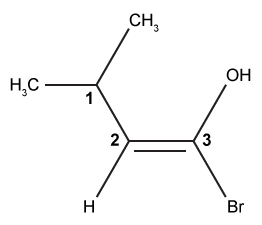 If Br2 and then water are added to the enol, which of the following carbon atoms will be brominated in the final product?
If Br2 and then water are added to the enol, which of the following carbon atoms will be brominated in the final product?
A)Carbons 1 and 2 only
B)Carbon 2 only
C)Carbons 1 and 3 only
D)Carbon 3 only
 If Br2 and then water are added to the enol, which of the following carbon atoms will be brominated in the final product?
If Br2 and then water are added to the enol, which of the following carbon atoms will be brominated in the final product?A)Carbons 1 and 2 only
B)Carbon 2 only
C)Carbons 1 and 3 only
D)Carbon 3 only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
76
Which of the following amino acids would most likely be used as a precursor in the synthesis of Compound X, shown below?  Compound X
Compound X
A)Pro
B)Trp
C)His
D)Tyr
 Compound X
Compound XA)Pro
B)Trp
C)His
D)Tyr

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
77
Passage
Fluorescent amino acids are useful for labeling proteins because they can be visualized spectroscopically, facilitating the study of protein structure and interactions. The noncanonical amino acid dansylalanine, a fluorescent amino acid that is sensitive to the hydrophobicity of its environment, is a good candidate to be incorporated into proteins because of its small size and fluorescent capabilities. Dansyl chloride and Compound 1 were used to synthesize dansylalanine, as shown in Reaction 1. Dansylalanine absorbs maximally at 340 nm and emits at approximately 540 nm.
 Reaction 1 Synthesis of dansylalanineA leucyl-tRNA synthetase from Escherichia coli was engineered to aminoacylate bacterial tRNAs containing a CUA anticodon with dansylalanine. After verifying that the tRNA/synthetase pair does not interact with endogenous amino acids or tRNAs, it was transfected into the yeast Saccharomyces cerevisiae along with a plasmid encoding a mutated human superoxide dismutase (hSOD). Using this system, dansylalanine was genetically incorporated into hSOD in place of either Gln-16 or Trp-33, and expressed in S. cerevisiae.Position 16 is located on the surface of hSOD at the N-terminus of a β-strand, and position 33 lies in the center of a strand within a β-barrel. To probe changes in the structure of hSOD, researchers exposed the purified protein to guanidinium chloride (GdmCl) to denature the protein at concentrations ranging 0-4.5 M. The experiment was conducted in a 35 mM sodium phosphate buffer at pH 7.2. The fluorescence of hSOD with dansylalanine at either position 16 or 33 was monitored at each GdmCl concentration, and the results of the unfolding experiment are shown in Figure 1.
Reaction 1 Synthesis of dansylalanineA leucyl-tRNA synthetase from Escherichia coli was engineered to aminoacylate bacterial tRNAs containing a CUA anticodon with dansylalanine. After verifying that the tRNA/synthetase pair does not interact with endogenous amino acids or tRNAs, it was transfected into the yeast Saccharomyces cerevisiae along with a plasmid encoding a mutated human superoxide dismutase (hSOD). Using this system, dansylalanine was genetically incorporated into hSOD in place of either Gln-16 or Trp-33, and expressed in S. cerevisiae.Position 16 is located on the surface of hSOD at the N-terminus of a β-strand, and position 33 lies in the center of a strand within a β-barrel. To probe changes in the structure of hSOD, researchers exposed the purified protein to guanidinium chloride (GdmCl) to denature the protein at concentrations ranging 0-4.5 M. The experiment was conducted in a 35 mM sodium phosphate buffer at pH 7.2. The fluorescence of hSOD with dansylalanine at either position 16 or 33 was monitored at each GdmCl concentration, and the results of the unfolding experiment are shown in Figure 1.
 Figure 1 Plot of dansylalanine fluorescence at different concentrations of GdmCl
Figure 1 Plot of dansylalanine fluorescence at different concentrations of GdmCl
Adapted from Summerer D, Chen S, Wu N, Deiters A, Chin JW, Schultz PG. A genetically encoded fluorescent amino acid. Proc Natl Acad Sci USA. 2006.
