Deck 5: General Chemistry
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/317
العب
ملء الشاشة (f)
Deck 5: General Chemistry
1
Passage
Magnetic resonance imaging (MRI) interprets the nuclear relaxation or relaxivity of hydrogen nuclei in resident water molecules. Hydrogen nuclei in water have two magnetic spin states: alpha and beta. In the presence of an applied magnetic field, these spins will align with the field in an "excited" state and then relax back to the "ground" state, producing a signal with intensity proportional to relaxivity. Frequently, an MRI contrast agent is used to increase the relaxation time in coordinated molecules, resulting in a more intense signal.Commonly used contrast agents include gadolinium-based agents. Depending on its environment, gadolinium (Gd) can have eight or nine sites in its coordination sphere, allowing Gd3+ to interact with eight water molecules as in Gd(H2O)83+. The electronic configuration of gadolinium is shown in Figure 1.
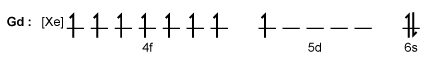 Figure 1 Electronic configuration of gadoliniumGd3+ alone is toxic to cells because its ionic radius is similar to that of Ca2+, allowing Gd3+ to displace Ca2+ in biologically important settings. Therefore, Gd3+ must be coordinated to an organic ligand such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to allow it to pass through the body safely. When chelated, DOTA displaces the water around gadolinium and leaves only one coordination site for a water molecule (Figure 2).
Figure 1 Electronic configuration of gadoliniumGd3+ alone is toxic to cells because its ionic radius is similar to that of Ca2+, allowing Gd3+ to displace Ca2+ in biologically important settings. Therefore, Gd3+ must be coordinated to an organic ligand such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to allow it to pass through the body safely. When chelated, DOTA displaces the water around gadolinium and leaves only one coordination site for a water molecule (Figure 2).
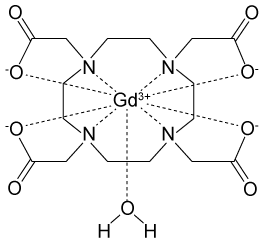 Figure 2 Gd-DOTA with a water coordinated to Gd3+Zinc ions (Zn2+) are required to store insulin and are co-released when insulin is secreted by the pancreas. To detect zinc ions in the body, researchers have designed a variation of Gd-DOTA called Gd-daa3 with two diaminoacetate (daa) arms that preferentially bind to Zn2+ ions (Figure 3). When Zn2+ is absent, these arms coordinate to Gd3+ and create a nine-coordinate complex that prohibits water from binding to Gd3+. Binding of diaminoacetate arms to Zn2+ frees Gd3+ to coordinate with water molecules. This configuration results in increased signal intensity in the parts of the cell where Zn2+ is located.
Figure 2 Gd-DOTA with a water coordinated to Gd3+Zinc ions (Zn2+) are required to store insulin and are co-released when insulin is secreted by the pancreas. To detect zinc ions in the body, researchers have designed a variation of Gd-DOTA called Gd-daa3 with two diaminoacetate (daa) arms that preferentially bind to Zn2+ ions (Figure 3). When Zn2+ is absent, these arms coordinate to Gd3+ and create a nine-coordinate complex that prohibits water from binding to Gd3+. Binding of diaminoacetate arms to Zn2+ frees Gd3+ to coordinate with water molecules. This configuration results in increased signal intensity in the parts of the cell where Zn2+ is located.
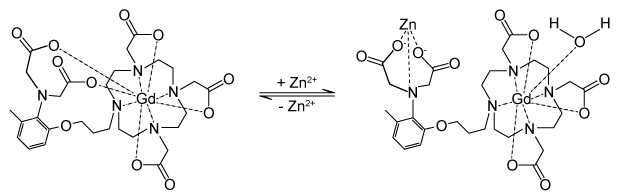 Figure 3 Gd-daa3 when Zn2+ is present
Figure 3 Gd-daa3 when Zn2+ is present
Adapted from Louie A. MRI biosensors: a short primer. J Magn Reson Imaging. 2013;38(3):530-9.
The Heisenberg uncertainty principle impacts the study of nuclei and small particles such as electrons. Which of the following is a consequence of this principle?
A)Two electrons in the same orbital cannot both have parallel alignment with the magnetic field.
B)Neutrons and protons are affected by the nuclear force almost identically.
C)Electrons in coordination bonds can be described only as probability distributions.
D)Particles will emit energy when transitioning from an excited state to a ground state.
Magnetic resonance imaging (MRI) interprets the nuclear relaxation or relaxivity of hydrogen nuclei in resident water molecules. Hydrogen nuclei in water have two magnetic spin states: alpha and beta. In the presence of an applied magnetic field, these spins will align with the field in an "excited" state and then relax back to the "ground" state, producing a signal with intensity proportional to relaxivity. Frequently, an MRI contrast agent is used to increase the relaxation time in coordinated molecules, resulting in a more intense signal.Commonly used contrast agents include gadolinium-based agents. Depending on its environment, gadolinium (Gd) can have eight or nine sites in its coordination sphere, allowing Gd3+ to interact with eight water molecules as in Gd(H2O)83+. The electronic configuration of gadolinium is shown in Figure 1.
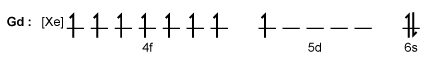 Figure 1 Electronic configuration of gadoliniumGd3+ alone is toxic to cells because its ionic radius is similar to that of Ca2+, allowing Gd3+ to displace Ca2+ in biologically important settings. Therefore, Gd3+ must be coordinated to an organic ligand such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to allow it to pass through the body safely. When chelated, DOTA displaces the water around gadolinium and leaves only one coordination site for a water molecule (Figure 2).
Figure 1 Electronic configuration of gadoliniumGd3+ alone is toxic to cells because its ionic radius is similar to that of Ca2+, allowing Gd3+ to displace Ca2+ in biologically important settings. Therefore, Gd3+ must be coordinated to an organic ligand such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to allow it to pass through the body safely. When chelated, DOTA displaces the water around gadolinium and leaves only one coordination site for a water molecule (Figure 2). Figure 2 Gd-DOTA with a water coordinated to Gd3+Zinc ions (Zn2+) are required to store insulin and are co-released when insulin is secreted by the pancreas. To detect zinc ions in the body, researchers have designed a variation of Gd-DOTA called Gd-daa3 with two diaminoacetate (daa) arms that preferentially bind to Zn2+ ions (Figure 3). When Zn2+ is absent, these arms coordinate to Gd3+ and create a nine-coordinate complex that prohibits water from binding to Gd3+. Binding of diaminoacetate arms to Zn2+ frees Gd3+ to coordinate with water molecules. This configuration results in increased signal intensity in the parts of the cell where Zn2+ is located.
Figure 2 Gd-DOTA with a water coordinated to Gd3+Zinc ions (Zn2+) are required to store insulin and are co-released when insulin is secreted by the pancreas. To detect zinc ions in the body, researchers have designed a variation of Gd-DOTA called Gd-daa3 with two diaminoacetate (daa) arms that preferentially bind to Zn2+ ions (Figure 3). When Zn2+ is absent, these arms coordinate to Gd3+ and create a nine-coordinate complex that prohibits water from binding to Gd3+. Binding of diaminoacetate arms to Zn2+ frees Gd3+ to coordinate with water molecules. This configuration results in increased signal intensity in the parts of the cell where Zn2+ is located. Figure 3 Gd-daa3 when Zn2+ is present
Figure 3 Gd-daa3 when Zn2+ is presentAdapted from Louie A. MRI biosensors: a short primer. J Magn Reson Imaging. 2013;38(3):530-9.
The Heisenberg uncertainty principle impacts the study of nuclei and small particles such as electrons. Which of the following is a consequence of this principle?
A)Two electrons in the same orbital cannot both have parallel alignment with the magnetic field.
B)Neutrons and protons are affected by the nuclear force almost identically.
C)Electrons in coordination bonds can be described only as probability distributions.
D)Particles will emit energy when transitioning from an excited state to a ground state.
Electrons in coordination bonds can be described only as probability distributions.
2
Passage
Magnetic resonance imaging (MRI) interprets the nuclear relaxation or relaxivity of hydrogen nuclei in resident water molecules. Hydrogen nuclei in water have two magnetic spin states: alpha and beta. In the presence of an applied magnetic field, these spins will align with the field in an "excited" state and then relax back to the "ground" state, producing a signal with intensity proportional to relaxivity. Frequently, an MRI contrast agent is used to increase the relaxation time in coordinated molecules, resulting in a more intense signal.Commonly used contrast agents include gadolinium-based agents. Depending on its environment, gadolinium (Gd) can have eight or nine sites in its coordination sphere, allowing Gd3+ to interact with eight water molecules as in Gd(H2O)83+. The electronic configuration of gadolinium is shown in Figure 1.
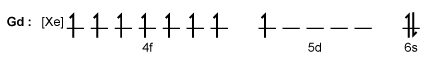 Figure 1 Electronic configuration of gadoliniumGd3+ alone is toxic to cells because its ionic radius is similar to that of Ca2+, allowing Gd3+ to displace Ca2+ in biologically important settings. Therefore, Gd3+ must be coordinated to an organic ligand such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to allow it to pass through the body safely. When chelated, DOTA displaces the water around gadolinium and leaves only one coordination site for a water molecule (Figure 2).
Figure 1 Electronic configuration of gadoliniumGd3+ alone is toxic to cells because its ionic radius is similar to that of Ca2+, allowing Gd3+ to displace Ca2+ in biologically important settings. Therefore, Gd3+ must be coordinated to an organic ligand such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to allow it to pass through the body safely. When chelated, DOTA displaces the water around gadolinium and leaves only one coordination site for a water molecule (Figure 2).
 Figure 2 Gd-DOTA with a water coordinated to Gd3+Zinc ions (Zn2+) are required to store insulin and are co-released when insulin is secreted by the pancreas. To detect zinc ions in the body, researchers have designed a variation of Gd-DOTA called Gd-daa3 with two diaminoacetate (daa) arms that preferentially bind to Zn2+ ions (Figure 3). When Zn2+ is absent, these arms coordinate to Gd3+ and create a nine-coordinate complex that prohibits water from binding to Gd3+. Binding of diaminoacetate arms to Zn2+ frees Gd3+ to coordinate with water molecules. This configuration results in increased signal intensity in the parts of the cell where Zn2+ is located.