In the synthesis of dansylalanine, the Boc group on Compound 1 most likely functions to:
A)protect the α-amino group from nucleophilic reaction.
B)protect the C-terminus from nucleophilic reaction.
C)make the N-terminus a better leaving group.
D)make the carboxyl a better leaving group.
Fluorescent amino acids are useful for labeling proteins because they can be visualized spectroscopically, facilitating the study of protein structure and interactions. The noncanonical amino acid dansylalanine, a fluorescent amino acid that is sensitive to the hydrophobicity of its environment, is a good candidate to be incorporated into proteins because of its small size and fluorescent capabilities. Dansyl chloride and Compound 1 were used to synthesize dansylalanine, as shown in Reaction 1. Dansylalanine absorbs maximally at 340 nm and emits at approximately 540 nm.
 Reaction 1 Synthesis of dansylalanineA leucyl-tRNA synthetase from Escherichia coli was engineered to aminoacylate bacterial tRNAs containing a CUA anticodon with dansylalanine. After verifying that the tRNA/synthetase pair does not interact with endogenous amino acids or tRNAs, it was transfected into the yeast Saccharomyces cerevisiae along with a plasmid encoding a mutated human superoxide dismutase (hSOD). Using this system, dansylalanine was genetically incorporated into hSOD in place of either Gln-16 or Trp-33, and expressed in S. cerevisiae.Position 16 is located on the surface of hSOD at the N-terminus of a β-strand, and position 33 lies in the center of a strand within a β-barrel. To probe changes in the structure of hSOD, researchers exposed the purified protein to guanidinium chloride (GdmCl) to denature the protein at concentrations ranging 0-4.5 M. The experiment was conducted in a 35 mM sodium phosphate buffer at pH 7.2. The fluorescence of hSOD with dansylalanine at either position 16 or 33 was monitored at each GdmCl concentration, and the results of the unfolding experiment are shown in Figure 1.
Reaction 1 Synthesis of dansylalanineA leucyl-tRNA synthetase from Escherichia coli was engineered to aminoacylate bacterial tRNAs containing a CUA anticodon with dansylalanine. After verifying that the tRNA/synthetase pair does not interact with endogenous amino acids or tRNAs, it was transfected into the yeast Saccharomyces cerevisiae along with a plasmid encoding a mutated human superoxide dismutase (hSOD). Using this system, dansylalanine was genetically incorporated into hSOD in place of either Gln-16 or Trp-33, and expressed in S. cerevisiae.Position 16 is located on the surface of hSOD at the N-terminus of a β-strand, and position 33 lies in the center of a strand within a β-barrel. To probe changes in the structure of hSOD, researchers exposed the purified protein to guanidinium chloride (GdmCl) to denature the protein at concentrations ranging 0-4.5 M. The experiment was conducted in a 35 mM sodium phosphate buffer at pH 7.2. The fluorescence of hSOD with dansylalanine at either position 16 or 33 was monitored at each GdmCl concentration, and the results of the unfolding experiment are shown in Figure 1. Figure 1 Plot of dansylalanine fluorescence at different concentrations of GdmCl
Figure 1 Plot of dansylalanine fluorescence at different concentrations of GdmClAdapted from Summerer D, Chen S, Wu N, Deiters A, Chin JW, Schultz PG. A genetically encoded fluorescent amino acid. Proc Natl Acad Sci USA. 2006.
In the synthesis of dansylalanine, the Boc group on Compound 1 most likely functions to:
A)protect the α-amino group from nucleophilic reaction.
B)protect the C-terminus from nucleophilic reaction.
C)make the N-terminus a better leaving group.
D)make the carboxyl a better leaving group.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
78
The (R)-enantiomer of the antiasthma drug albuterol is the active isomer. If a researcher wants to separate a racemic mixture of albuterol, which of the following methods will most likely separate the enantiomers?
A)Extraction with dilute base
B)Thin-layer chromatography
C)Fractional distillation
D)Addition of a resolving agent
A)Extraction with dilute base
B)Thin-layer chromatography
C)Fractional distillation
D)Addition of a resolving agent

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
79
A solution of Compound 1, shown below, absorbs light maximally at 448 nm in the absence of copper(II) ions but shifts to a 623 nm absorption maximum upon the addition of Cu2+. Which of the following best describes this process? 
A)Changes in electronic structure cause the solution to change from yellow to blue.
B)Changes in vibrational modes cause the solution to change from green to yellow.
C)Changes in the mass-to-charge ratio (m/z) cause the solution to change from violet to orange.
D)Changes in nuclear spin cause the solution to change from colorless to violet.

A)Changes in electronic structure cause the solution to change from yellow to blue.
B)Changes in vibrational modes cause the solution to change from green to yellow.
C)Changes in the mass-to-charge ratio (m/z) cause the solution to change from violet to orange.
D)Changes in nuclear spin cause the solution to change from colorless to violet.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck
80
Passage
Fluorescent amino acids are useful for labeling proteins because they can be visualized spectroscopically, facilitating the study of protein structure and interactions. The noncanonical amino acid dansylalanine, a fluorescent amino acid that is sensitive to the hydrophobicity of its environment, is a good candidate to be incorporated into proteins because of its small size and fluorescent capabilities. Dansyl chloride and Compound 1 were used to synthesize dansylalanine, as shown in Reaction 1. Dansylalanine absorbs maximally at 340 nm and emits at approximately 540 nm.
 Reaction 1 Synthesis of dansylalanineA leucyl-tRNA synthetase from Escherichia coli was engineered to aminoacylate bacterial tRNAs containing a CUA anticodon with dansylalanine. After verifying that the tRNA/synthetase pair does not interact with endogenous amino acids or tRNAs, it was transfected into the yeast Saccharomyces cerevisiae along with a plasmid encoding a mutated human superoxide dismutase (hSOD). Using this system, dansylalanine was genetically incorporated into hSOD in place of either Gln-16 or Trp-33, and expressed in S. cerevisiae.Position 16 is located on the surface of hSOD at the N-terminus of a β-strand, and position 33 lies in the center of a strand within a β-barrel. To probe changes in the structure of hSOD, researchers exposed the purified protein to guanidinium chloride (GdmCl) to denature the protein at concentrations ranging 0-4.5 M. The experiment was conducted in a 35 mM sodium phosphate buffer at pH 7.2. The fluorescence of hSOD with dansylalanine at either position 16 or 33 was monitored at each GdmCl concentration, and the results of the unfolding experiment are shown in Figure 1.
Reaction 1 Synthesis of dansylalanineA leucyl-tRNA synthetase from Escherichia coli was engineered to aminoacylate bacterial tRNAs containing a CUA anticodon with dansylalanine. After verifying that the tRNA/synthetase pair does not interact with endogenous amino acids or tRNAs, it was transfected into the yeast Saccharomyces cerevisiae along with a plasmid encoding a mutated human superoxide dismutase (hSOD). Using this system, dansylalanine was genetically incorporated into hSOD in place of either Gln-16 or Trp-33, and expressed in S. cerevisiae.Position 16 is located on the surface of hSOD at the N-terminus of a β-strand, and position 33 lies in the center of a strand within a β-barrel. To probe changes in the structure of hSOD, researchers exposed the purified protein to guanidinium chloride (GdmCl) to denature the protein at concentrations ranging 0-4.5 M. The experiment was conducted in a 35 mM sodium phosphate buffer at pH 7.2. The fluorescence of hSOD with dansylalanine at either position 16 or 33 was monitored at each GdmCl concentration, and the results of the unfolding experiment are shown in Figure 1.
 Figure 1 Plot of dansylalanine fluorescence at different concentrations of GdmCl
Figure 1 Plot of dansylalanine fluorescence at different concentrations of GdmCl
Adapted from Summerer D, Chen S, Wu N, Deiters A, Chin JW, Schultz PG. A genetically encoded fluorescent amino acid. Proc Natl Acad Sci USA. 2006.