Figure 2 Gd-DOTA with a water coordinated to Gd3+Zinc ions (Zn2+) are required to store insulin and are co-released when insulin is secreted by the pancreas. To detect zinc ions in the body, researchers have designed a variation of Gd-DOTA called Gd-daa3 with two diaminoacetate (daa) arms that preferentially bind to Zn2+ ions (Figure 3). When Zn2+ is absent, these arms coordinate to Gd3+ and create a nine-coordinate complex that prohibits water from binding to Gd3+. Binding of diaminoacetate arms to Zn2+ frees Gd3+ to coordinate with water molecules. This configuration results in increased signal intensity in the parts of the cell where Zn2+ is located.
 Figure 3 Gd-daa3 when Zn2+ is present
Figure 3 Gd-daa3 when Zn2+ is present
Adapted from Louie A. MRI biosensors: a short primer. J Magn Reson Imaging. 2013;38(3):530-9.
Two MRI images of the pancreas were taken at different times (Time A and Time B) in a mouse injected with Gd-daa3. Greater relaxivity was detected at Time B, suggesting all of the following EXCEPT:
A)Time B was soon after a meal.
B)fewer daa arms were bound to gadolinium ions at Time B than at Time A.
C)the relaxivity of water molecule nuclei was increased at Time B.
D)Gd3+ are bound to Zn2+ at Time B.
Magnetic resonance imaging (MRI) interprets the nuclear relaxation or relaxivity of hydrogen nuclei in resident water molecules. Hydrogen nuclei in water have two magnetic spin states: alpha and beta. In the presence of an applied magnetic field, these spins will align with the field in an "excited" state and then relax back to the "ground" state, producing a signal with intensity proportional to relaxivity. Frequently, an MRI contrast agent is used to increase the relaxation time in coordinated molecules, resulting in a more intense signal.Commonly used contrast agents include gadolinium-based agents. Depending on its environment, gadolinium (Gd) can have eight or nine sites in its coordination sphere, allowing Gd3+ to interact with eight water molecules as in Gd(H2O)83+. The electronic configuration of gadolinium is shown in Figure 1.
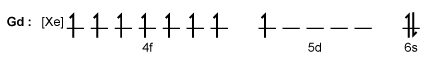 Figure 1 Electronic configuration of gadoliniumGd3+ alone is toxic to cells because its ionic radius is similar to that of Ca2+, allowing Gd3+ to displace Ca2+ in biologically important settings. Therefore, Gd3+ must be coordinated to an organic ligand such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to allow it to pass through the body safely. When chelated, DOTA displaces the water around gadolinium and leaves only one coordination site for a water molecule (Figure 2).
Figure 1 Electronic configuration of gadoliniumGd3+ alone is toxic to cells because its ionic radius is similar to that of Ca2+, allowing Gd3+ to displace Ca2+ in biologically important settings. Therefore, Gd3+ must be coordinated to an organic ligand such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to allow it to pass through the body safely. When chelated, DOTA displaces the water around gadolinium and leaves only one coordination site for a water molecule (Figure 2). Figure 2 Gd-DOTA with a water coordinated to Gd3+Zinc ions (Zn2+) are required to store insulin and are co-released when insulin is secreted by the pancreas. To detect zinc ions in the body, researchers have designed a variation of Gd-DOTA called Gd-daa3 with two diaminoacetate (daa) arms that preferentially bind to Zn2+ ions (Figure 3). When Zn2+ is absent, these arms coordinate to Gd3+ and create a nine-coordinate complex that prohibits water from binding to Gd3+. Binding of diaminoacetate arms to Zn2+ frees Gd3+ to coordinate with water molecules. This configuration results in increased signal intensity in the parts of the cell where Zn2+ is located.
Figure 2 Gd-DOTA with a water coordinated to Gd3+Zinc ions (Zn2+) are required to store insulin and are co-released when insulin is secreted by the pancreas. To detect zinc ions in the body, researchers have designed a variation of Gd-DOTA called Gd-daa3 with two diaminoacetate (daa) arms that preferentially bind to Zn2+ ions (Figure 3). When Zn2+ is absent, these arms coordinate to Gd3+ and create a nine-coordinate complex that prohibits water from binding to Gd3+. Binding of diaminoacetate arms to Zn2+ frees Gd3+ to coordinate with water molecules. This configuration results in increased signal intensity in the parts of the cell where Zn2+ is located. Figure 3 Gd-daa3 when Zn2+ is present
Figure 3 Gd-daa3 when Zn2+ is presentAdapted from Louie A. MRI biosensors: a short primer. J Magn Reson Imaging. 2013;38(3):530-9.
Two MRI images of the pancreas were taken at different times (Time A and Time B) in a mouse injected with Gd-daa3. Greater relaxivity was detected at Time B, suggesting all of the following EXCEPT:
A)Time B was soon after a meal.
B)fewer daa arms were bound to gadolinium ions at Time B than at Time A.
C)the relaxivity of water molecule nuclei was increased at Time B.
D)Gd3+ are bound to Zn2+ at Time B.
Gd3+ are bound to Zn2+ at Time B.
3
Passage
Nuclear medicine uses radiopharmaceuticals for disease treatment and as tracers in medical imaging studies. Yttrium-90 (90Y) is a radiopharmaceutical agent used to treat overgrown joint lining known as pigmented villonodular synovitis, as well as some forms of liver cancer. It has a half-life of about 64 hours.Although the relatively short half-life of 90Y is optimal for use in medical applications, transportation and storage of the radiopharmaceutical are not feasible. As a result, strontium-90 (90Sr), with a half-life of 28.8 years, is the most common source of 90Y. 90Sr is created by a nuclear fission process that begins with uranium-235 (235U) in a nuclear reactor as shown in Reaction 1.235U → 90Sr + ZReaction 1Fission of 235U produces 90Sr as well as additional fission products (Z) including, but not limited to, technetium-99 (99Tc), iodine-129 (129I), and zirconium-93 (93Zr). Figure 1 shows the distribution of fission products.
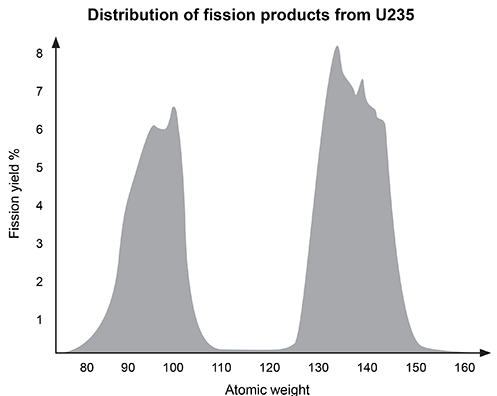 Figure 1 Distribution of 235U fission products by atomic weightTo create the final 90Y required for nuclear medicine studies, 90Sr decay is carried out in a controlled 90Y generator. 90Y is then separated from residual 90Sr for use in clinical applications.
Figure 1 Distribution of 235U fission products by atomic weightTo create the final 90Y required for nuclear medicine studies, 90Sr decay is carried out in a controlled 90Y generator. 90Y is then separated from residual 90Sr for use in clinical applications.
Adapted from Wheeler CE. Comments on vaccines, August 1987. J Am Acad Dermatol. 1988;18(1 Pt 2):232-4.
A patient is injected with 20 ng of 90Y. Assuming yttrium is not excreted, how long will it take for the initial amount of 90Y to be reduced to 2.5 ng?
A)64 hours
B)128 hours
C)192 hours
D)256 hours
Nuclear medicine uses radiopharmaceuticals for disease treatment and as tracers in medical imaging studies. Yttrium-90 (90Y) is a radiopharmaceutical agent used to treat overgrown joint lining known as pigmented villonodular synovitis, as well as some forms of liver cancer. It has a half-life of about 64 hours.Although the relatively short half-life of 90Y is optimal for use in medical applications, transportation and storage of the radiopharmaceutical are not feasible. As a result, strontium-90 (90Sr), with a half-life of 28.8 years, is the most common source of 90Y. 90Sr is created by a nuclear fission process that begins with uranium-235 (235U) in a nuclear reactor as shown in Reaction 1.235U → 90Sr + ZReaction 1Fission of 235U produces 90Sr as well as additional fission products (Z) including, but not limited to, technetium-99 (99Tc), iodine-129 (129I), and zirconium-93 (93Zr). Figure 1 shows the distribution of fission products.
 Figure 1 Distribution of 235U fission products by atomic weightTo create the final 90Y required for nuclear medicine studies, 90Sr decay is carried out in a controlled 90Y generator. 90Y is then separated from residual 90Sr for use in clinical applications.
Figure 1 Distribution of 235U fission products by atomic weightTo create the final 90Y required for nuclear medicine studies, 90Sr decay is carried out in a controlled 90Y generator. 90Y is then separated from residual 90Sr for use in clinical applications.Adapted from Wheeler CE. Comments on vaccines, August 1987. J Am Acad Dermatol. 1988;18(1 Pt 2):232-4.
A patient is injected with 20 ng of 90Y. Assuming yttrium is not excreted, how long will it take for the initial amount of 90Y to be reduced to 2.5 ng?
A)64 hours
B)128 hours
C)192 hours
D)256 hours
192 hours
4
Passage
Depending on the active compound's specific method of action, sunscreens are broadly classified into two groups: organic and inorganic. Organic sunscreens typically contain aromatic compounds that absorb ultraviolet (UV) radiation. The absorbed energy from the UV radiation causes these compounds to enter electronically excited states which then relax back to the ground state and harmlessly re-emit the absorbed radiation as heat. Alternatively, inorganic sunscreens contain metallic nanoparticles that serve to reflect, scatter, and absorb incident radiation. The mechanism for UV protection of each group is shown in Figure 1.
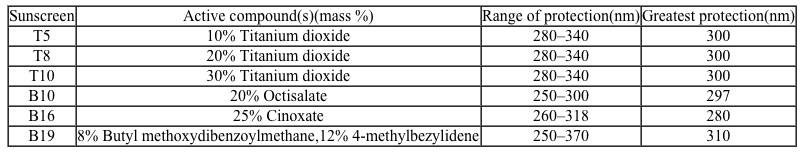
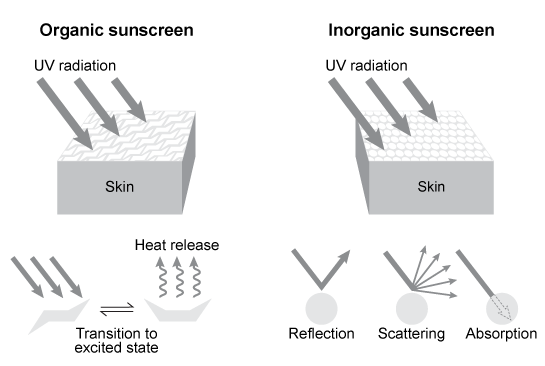 Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF). However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens
Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF). However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens
 In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested.
In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested.
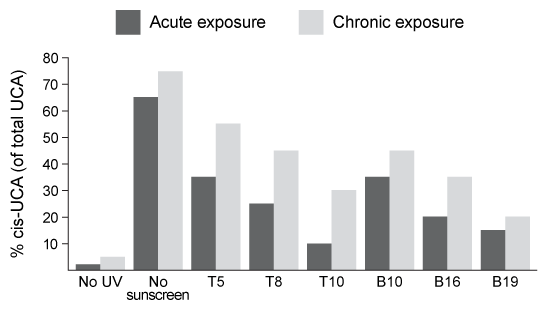 Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreens
Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreens
Adapted from Van der molen RG, Out-luiting C, Driller H, Claas FH, Koerten HK, Mommaas AM. Broad-spectrum sunscreens offer protection against urocanic acid photoisomerization by artificial ultraviolet radiation in human skin. J Invest Dermatol. 2000;115(3):421-6.
What is the molarity of cinoxate (0.250 kg/mol) in 100 mL of sunscreen B16 (density = 1 g/mL)?
A)100 M
B)0.0010 M
C)1.0 M
D)1000 M
Depending on the active compound's specific method of action, sunscreens are broadly classified into two groups: organic and inorganic. Organic sunscreens typically contain aromatic compounds that absorb ultraviolet (UV) radiation. The absorbed energy from the UV radiation causes these compounds to enter electronically excited states which then relax back to the ground state and harmlessly re-emit the absorbed radiation as heat. Alternatively, inorganic sunscreens contain metallic nanoparticles that serve to reflect, scatter, and absorb incident radiation. The mechanism for UV protection of each group is shown in Figure 1.
 Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF). However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens
Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF). However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested.
In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested. Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreens
Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreensAdapted from Van der molen RG, Out-luiting C, Driller H, Claas FH, Koerten HK, Mommaas AM. Broad-spectrum sunscreens offer protection against urocanic acid photoisomerization by artificial ultraviolet radiation in human skin. J Invest Dermatol. 2000;115(3):421-6.
What is the molarity of cinoxate (0.250 kg/mol) in 100 mL of sunscreen B16 (density = 1 g/mL)?
A)100 M
B)0.0010 M
C)1.0 M
D)1000 M

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
5
Passage
Nuclear medicine uses radiopharmaceuticals for disease treatment and as tracers in medical imaging studies. Yttrium-90 (90Y) is a radiopharmaceutical agent used to treat overgrown joint lining known as pigmented villonodular synovitis, as well as some forms of liver cancer. It has a half-life of about 64 hours.Although the relatively short half-life of 90Y is optimal for use in medical applications, transportation and storage of the radiopharmaceutical are not feasible. As a result, strontium-90 (90Sr), with a half-life of 28.8 years, is the most common source of 90Y. 90Sr is created by a nuclear fission process that begins with uranium-235 (235U) in a nuclear reactor as shown in Reaction 1.235U → 90Sr + ZReaction 1Fission of 235U produces 90Sr as well as additional fission products (Z) including, but not limited to, technetium-99 (99Tc), iodine-129 (129I), and zirconium-93 (93Zr). Figure 1 shows the distribution of fission products.
 Figure 1 Distribution of 235U fission products by atomic weightTo create the final 90Y required for nuclear medicine studies, 90Sr decay is carried out in a controlled 90Y generator. 90Y is then separated from residual 90Sr for use in clinical applications.
Figure 1 Distribution of 235U fission products by atomic weightTo create the final 90Y required for nuclear medicine studies, 90Sr decay is carried out in a controlled 90Y generator. 90Y is then separated from residual 90Sr for use in clinical applications.
Adapted from Wheeler CE. Comments on vaccines, August 1987. J Am Acad Dermatol. 1988;18(1 Pt 2):232-4.
Fission of 235U produces multiple elements. Based on Figure 1, which of the following is the atomic number of the most common fission product?
A)38
B)55
C)92
D)133
Nuclear medicine uses radiopharmaceuticals for disease treatment and as tracers in medical imaging studies. Yttrium-90 (90Y) is a radiopharmaceutical agent used to treat overgrown joint lining known as pigmented villonodular synovitis, as well as some forms of liver cancer. It has a half-life of about 64 hours.Although the relatively short half-life of 90Y is optimal for use in medical applications, transportation and storage of the radiopharmaceutical are not feasible. As a result, strontium-90 (90Sr), with a half-life of 28.8 years, is the most common source of 90Y. 90Sr is created by a nuclear fission process that begins with uranium-235 (235U) in a nuclear reactor as shown in Reaction 1.235U → 90Sr + ZReaction 1Fission of 235U produces 90Sr as well as additional fission products (Z) including, but not limited to, technetium-99 (99Tc), iodine-129 (129I), and zirconium-93 (93Zr). Figure 1 shows the distribution of fission products.
 Figure 1 Distribution of 235U fission products by atomic weightTo create the final 90Y required for nuclear medicine studies, 90Sr decay is carried out in a controlled 90Y generator. 90Y is then separated from residual 90Sr for use in clinical applications.
Figure 1 Distribution of 235U fission products by atomic weightTo create the final 90Y required for nuclear medicine studies, 90Sr decay is carried out in a controlled 90Y generator. 90Y is then separated from residual 90Sr for use in clinical applications.Adapted from Wheeler CE. Comments on vaccines, August 1987. J Am Acad Dermatol. 1988;18(1 Pt 2):232-4.
Fission of 235U produces multiple elements. Based on Figure 1, which of the following is the atomic number of the most common fission product?
A)38
B)55
C)92
D)133

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
6
Passage
The bicarbonate (HCO3−) buffer system (Reaction 1) helps to maintain acid-base homeostasis and a blood pH near 7.4. Therefore, concentrations of carbon dioxide (CO2) and HCO3− must be tightly regulated through adaptations in respiratory and renal physiology. For patients with suspected acid-base imbalance, these concentrations can be monitored by blood gas analysis performed on arterial or venous blood.
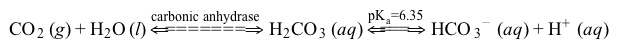 CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1). The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample.
CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1). The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample.
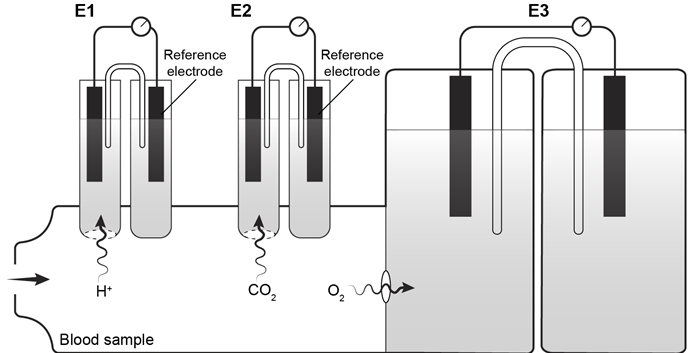 Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3), reported in milliequivalents per liter (mEq/L), can be calculated from PaCO2 by Equation 1:
Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3), reported in milliequivalents per liter (mEq/L), can be calculated from PaCO2 by Equation 1:
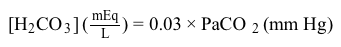 H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.
H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.
In the Clark electrode, O2 from the blood is:
A)oxidized at the anode.
B)oxidized at the cathode.
C)reduced at the anode.
D)reduced at the cathode.
The bicarbonate (HCO3−) buffer system (Reaction 1) helps to maintain acid-base homeostasis and a blood pH near 7.4. Therefore, concentrations of carbon dioxide (CO2) and HCO3− must be tightly regulated through adaptations in respiratory and renal physiology. For patients with suspected acid-base imbalance, these concentrations can be monitored by blood gas analysis performed on arterial or venous blood.
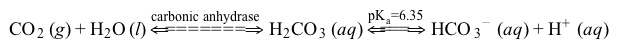 CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1). The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample.
CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1). The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample. Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3), reported in milliequivalents per liter (mEq/L), can be calculated from PaCO2 by Equation 1:
Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3), reported in milliequivalents per liter (mEq/L), can be calculated from PaCO2 by Equation 1: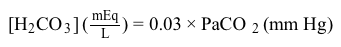 H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.
H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.In the Clark electrode, O2 from the blood is:
A)oxidized at the anode.
B)oxidized at the cathode.
C)reduced at the anode.
D)reduced at the cathode.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
7
Passage
Magnetic resonance imaging (MRI) interprets the nuclear relaxation or relaxivity of hydrogen nuclei in resident water molecules. Hydrogen nuclei in water have two magnetic spin states: alpha and beta. In the presence of an applied magnetic field, these spins will align with the field in an "excited" state and then relax back to the "ground" state, producing a signal with intensity proportional to relaxivity. Frequently, an MRI contrast agent is used to increase the relaxation time in coordinated molecules, resulting in a more intense signal.Commonly used contrast agents include gadolinium-based agents. Depending on its environment, gadolinium (Gd) can have eight or nine sites in its coordination sphere, allowing Gd3+ to interact with eight water molecules as in Gd(H2O)83+. The electronic configuration of gadolinium is shown in Figure 1.
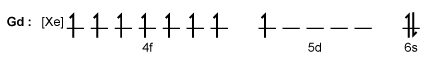 Figure 1 Electronic configuration of gadoliniumGd3+ alone is toxic to cells because its ionic radius is similar to that of Ca2+, allowing Gd3+ to displace Ca2+ in biologically important settings. Therefore, Gd3+ must be coordinated to an organic ligand such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to allow it to pass through the body safely. When chelated, DOTA displaces the water around gadolinium and leaves only one coordination site for a water molecule (Figure 2).