Of the positions where dansylalanine was incorporated, which one most likely experiences the greatest environmental change upon unfolding of the protein?
A)Position 16, because the fluorescence intensity slightly decreases as the concentration of GdmCl increases
B)Position 16, because it exhibits the highest fluorescence intensity at 4.5 M GdmCl
C)Position 33, because the fluorescence intensity greatly decreases as the concentration of GdmCl increases
D)Position 33, because it exhibits the lowest fluorescence intensity at 3.0 M GdmCl
Fluorescent amino acids are useful for labeling proteins because they can be visualized spectroscopically, facilitating the study of protein structure and interactions. The noncanonical amino acid dansylalanine, a fluorescent amino acid that is sensitive to the hydrophobicity of its environment, is a good candidate to be incorporated into proteins because of its small size and fluorescent capabilities. Dansyl chloride and Compound 1 were used to synthesize dansylalanine, as shown in Reaction 1. Dansylalanine absorbs maximally at 340 nm and emits at approximately 540 nm.
 Reaction 1 Synthesis of dansylalanineA leucyl-tRNA synthetase from Escherichia coli was engineered to aminoacylate bacterial tRNAs containing a CUA anticodon with dansylalanine. After verifying that the tRNA/synthetase pair does not interact with endogenous amino acids or tRNAs, it was transfected into the yeast Saccharomyces cerevisiae along with a plasmid encoding a mutated human superoxide dismutase (hSOD). Using this system, dansylalanine was genetically incorporated into hSOD in place of either Gln-16 or Trp-33, and expressed in S. cerevisiae.Position 16 is located on the surface of hSOD at the N-terminus of a β-strand, and position 33 lies in the center of a strand within a β-barrel. To probe changes in the structure of hSOD, researchers exposed the purified protein to guanidinium chloride (GdmCl) to denature the protein at concentrations ranging 0-4.5 M. The experiment was conducted in a 35 mM sodium phosphate buffer at pH 7.2. The fluorescence of hSOD with dansylalanine at either position 16 or 33 was monitored at each GdmCl concentration, and the results of the unfolding experiment are shown in Figure 1.
Reaction 1 Synthesis of dansylalanineA leucyl-tRNA synthetase from Escherichia coli was engineered to aminoacylate bacterial tRNAs containing a CUA anticodon with dansylalanine. After verifying that the tRNA/synthetase pair does not interact with endogenous amino acids or tRNAs, it was transfected into the yeast Saccharomyces cerevisiae along with a plasmid encoding a mutated human superoxide dismutase (hSOD). Using this system, dansylalanine was genetically incorporated into hSOD in place of either Gln-16 or Trp-33, and expressed in S. cerevisiae.Position 16 is located on the surface of hSOD at the N-terminus of a β-strand, and position 33 lies in the center of a strand within a β-barrel. To probe changes in the structure of hSOD, researchers exposed the purified protein to guanidinium chloride (GdmCl) to denature the protein at concentrations ranging 0-4.5 M. The experiment was conducted in a 35 mM sodium phosphate buffer at pH 7.2. The fluorescence of hSOD with dansylalanine at either position 16 or 33 was monitored at each GdmCl concentration, and the results of the unfolding experiment are shown in Figure 1. Figure 1 Plot of dansylalanine fluorescence at different concentrations of GdmCl
Figure 1 Plot of dansylalanine fluorescence at different concentrations of GdmClAdapted from Summerer D, Chen S, Wu N, Deiters A, Chin JW, Schultz PG. A genetically encoded fluorescent amino acid. Proc Natl Acad Sci USA. 2006.
Of the positions where dansylalanine was incorporated, which one most likely experiences the greatest environmental change upon unfolding of the protein?
A)Position 16, because the fluorescence intensity slightly decreases as the concentration of GdmCl increases
B)Position 16, because it exhibits the highest fluorescence intensity at 4.5 M GdmCl
C)Position 33, because the fluorescence intensity greatly decreases as the concentration of GdmCl increases
D)Position 33, because it exhibits the lowest fluorescence intensity at 3.0 M GdmCl

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 191 في هذه المجموعة.
فتح الحزمة
k this deck