Figure 1 Electronic configuration of gadoliniumGd3+ alone is toxic to cells because its ionic radius is similar to that of Ca2+, allowing Gd3+ to displace Ca2+ in biologically important settings. Therefore, Gd3+ must be coordinated to an organic ligand such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to allow it to pass through the body safely. When chelated, DOTA displaces the water around gadolinium and leaves only one coordination site for a water molecule (Figure 2).
 Figure 2 Gd-DOTA with a water coordinated to Gd3+Zinc ions (Zn2+) are required to store insulin and are co-released when insulin is secreted by the pancreas. To detect zinc ions in the body, researchers have designed a variation of Gd-DOTA called Gd-daa3 with two diaminoacetate (daa) arms that preferentially bind to Zn2+ ions (Figure 3). When Zn2+ is absent, these arms coordinate to Gd3+ and create a nine-coordinate complex that prohibits water from binding to Gd3+. Binding of diaminoacetate arms to Zn2+ frees Gd3+ to coordinate with water molecules. This configuration results in increased signal intensity in the parts of the cell where Zn2+ is located.
Figure 2 Gd-DOTA with a water coordinated to Gd3+Zinc ions (Zn2+) are required to store insulin and are co-released when insulin is secreted by the pancreas. To detect zinc ions in the body, researchers have designed a variation of Gd-DOTA called Gd-daa3 with two diaminoacetate (daa) arms that preferentially bind to Zn2+ ions (Figure 3). When Zn2+ is absent, these arms coordinate to Gd3+ and create a nine-coordinate complex that prohibits water from binding to Gd3+. Binding of diaminoacetate arms to Zn2+ frees Gd3+ to coordinate with water molecules. This configuration results in increased signal intensity in the parts of the cell where Zn2+ is located.
 Figure 3 Gd-daa3 when Zn2+ is present
Figure 3 Gd-daa3 when Zn2+ is present
Adapted from Louie A. MRI biosensors: a short primer. J Magn Reson Imaging. 2013;38(3):530-9.
To determine whether Gd-daa3 is selective for Zn2+, researchers compared relaxivity measurements of Gd-daa3 in the presence of different cations. Varying amounts of XCl2 (where X is Zn, Ca, or Mg) were added to a 0.1 mM solution of Gd-daa3 plus 10 mM KCl/10 mM HEPES buffer at pH 7.4. What is the most appropriate control for this experiment?
A)Relaxivity measurements without XCl2
B)Relaxivity measurements with ZnCl2
C)Relaxivity measurements with BeCl2
D)Relaxivity measurements without Gd-daa3
Magnetic resonance imaging (MRI) interprets the nuclear relaxation or relaxivity of hydrogen nuclei in resident water molecules. Hydrogen nuclei in water have two magnetic spin states: alpha and beta. In the presence of an applied magnetic field, these spins will align with the field in an "excited" state and then relax back to the "ground" state, producing a signal with intensity proportional to relaxivity. Frequently, an MRI contrast agent is used to increase the relaxation time in coordinated molecules, resulting in a more intense signal.Commonly used contrast agents include gadolinium-based agents. Depending on its environment, gadolinium (Gd) can have eight or nine sites in its coordination sphere, allowing Gd3+ to interact with eight water molecules as in Gd(H2O)83+. The electronic configuration of gadolinium is shown in Figure 1.
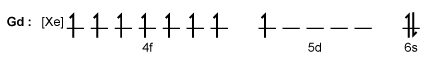 Figure 1 Electronic configuration of gadoliniumGd3+ alone is toxic to cells because its ionic radius is similar to that of Ca2+, allowing Gd3+ to displace Ca2+ in biologically important settings. Therefore, Gd3+ must be coordinated to an organic ligand such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to allow it to pass through the body safely. When chelated, DOTA displaces the water around gadolinium and leaves only one coordination site for a water molecule (Figure 2).
Figure 1 Electronic configuration of gadoliniumGd3+ alone is toxic to cells because its ionic radius is similar to that of Ca2+, allowing Gd3+ to displace Ca2+ in biologically important settings. Therefore, Gd3+ must be coordinated to an organic ligand such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to allow it to pass through the body safely. When chelated, DOTA displaces the water around gadolinium and leaves only one coordination site for a water molecule (Figure 2). Figure 2 Gd-DOTA with a water coordinated to Gd3+Zinc ions (Zn2+) are required to store insulin and are co-released when insulin is secreted by the pancreas. To detect zinc ions in the body, researchers have designed a variation of Gd-DOTA called Gd-daa3 with two diaminoacetate (daa) arms that preferentially bind to Zn2+ ions (Figure 3). When Zn2+ is absent, these arms coordinate to Gd3+ and create a nine-coordinate complex that prohibits water from binding to Gd3+. Binding of diaminoacetate arms to Zn2+ frees Gd3+ to coordinate with water molecules. This configuration results in increased signal intensity in the parts of the cell where Zn2+ is located.
Figure 2 Gd-DOTA with a water coordinated to Gd3+Zinc ions (Zn2+) are required to store insulin and are co-released when insulin is secreted by the pancreas. To detect zinc ions in the body, researchers have designed a variation of Gd-DOTA called Gd-daa3 with two diaminoacetate (daa) arms that preferentially bind to Zn2+ ions (Figure 3). When Zn2+ is absent, these arms coordinate to Gd3+ and create a nine-coordinate complex that prohibits water from binding to Gd3+. Binding of diaminoacetate arms to Zn2+ frees Gd3+ to coordinate with water molecules. This configuration results in increased signal intensity in the parts of the cell where Zn2+ is located. Figure 3 Gd-daa3 when Zn2+ is present
Figure 3 Gd-daa3 when Zn2+ is presentAdapted from Louie A. MRI biosensors: a short primer. J Magn Reson Imaging. 2013;38(3):530-9.
To determine whether Gd-daa3 is selective for Zn2+, researchers compared relaxivity measurements of Gd-daa3 in the presence of different cations. Varying amounts of XCl2 (where X is Zn, Ca, or Mg) were added to a 0.1 mM solution of Gd-daa3 plus 10 mM KCl/10 mM HEPES buffer at pH 7.4. What is the most appropriate control for this experiment?
A)Relaxivity measurements without XCl2
B)Relaxivity measurements with ZnCl2
C)Relaxivity measurements with BeCl2
D)Relaxivity measurements without Gd-daa3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
8
Passage
Magnetic resonance imaging (MRI) interprets the nuclear relaxation or relaxivity of hydrogen nuclei in resident water molecules. Hydrogen nuclei in water have two magnetic spin states: alpha and beta. In the presence of an applied magnetic field, these spins will align with the field in an "excited" state and then relax back to the "ground" state, producing a signal with intensity proportional to relaxivity. Frequently, an MRI contrast agent is used to increase the relaxation time in coordinated molecules, resulting in a more intense signal.Commonly used contrast agents include gadolinium-based agents. Depending on its environment, gadolinium (Gd) can have eight or nine sites in its coordination sphere, allowing Gd3+ to interact with eight water molecules as in Gd(H2O)83+. The electronic configuration of gadolinium is shown in Figure 1.
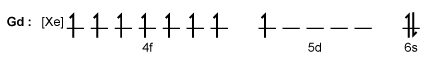 Figure 1 Electronic configuration of gadoliniumGd3+ alone is toxic to cells because its ionic radius is similar to that of Ca2+, allowing Gd3+ to displace Ca2+ in biologically important settings. Therefore, Gd3+ must be coordinated to an organic ligand such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to allow it to pass through the body safely. When chelated, DOTA displaces the water around gadolinium and leaves only one coordination site for a water molecule (Figure 2).
Figure 1 Electronic configuration of gadoliniumGd3+ alone is toxic to cells because its ionic radius is similar to that of Ca2+, allowing Gd3+ to displace Ca2+ in biologically important settings. Therefore, Gd3+ must be coordinated to an organic ligand such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to allow it to pass through the body safely. When chelated, DOTA displaces the water around gadolinium and leaves only one coordination site for a water molecule (Figure 2).
 Figure 2 Gd-DOTA with a water coordinated to Gd3+Zinc ions (Zn2+) are required to store insulin and are co-released when insulin is secreted by the pancreas. To detect zinc ions in the body, researchers have designed a variation of Gd-DOTA called Gd-daa3 with two diaminoacetate (daa) arms that preferentially bind to Zn2+ ions (Figure 3). When Zn2+ is absent, these arms coordinate to Gd3+ and create a nine-coordinate complex that prohibits water from binding to Gd3+. Binding of diaminoacetate arms to Zn2+ frees Gd3+ to coordinate with water molecules. This configuration results in increased signal intensity in the parts of the cell where Zn2+ is located.
Figure 2 Gd-DOTA with a water coordinated to Gd3+Zinc ions (Zn2+) are required to store insulin and are co-released when insulin is secreted by the pancreas. To detect zinc ions in the body, researchers have designed a variation of Gd-DOTA called Gd-daa3 with two diaminoacetate (daa) arms that preferentially bind to Zn2+ ions (Figure 3). When Zn2+ is absent, these arms coordinate to Gd3+ and create a nine-coordinate complex that prohibits water from binding to Gd3+. Binding of diaminoacetate arms to Zn2+ frees Gd3+ to coordinate with water molecules. This configuration results in increased signal intensity in the parts of the cell where Zn2+ is located.
 Figure 3 Gd-daa3 when Zn2+ is present
Figure 3 Gd-daa3 when Zn2+ is present
Adapted from Louie A. MRI biosensors: a short primer. J Magn Reson Imaging. 2013;38(3):530-9.
When Gd3+ binds to DOTA, Gd3+ is acting as a(n):
A)Lewis acid
B)Lewis base
C)Arrhenius acid
D)Arrhenius base
Magnetic resonance imaging (MRI) interprets the nuclear relaxation or relaxivity of hydrogen nuclei in resident water molecules. Hydrogen nuclei in water have two magnetic spin states: alpha and beta. In the presence of an applied magnetic field, these spins will align with the field in an "excited" state and then relax back to the "ground" state, producing a signal with intensity proportional to relaxivity. Frequently, an MRI contrast agent is used to increase the relaxation time in coordinated molecules, resulting in a more intense signal.Commonly used contrast agents include gadolinium-based agents. Depending on its environment, gadolinium (Gd) can have eight or nine sites in its coordination sphere, allowing Gd3+ to interact with eight water molecules as in Gd(H2O)83+. The electronic configuration of gadolinium is shown in Figure 1.
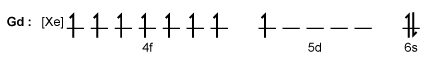 Figure 1 Electronic configuration of gadoliniumGd3+ alone is toxic to cells because its ionic radius is similar to that of Ca2+, allowing Gd3+ to displace Ca2+ in biologically important settings. Therefore, Gd3+ must be coordinated to an organic ligand such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to allow it to pass through the body safely. When chelated, DOTA displaces the water around gadolinium and leaves only one coordination site for a water molecule (Figure 2).
Figure 1 Electronic configuration of gadoliniumGd3+ alone is toxic to cells because its ionic radius is similar to that of Ca2+, allowing Gd3+ to displace Ca2+ in biologically important settings. Therefore, Gd3+ must be coordinated to an organic ligand such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to allow it to pass through the body safely. When chelated, DOTA displaces the water around gadolinium and leaves only one coordination site for a water molecule (Figure 2). Figure 2 Gd-DOTA with a water coordinated to Gd3+Zinc ions (Zn2+) are required to store insulin and are co-released when insulin is secreted by the pancreas. To detect zinc ions in the body, researchers have designed a variation of Gd-DOTA called Gd-daa3 with two diaminoacetate (daa) arms that preferentially bind to Zn2+ ions (Figure 3). When Zn2+ is absent, these arms coordinate to Gd3+ and create a nine-coordinate complex that prohibits water from binding to Gd3+. Binding of diaminoacetate arms to Zn2+ frees Gd3+ to coordinate with water molecules. This configuration results in increased signal intensity in the parts of the cell where Zn2+ is located.
Figure 2 Gd-DOTA with a water coordinated to Gd3+Zinc ions (Zn2+) are required to store insulin and are co-released when insulin is secreted by the pancreas. To detect zinc ions in the body, researchers have designed a variation of Gd-DOTA called Gd-daa3 with two diaminoacetate (daa) arms that preferentially bind to Zn2+ ions (Figure 3). When Zn2+ is absent, these arms coordinate to Gd3+ and create a nine-coordinate complex that prohibits water from binding to Gd3+. Binding of diaminoacetate arms to Zn2+ frees Gd3+ to coordinate with water molecules. This configuration results in increased signal intensity in the parts of the cell where Zn2+ is located. Figure 3 Gd-daa3 when Zn2+ is present
Figure 3 Gd-daa3 when Zn2+ is presentAdapted from Louie A. MRI biosensors: a short primer. J Magn Reson Imaging. 2013;38(3):530-9.
When Gd3+ binds to DOTA, Gd3+ is acting as a(n):
A)Lewis acid
B)Lewis base
C)Arrhenius acid
D)Arrhenius base

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
9
Passage
The bicarbonate (HCO3−) buffer system (Reaction 1) helps to maintain acid-base homeostasis and a blood pH near 7.4. Therefore, concentrations of carbon dioxide (CO2) and HCO3− must be tightly regulated through adaptations in respiratory and renal physiology. For patients with suspected acid-base imbalance, these concentrations can be monitored by blood gas analysis performed on arterial or venous blood.
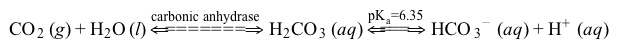 CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1). The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample.
CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1). The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample.
 Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3), reported in milliequivalents per liter (mEq/L), can be calculated from PaCO2 by Equation 1:
Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3), reported in milliequivalents per liter (mEq/L), can be calculated from PaCO2 by Equation 1:
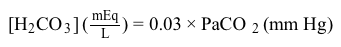 H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.
H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.
If a 0.2-mL blood sample with a PaCO2 of 40 mm Hg diffuses across a membrane into the E2 electrode containing 0.3 mL of solution, the final PaCO2 in the blood sample will be:
A)exactly 0 mm Hg.
B)less than 16 mm Hg.
C)exactly 16 mm Hg.
D)greater than 16 mm Hg.
The bicarbonate (HCO3−) buffer system (Reaction 1) helps to maintain acid-base homeostasis and a blood pH near 7.4. Therefore, concentrations of carbon dioxide (CO2) and HCO3− must be tightly regulated through adaptations in respiratory and renal physiology. For patients with suspected acid-base imbalance, these concentrations can be monitored by blood gas analysis performed on arterial or venous blood.
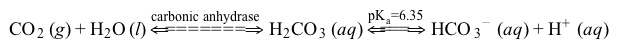 CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1). The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample.
CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1). The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample. Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3), reported in milliequivalents per liter (mEq/L), can be calculated from PaCO2 by Equation 1:
Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3), reported in milliequivalents per liter (mEq/L), can be calculated from PaCO2 by Equation 1: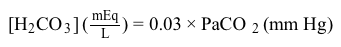 H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.
H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.If a 0.2-mL blood sample with a PaCO2 of 40 mm Hg diffuses across a membrane into the E2 electrode containing 0.3 mL of solution, the final PaCO2 in the blood sample will be:
A)exactly 0 mm Hg.
B)less than 16 mm Hg.
C)exactly 16 mm Hg.
D)greater than 16 mm Hg.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
10
Passage
Magnetic resonance imaging (MRI) interprets the nuclear relaxation or relaxivity of hydrogen nuclei in resident water molecules. Hydrogen nuclei in water have two magnetic spin states: alpha and beta. In the presence of an applied magnetic field, these spins will align with the field in an "excited" state and then relax back to the "ground" state, producing a signal with intensity proportional to relaxivity. Frequently, an MRI contrast agent is used to increase the relaxation time in coordinated molecules, resulting in a more intense signal.Commonly used contrast agents include gadolinium-based agents. Depending on its environment, gadolinium (Gd) can have eight or nine sites in its coordination sphere, allowing Gd3+ to interact with eight water molecules as in Gd(H2O)83+. The electronic configuration of gadolinium is shown in Figure 1.
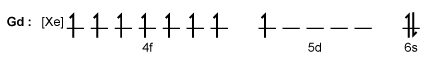 Figure 1 Electronic configuration of gadoliniumGd3+ alone is toxic to cells because its ionic radius is similar to that of Ca2+, allowing Gd3+ to displace Ca2+ in biologically important settings. Therefore, Gd3+ must be coordinated to an organic ligand such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to allow it to pass through the body safely. When chelated, DOTA displaces the water around gadolinium and leaves only one coordination site for a water molecule (Figure 2).
Figure 1 Electronic configuration of gadoliniumGd3+ alone is toxic to cells because its ionic radius is similar to that of Ca2+, allowing Gd3+ to displace Ca2+ in biologically important settings. Therefore, Gd3+ must be coordinated to an organic ligand such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to allow it to pass through the body safely. When chelated, DOTA displaces the water around gadolinium and leaves only one coordination site for a water molecule (Figure 2).
 Figure 2 Gd-DOTA with a water coordinated to Gd3+Zinc ions (Zn2+) are required to store insulin and are co-released when insulin is secreted by the pancreas. To detect zinc ions in the body, researchers have designed a variation of Gd-DOTA called Gd-daa3 with two diaminoacetate (daa) arms that preferentially bind to Zn2+ ions (Figure 3). When Zn2+ is absent, these arms coordinate to Gd3+ and create a nine-coordinate complex that prohibits water from binding to Gd3+. Binding of diaminoacetate arms to Zn2+ frees Gd3+ to coordinate with water molecules. This configuration results in increased signal intensity in the parts of the cell where Zn2+ is located.
Figure 2 Gd-DOTA with a water coordinated to Gd3+Zinc ions (Zn2+) are required to store insulin and are co-released when insulin is secreted by the pancreas. To detect zinc ions in the body, researchers have designed a variation of Gd-DOTA called Gd-daa3 with two diaminoacetate (daa) arms that preferentially bind to Zn2+ ions (Figure 3). When Zn2+ is absent, these arms coordinate to Gd3+ and create a nine-coordinate complex that prohibits water from binding to Gd3+. Binding of diaminoacetate arms to Zn2+ frees Gd3+ to coordinate with water molecules. This configuration results in increased signal intensity in the parts of the cell where Zn2+ is located.
 Figure 3 Gd-daa3 when Zn2+ is present
Figure 3 Gd-daa3 when Zn2+ is present
Adapted from Louie A. MRI biosensors: a short primer. J Magn Reson Imaging. 2013;38(3):530-9.
Which of the following accurately describes the magnetic properties of Gd3+ ions?
A)Gd3+ is diamagnetic because of unpaired electrons.
B)Gd3+ is paramagnetic and its electron spins align parallel to the applied magnetic field.
C)Gd3+ is diamagnetic because ions are attracted to the magnetic field due to their positive charge.
D)Gd3+ is paramagnetic and therefore repels magnetic field lines.
Magnetic resonance imaging (MRI) interprets the nuclear relaxation or relaxivity of hydrogen nuclei in resident water molecules. Hydrogen nuclei in water have two magnetic spin states: alpha and beta. In the presence of an applied magnetic field, these spins will align with the field in an "excited" state and then relax back to the "ground" state, producing a signal with intensity proportional to relaxivity. Frequently, an MRI contrast agent is used to increase the relaxation time in coordinated molecules, resulting in a more intense signal.Commonly used contrast agents include gadolinium-based agents. Depending on its environment, gadolinium (Gd) can have eight or nine sites in its coordination sphere, allowing Gd3+ to interact with eight water molecules as in Gd(H2O)83+. The electronic configuration of gadolinium is shown in Figure 1.
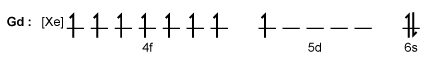 Figure 1 Electronic configuration of gadoliniumGd3+ alone is toxic to cells because its ionic radius is similar to that of Ca2+, allowing Gd3+ to displace Ca2+ in biologically important settings. Therefore, Gd3+ must be coordinated to an organic ligand such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to allow it to pass through the body safely. When chelated, DOTA displaces the water around gadolinium and leaves only one coordination site for a water molecule (Figure 2).
Figure 1 Electronic configuration of gadoliniumGd3+ alone is toxic to cells because its ionic radius is similar to that of Ca2+, allowing Gd3+ to displace Ca2+ in biologically important settings. Therefore, Gd3+ must be coordinated to an organic ligand such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to allow it to pass through the body safely. When chelated, DOTA displaces the water around gadolinium and leaves only one coordination site for a water molecule (Figure 2). Figure 2 Gd-DOTA with a water coordinated to Gd3+Zinc ions (Zn2+) are required to store insulin and are co-released when insulin is secreted by the pancreas. To detect zinc ions in the body, researchers have designed a variation of Gd-DOTA called Gd-daa3 with two diaminoacetate (daa) arms that preferentially bind to Zn2+ ions (Figure 3). When Zn2+ is absent, these arms coordinate to Gd3+ and create a nine-coordinate complex that prohibits water from binding to Gd3+. Binding of diaminoacetate arms to Zn2+ frees Gd3+ to coordinate with water molecules. This configuration results in increased signal intensity in the parts of the cell where Zn2+ is located.
Figure 2 Gd-DOTA with a water coordinated to Gd3+Zinc ions (Zn2+) are required to store insulin and are co-released when insulin is secreted by the pancreas. To detect zinc ions in the body, researchers have designed a variation of Gd-DOTA called Gd-daa3 with two diaminoacetate (daa) arms that preferentially bind to Zn2+ ions (Figure 3). When Zn2+ is absent, these arms coordinate to Gd3+ and create a nine-coordinate complex that prohibits water from binding to Gd3+. Binding of diaminoacetate arms to Zn2+ frees Gd3+ to coordinate with water molecules. This configuration results in increased signal intensity in the parts of the cell where Zn2+ is located. Figure 3 Gd-daa3 when Zn2+ is present
Figure 3 Gd-daa3 when Zn2+ is presentAdapted from Louie A. MRI biosensors: a short primer. J Magn Reson Imaging. 2013;38(3):530-9.
Which of the following accurately describes the magnetic properties of Gd3+ ions?
A)Gd3+ is diamagnetic because of unpaired electrons.
B)Gd3+ is paramagnetic and its electron spins align parallel to the applied magnetic field.
C)Gd3+ is diamagnetic because ions are attracted to the magnetic field due to their positive charge.
D)Gd3+ is paramagnetic and therefore repels magnetic field lines.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
11
Passage
The bicarbonate (HCO3−) buffer system (Reaction 1) helps to maintain acid-base homeostasis and a blood pH near 7.4. Therefore, concentrations of carbon dioxide (CO2) and HCO3− must be tightly regulated through adaptations in respiratory and renal physiology. For patients with suspected acid-base imbalance, these concentrations can be monitored by blood gas analysis performed on arterial or venous blood.
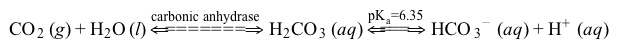 CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1). The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample.
CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1). The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample.
 Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3), reported in milliequivalents per liter (mEq/L), can be calculated from PaCO2 by Equation 1:
Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3), reported in milliequivalents per liter (mEq/L), can be calculated from PaCO2 by Equation 1:
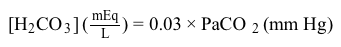 H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.
H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.
The reference electrode for E2 should be maintained at a known, stable concentration of:
A)H+(aq).
B)HCO3−(aq).
C)dissolved CO2.
D)dissolved O2.
The bicarbonate (HCO3−) buffer system (Reaction 1) helps to maintain acid-base homeostasis and a blood pH near 7.4. Therefore, concentrations of carbon dioxide (CO2) and HCO3− must be tightly regulated through adaptations in respiratory and renal physiology. For patients with suspected acid-base imbalance, these concentrations can be monitored by blood gas analysis performed on arterial or venous blood.
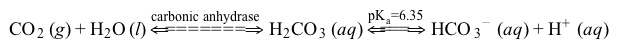 CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1). The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample.
CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1). The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample. Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3), reported in milliequivalents per liter (mEq/L), can be calculated from PaCO2 by Equation 1:
Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3), reported in milliequivalents per liter (mEq/L), can be calculated from PaCO2 by Equation 1: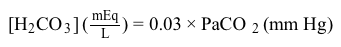 H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.
H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.The reference electrode for E2 should be maintained at a known, stable concentration of:
A)H+(aq).
B)HCO3−(aq).
C)dissolved CO2.
D)dissolved O2.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
12
Passage
Magnetic resonance imaging (MRI) interprets the nuclear relaxation or relaxivity of hydrogen nuclei in resident water molecules. Hydrogen nuclei in water have two magnetic spin states: alpha and beta. In the presence of an applied magnetic field, these spins will align with the field in an "excited" state and then relax back to the "ground" state, producing a signal with intensity proportional to relaxivity. Frequently, an MRI contrast agent is used to increase the relaxation time in coordinated molecules, resulting in a more intense signal.Commonly used contrast agents include gadolinium-based agents. Depending on its environment, gadolinium (Gd) can have eight or nine sites in its coordination sphere, allowing Gd3+ to interact with eight water molecules as in Gd(H2O)83+. The electronic configuration of gadolinium is shown in Figure 1.
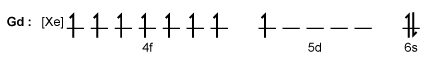 Figure 1 Electronic configuration of gadoliniumGd3+ alone is toxic to cells because its ionic radius is similar to that of Ca2+, allowing Gd3+ to displace Ca2+ in biologically important settings. Therefore, Gd3+ must be coordinated to an organic ligand such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to allow it to pass through the body safely. When chelated, DOTA displaces the water around gadolinium and leaves only one coordination site for a water molecule (Figure 2).
Figure 1 Electronic configuration of gadoliniumGd3+ alone is toxic to cells because its ionic radius is similar to that of Ca2+, allowing Gd3+ to displace Ca2+ in biologically important settings. Therefore, Gd3+ must be coordinated to an organic ligand such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to allow it to pass through the body safely. When chelated, DOTA displaces the water around gadolinium and leaves only one coordination site for a water molecule (Figure 2).
 Figure 2 Gd-DOTA with a water coordinated to Gd3+Zinc ions (Zn2+) are required to store insulin and are co-released when insulin is secreted by the pancreas. To detect zinc ions in the body, researchers have designed a variation of Gd-DOTA called Gd-daa3 with two diaminoacetate (daa) arms that preferentially bind to Zn2+ ions (Figure 3). When Zn2+ is absent, these arms coordinate to Gd3+ and create a nine-coordinate complex that prohibits water from binding to Gd3+. Binding of diaminoacetate arms to Zn2+ frees Gd3+ to coordinate with water molecules. This configuration results in increased signal intensity in the parts of the cell where Zn2+ is located.
Figure 2 Gd-DOTA with a water coordinated to Gd3+Zinc ions (Zn2+) are required to store insulin and are co-released when insulin is secreted by the pancreas. To detect zinc ions in the body, researchers have designed a variation of Gd-DOTA called Gd-daa3 with two diaminoacetate (daa) arms that preferentially bind to Zn2+ ions (Figure 3). When Zn2+ is absent, these arms coordinate to Gd3+ and create a nine-coordinate complex that prohibits water from binding to Gd3+. Binding of diaminoacetate arms to Zn2+ frees Gd3+ to coordinate with water molecules. This configuration results in increased signal intensity in the parts of the cell where Zn2+ is located.
 Figure 3 Gd-daa3 when Zn2+ is present
Figure 3 Gd-daa3 when Zn2+ is present
Adapted from Louie A. MRI biosensors: a short primer. J Magn Reson Imaging. 2013;38(3):530-9.
Gadolinium becomes ionized to Gd3+ when it loses electrons from which orbital(s)?
A)4f, 4d, and 5s
B)4f
C)4f, 5d, and 6s
D)5d and 6s
Magnetic resonance imaging (MRI) interprets the nuclear relaxation or relaxivity of hydrogen nuclei in resident water molecules. Hydrogen nuclei in water have two magnetic spin states: alpha and beta. In the presence of an applied magnetic field, these spins will align with the field in an "excited" state and then relax back to the "ground" state, producing a signal with intensity proportional to relaxivity. Frequently, an MRI contrast agent is used to increase the relaxation time in coordinated molecules, resulting in a more intense signal.Commonly used contrast agents include gadolinium-based agents. Depending on its environment, gadolinium (Gd) can have eight or nine sites in its coordination sphere, allowing Gd3+ to interact with eight water molecules as in Gd(H2O)83+. The electronic configuration of gadolinium is shown in Figure 1.
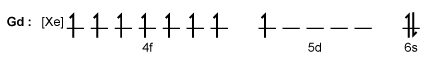 Figure 1 Electronic configuration of gadoliniumGd3+ alone is toxic to cells because its ionic radius is similar to that of Ca2+, allowing Gd3+ to displace Ca2+ in biologically important settings. Therefore, Gd3+ must be coordinated to an organic ligand such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to allow it to pass through the body safely. When chelated, DOTA displaces the water around gadolinium and leaves only one coordination site for a water molecule (Figure 2).
Figure 1 Electronic configuration of gadoliniumGd3+ alone is toxic to cells because its ionic radius is similar to that of Ca2+, allowing Gd3+ to displace Ca2+ in biologically important settings. Therefore, Gd3+ must be coordinated to an organic ligand such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to allow it to pass through the body safely. When chelated, DOTA displaces the water around gadolinium and leaves only one coordination site for a water molecule (Figure 2). Figure 2 Gd-DOTA with a water coordinated to Gd3+Zinc ions (Zn2+) are required to store insulin and are co-released when insulin is secreted by the pancreas. To detect zinc ions in the body, researchers have designed a variation of Gd-DOTA called Gd-daa3 with two diaminoacetate (daa) arms that preferentially bind to Zn2+ ions (Figure 3). When Zn2+ is absent, these arms coordinate to Gd3+ and create a nine-coordinate complex that prohibits water from binding to Gd3+. Binding of diaminoacetate arms to Zn2+ frees Gd3+ to coordinate with water molecules. This configuration results in increased signal intensity in the parts of the cell where Zn2+ is located.
Figure 2 Gd-DOTA with a water coordinated to Gd3+Zinc ions (Zn2+) are required to store insulin and are co-released when insulin is secreted by the pancreas. To detect zinc ions in the body, researchers have designed a variation of Gd-DOTA called Gd-daa3 with two diaminoacetate (daa) arms that preferentially bind to Zn2+ ions (Figure 3). When Zn2+ is absent, these arms coordinate to Gd3+ and create a nine-coordinate complex that prohibits water from binding to Gd3+. Binding of diaminoacetate arms to Zn2+ frees Gd3+ to coordinate with water molecules. This configuration results in increased signal intensity in the parts of the cell where Zn2+ is located. Figure 3 Gd-daa3 when Zn2+ is present
Figure 3 Gd-daa3 when Zn2+ is presentAdapted from Louie A. MRI biosensors: a short primer. J Magn Reson Imaging. 2013;38(3):530-9.
Gadolinium becomes ionized to Gd3+ when it loses electrons from which orbital(s)?
A)4f, 4d, and 5s
B)4f
C)4f, 5d, and 6s
D)5d and 6s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
13
Passage
The bicarbonate (HCO3−) buffer system (Reaction 1) helps to maintain acid-base homeostasis and a blood pH near 7.4. Therefore, concentrations of carbon dioxide (CO2) and HCO3− must be tightly regulated through adaptations in respiratory and renal physiology. For patients with suspected acid-base imbalance, these concentrations can be monitored by blood gas analysis performed on arterial or venous blood.
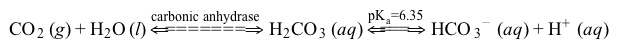 CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1). The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample.
CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1). The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample.
 Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3), reported in milliequivalents per liter (mEq/L), can be calculated from PaCO2 by Equation 1:
Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3), reported in milliequivalents per liter (mEq/L), can be calculated from PaCO2 by Equation 1:
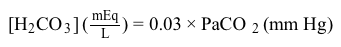 H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.
H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.
In the alveoli, hypoventilation (decreased gas exchange) and hyperventilation (increased gas exchange) can lead to abnormalities in acid-base homeostasis. Which of the following is true of the immediate effects of abnormal ventilation?
A)Hyperventilation decreases blood pH and decreases HCO3− levels.
B)Hyperventilation increases blood pH and decreases HCO3− levels.
C)Hypoventilation increases blood pH and increases HCO3− levels.
D)Hypoventilation decreases blood pH and decreases HCO3− levels.
The bicarbonate (HCO3−) buffer system (Reaction 1) helps to maintain acid-base homeostasis and a blood pH near 7.4. Therefore, concentrations of carbon dioxide (CO2) and HCO3− must be tightly regulated through adaptations in respiratory and renal physiology. For patients with suspected acid-base imbalance, these concentrations can be monitored by blood gas analysis performed on arterial or venous blood.
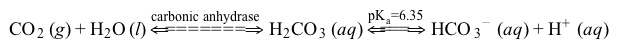 CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1). The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample.
CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1). The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample. Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3), reported in milliequivalents per liter (mEq/L), can be calculated from PaCO2 by Equation 1:
Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3), reported in milliequivalents per liter (mEq/L), can be calculated from PaCO2 by Equation 1: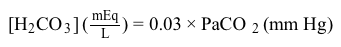 H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.
H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.In the alveoli, hypoventilation (decreased gas exchange) and hyperventilation (increased gas exchange) can lead to abnormalities in acid-base homeostasis. Which of the following is true of the immediate effects of abnormal ventilation?
A)Hyperventilation decreases blood pH and decreases HCO3− levels.
B)Hyperventilation increases blood pH and decreases HCO3− levels.
C)Hypoventilation increases blood pH and increases HCO3− levels.
D)Hypoventilation decreases blood pH and decreases HCO3− levels.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
14
Passage
Depending on the active compound's specific method of action, sunscreens are broadly classified into two groups: organic and inorganic. Organic sunscreens typically contain aromatic compounds that absorb ultraviolet (UV) radiation. The absorbed energy from the UV radiation causes these compounds to enter electronically excited states which then relax back to the ground state and harmlessly re-emit the absorbed radiation as heat. Alternatively, inorganic sunscreens contain metallic nanoparticles that serve to reflect, scatter, and absorb incident radiation. The mechanism for UV protection of each group is shown in Figure 1.
 Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF). However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens
Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF). However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens
 In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested.
In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested.
 Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreens
Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreens
Adapted from Van der molen RG, Out-luiting C, Driller H, Claas FH, Koerten HK, Mommaas AM. Broad-spectrum sunscreens offer protection against urocanic acid photoisomerization by artificial ultraviolet radiation in human skin. J Invest Dermatol. 2000;115(3):421-6.
Which of the following statements is true concerning sunscreens?
A)Organic sunscreens' method of protection involves relaxation to the ground state.
B)Sunscreens with titanium dioxide offer the greatest range of protection.
C)For chronic exposure to UV radiation, titanium dioxide provides better protection from photo-isomerization of trans-UCA to cis-UCA than does octisalate at an equal concentration.
D)A sunscreen's SPF can be used to measure its efficacy in protection from UV-induced immunosuppression.
Depending on the active compound's specific method of action, sunscreens are broadly classified into two groups: organic and inorganic. Organic sunscreens typically contain aromatic compounds that absorb ultraviolet (UV) radiation. The absorbed energy from the UV radiation causes these compounds to enter electronically excited states which then relax back to the ground state and harmlessly re-emit the absorbed radiation as heat. Alternatively, inorganic sunscreens contain metallic nanoparticles that serve to reflect, scatter, and absorb incident radiation. The mechanism for UV protection of each group is shown in Figure 1.
 Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF). However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens
Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF). However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested.
In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested. Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreens
Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreensAdapted from Van der molen RG, Out-luiting C, Driller H, Claas FH, Koerten HK, Mommaas AM. Broad-spectrum sunscreens offer protection against urocanic acid photoisomerization by artificial ultraviolet radiation in human skin. J Invest Dermatol. 2000;115(3):421-6.
Which of the following statements is true concerning sunscreens?
A)Organic sunscreens' method of protection involves relaxation to the ground state.
B)Sunscreens with titanium dioxide offer the greatest range of protection.
C)For chronic exposure to UV radiation, titanium dioxide provides better protection from photo-isomerization of trans-UCA to cis-UCA than does octisalate at an equal concentration.
D)A sunscreen's SPF can be used to measure its efficacy in protection from UV-induced immunosuppression.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
15
Passage
Nuclear medicine uses radiopharmaceuticals for disease treatment and as tracers in medical imaging studies. Yttrium-90 (90Y) is a radiopharmaceutical agent used to treat overgrown joint lining known as pigmented villonodular synovitis, as well as some forms of liver cancer. It has a half-life of about 64 hours.Although the relatively short half-life of 90Y is optimal for use in medical applications, transportation and storage of the radiopharmaceutical are not feasible. As a result, strontium-90 (90Sr), with a half-life of 28.8 years, is the most common source of 90Y. 90Sr is created by a nuclear fission process that begins with uranium-235 (235U) in a nuclear reactor as shown in Reaction 1.235U → 90Sr + ZReaction 1Fission of 235U produces 90Sr as well as additional fission products (Z) including, but not limited to, technetium-99 (99Tc), iodine-129 (129I), and zirconium-93 (93Zr). Figure 1 shows the distribution of fission products.
 Figure 1 Distribution of 235U fission products by atomic weightTo create the final 90Y required for nuclear medicine studies, 90Sr decay is carried out in a controlled 90Y generator. 90Y is then separated from residual 90Sr for use in clinical applications.
Figure 1 Distribution of 235U fission products by atomic weightTo create the final 90Y required for nuclear medicine studies, 90Sr decay is carried out in a controlled 90Y generator. 90Y is then separated from residual 90Sr for use in clinical applications.
Adapted from Wheeler CE. Comments on vaccines, August 1987. J Am Acad Dermatol. 1988;18(1 Pt 2):232-4.
Strontium is converted to yttrium by which of the following processes?
A)Electron capture
B)Positron emission
C)Gamma ray emission
D)Beta emission
Nuclear medicine uses radiopharmaceuticals for disease treatment and as tracers in medical imaging studies. Yttrium-90 (90Y) is a radiopharmaceutical agent used to treat overgrown joint lining known as pigmented villonodular synovitis, as well as some forms of liver cancer. It has a half-life of about 64 hours.Although the relatively short half-life of 90Y is optimal for use in medical applications, transportation and storage of the radiopharmaceutical are not feasible. As a result, strontium-90 (90Sr), with a half-life of 28.8 years, is the most common source of 90Y. 90Sr is created by a nuclear fission process that begins with uranium-235 (235U) in a nuclear reactor as shown in Reaction 1.235U → 90Sr + ZReaction 1Fission of 235U produces 90Sr as well as additional fission products (Z) including, but not limited to, technetium-99 (99Tc), iodine-129 (129I), and zirconium-93 (93Zr). Figure 1 shows the distribution of fission products.
 Figure 1 Distribution of 235U fission products by atomic weightTo create the final 90Y required for nuclear medicine studies, 90Sr decay is carried out in a controlled 90Y generator. 90Y is then separated from residual 90Sr for use in clinical applications.
Figure 1 Distribution of 235U fission products by atomic weightTo create the final 90Y required for nuclear medicine studies, 90Sr decay is carried out in a controlled 90Y generator. 90Y is then separated from residual 90Sr for use in clinical applications.Adapted from Wheeler CE. Comments on vaccines, August 1987. J Am Acad Dermatol. 1988;18(1 Pt 2):232-4.
Strontium is converted to yttrium by which of the following processes?
A)Electron capture
B)Positron emission
C)Gamma ray emission
D)Beta emission

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
16
Passage
Depending on the active compound's specific method of action, sunscreens are broadly classified into two groups: organic and inorganic. Organic sunscreens typically contain aromatic compounds that absorb ultraviolet (UV) radiation. The absorbed energy from the UV radiation causes these compounds to enter electronically excited states which then relax back to the ground state and harmlessly re-emit the absorbed radiation as heat. Alternatively, inorganic sunscreens contain metallic nanoparticles that serve to reflect, scatter, and absorb incident radiation. The mechanism for UV protection of each group is shown in Figure 1.
 Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF). However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens
Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF). However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens
 In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested.
In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested.
 Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreens
Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreens
Adapted from Van der molen RG, Out-luiting C, Driller H, Claas FH, Koerten HK, Mommaas AM. Broad-spectrum sunscreens offer protection against urocanic acid photoisomerization by artificial ultraviolet radiation in human skin. J Invest Dermatol. 2000;115(3):421-6.
Which of the following is the correct Lewis dot structure for the common inorganic sunscreen compound titanium dioxide (TiO2) given that titanium has four valence electrons?
A)
B)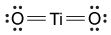
C)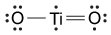
D)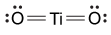
Depending on the active compound's specific method of action, sunscreens are broadly classified into two groups: organic and inorganic. Organic sunscreens typically contain aromatic compounds that absorb ultraviolet (UV) radiation. The absorbed energy from the UV radiation causes these compounds to enter electronically excited states which then relax back to the ground state and harmlessly re-emit the absorbed radiation as heat. Alternatively, inorganic sunscreens contain metallic nanoparticles that serve to reflect, scatter, and absorb incident radiation. The mechanism for UV protection of each group is shown in Figure 1.
 Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF). However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens
Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF). However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested.
In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested. Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreens
Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreensAdapted from Van der molen RG, Out-luiting C, Driller H, Claas FH, Koerten HK, Mommaas AM. Broad-spectrum sunscreens offer protection against urocanic acid photoisomerization by artificial ultraviolet radiation in human skin. J Invest Dermatol. 2000;115(3):421-6.
Which of the following is the correct Lewis dot structure for the common inorganic sunscreen compound titanium dioxide (TiO2) given that titanium has four valence electrons?
A)
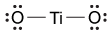
B)
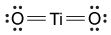
C)
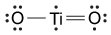
D)
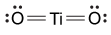

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
17
Passage
Nuclear medicine uses radiopharmaceuticals for disease treatment and as tracers in medical imaging studies. Yttrium-90 (90Y) is a radiopharmaceutical agent used to treat overgrown joint lining known as pigmented villonodular synovitis, as well as some forms of liver cancer. It has a half-life of about 64 hours.Although the relatively short half-life of 90Y is optimal for use in medical applications, transportation and storage of the radiopharmaceutical are not feasible. As a result, strontium-90 (90Sr), with a half-life of 28.8 years, is the most common source of 90Y. 90Sr is created by a nuclear fission process that begins with uranium-235 (235U) in a nuclear reactor as shown in Reaction 1.235U → 90Sr + ZReaction 1Fission of 235U produces 90Sr as well as additional fission products (Z) including, but not limited to, technetium-99 (99Tc), iodine-129 (129I), and zirconium-93 (93Zr). Figure 1 shows the distribution of fission products.
 Figure 1 Distribution of 235U fission products by atomic weightTo create the final 90Y required for nuclear medicine studies, 90Sr decay is carried out in a controlled 90Y generator. 90Y is then separated from residual 90Sr for use in clinical applications.
Figure 1 Distribution of 235U fission products by atomic weightTo create the final 90Y required for nuclear medicine studies, 90Sr decay is carried out in a controlled 90Y generator. 90Y is then separated from residual 90Sr for use in clinical applications.
Adapted from Wheeler CE. Comments on vaccines, August 1987. J Am Acad Dermatol. 1988;18(1 Pt 2):232-4.
The type of uranium required for Reaction 1 can be formed by the emission of an alpha particle from which of the following nuclei?
A)239U
B)239Pu
C)235U
D)235Pa
Nuclear medicine uses radiopharmaceuticals for disease treatment and as tracers in medical imaging studies. Yttrium-90 (90Y) is a radiopharmaceutical agent used to treat overgrown joint lining known as pigmented villonodular synovitis, as well as some forms of liver cancer. It has a half-life of about 64 hours.Although the relatively short half-life of 90Y is optimal for use in medical applications, transportation and storage of the radiopharmaceutical are not feasible. As a result, strontium-90 (90Sr), with a half-life of 28.8 years, is the most common source of 90Y. 90Sr is created by a nuclear fission process that begins with uranium-235 (235U) in a nuclear reactor as shown in Reaction 1.235U → 90Sr + ZReaction 1Fission of 235U produces 90Sr as well as additional fission products (Z) including, but not limited to, technetium-99 (99Tc), iodine-129 (129I), and zirconium-93 (93Zr). Figure 1 shows the distribution of fission products.
 Figure 1 Distribution of 235U fission products by atomic weightTo create the final 90Y required for nuclear medicine studies, 90Sr decay is carried out in a controlled 90Y generator. 90Y is then separated from residual 90Sr for use in clinical applications.
Figure 1 Distribution of 235U fission products by atomic weightTo create the final 90Y required for nuclear medicine studies, 90Sr decay is carried out in a controlled 90Y generator. 90Y is then separated from residual 90Sr for use in clinical applications.Adapted from Wheeler CE. Comments on vaccines, August 1987. J Am Acad Dermatol. 1988;18(1 Pt 2):232-4.
The type of uranium required for Reaction 1 can be formed by the emission of an alpha particle from which of the following nuclei?
A)239U
B)239Pu
C)235U
D)235Pa

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
18
Passage
The bicarbonate (HCO3−) buffer system (Reaction 1) helps to maintain acid-base homeostasis and a blood pH near 7.4. Therefore, concentrations of carbon dioxide (CO2) and HCO3− must be tightly regulated through adaptations in respiratory and renal physiology. For patients with suspected acid-base imbalance, these concentrations can be monitored by blood gas analysis performed on arterial or venous blood.
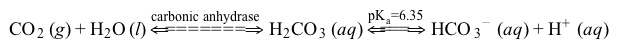 CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1). The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample.
CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1). The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample.
 Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3), reported in milliequivalents per liter (mEq/L), can be calculated from PaCO2 by Equation 1:
Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3), reported in milliequivalents per liter (mEq/L), can be calculated from PaCO2 by Equation 1:
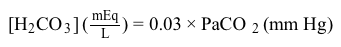 H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.
H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.
Blood gas analysis results are listed below. From the data, determine the HCO3− concentration in the blood sample resulting from the decomposition of carbonic acid.
A)0.12 mEq/L
B)1.2 mEq/L
C)2.4 mEq/L
D)12.0 mEq/L
The bicarbonate (HCO3−) buffer system (Reaction 1) helps to maintain acid-base homeostasis and a blood pH near 7.4. Therefore, concentrations of carbon dioxide (CO2) and HCO3− must be tightly regulated through adaptations in respiratory and renal physiology. For patients with suspected acid-base imbalance, these concentrations can be monitored by blood gas analysis performed on arterial or venous blood.
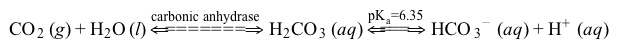 CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1). The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample.
CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1). The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample. Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3), reported in milliequivalents per liter (mEq/L), can be calculated from PaCO2 by Equation 1:
Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3), reported in milliequivalents per liter (mEq/L), can be calculated from PaCO2 by Equation 1: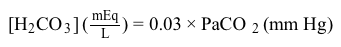 H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.
H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.Blood gas analysis results are listed below. From the data, determine the HCO3− concentration in the blood sample resulting from the decomposition of carbonic acid.

A)0.12 mEq/L
B)1.2 mEq/L
C)2.4 mEq/L
D)12.0 mEq/L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
19
Passage
The bicarbonate (HCO3−) buffer system (Reaction 1) helps to maintain acid-base homeostasis and a blood pH near 7.4. Therefore, concentrations of carbon dioxide (CO2) and HCO3− must be tightly regulated through adaptations in respiratory and renal physiology. For patients with suspected acid-base imbalance, these concentrations can be monitored by blood gas analysis performed on arterial or venous blood.
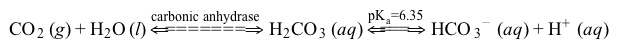 CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1). The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample.
CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1). The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample.
 Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3), reported in milliequivalents per liter (mEq/L), can be calculated from PaCO2 by Equation 1:
Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3), reported in milliequivalents per liter (mEq/L), can be calculated from PaCO2 by Equation 1:
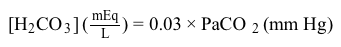 H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.
H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.
A 50 mL solution of 0.1 M carbonic acid is titrated with a solution of 0.2 M sodium hydroxide (NaOH). Which of the following represents the curve for this titration?
A)
B)
C)
D)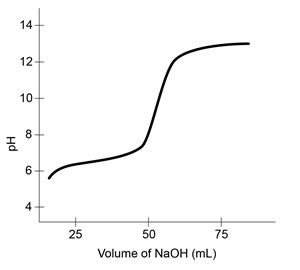
The bicarbonate (HCO3−) buffer system (Reaction 1) helps to maintain acid-base homeostasis and a blood pH near 7.4. Therefore, concentrations of carbon dioxide (CO2) and HCO3− must be tightly regulated through adaptations in respiratory and renal physiology. For patients with suspected acid-base imbalance, these concentrations can be monitored by blood gas analysis performed on arterial or venous blood.
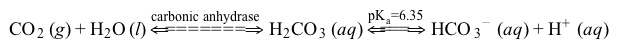 CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1). The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample.
CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1). The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample. Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3), reported in milliequivalents per liter (mEq/L), can be calculated from PaCO2 by Equation 1:
Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3), reported in milliequivalents per liter (mEq/L), can be calculated from PaCO2 by Equation 1: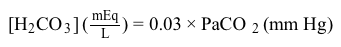 H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.
H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.A 50 mL solution of 0.1 M carbonic acid is titrated with a solution of 0.2 M sodium hydroxide (NaOH). Which of the following represents the curve for this titration?
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
20
Passage
Nuclear medicine uses radiopharmaceuticals for disease treatment and as tracers in medical imaging studies. Yttrium-90 (90Y) is a radiopharmaceutical agent used to treat overgrown joint lining known as pigmented villonodular synovitis, as well as some forms of liver cancer. It has a half-life of about 64 hours.Although the relatively short half-life of 90Y is optimal for use in medical applications, transportation and storage of the radiopharmaceutical are not feasible. As a result, strontium-90 (90Sr), with a half-life of 28.8 years, is the most common source of 90Y. 90Sr is created by a nuclear fission process that begins with uranium-235 (235U) in a nuclear reactor as shown in Reaction 1.235U → 90Sr + ZReaction 1Fission of 235U produces 90Sr as well as additional fission products (Z) including, but not limited to, technetium-99 (99Tc), iodine-129 (129I), and zirconium-93 (93Zr). Figure 1 shows the distribution of fission products.
 Figure 1 Distribution of 235U fission products by atomic weightTo create the final 90Y required for nuclear medicine studies, 90Sr decay is carried out in a controlled 90Y generator. 90Y is then separated from residual 90Sr for use in clinical applications.
Figure 1 Distribution of 235U fission products by atomic weightTo create the final 90Y required for nuclear medicine studies, 90Sr decay is carried out in a controlled 90Y generator. 90Y is then separated from residual 90Sr for use in clinical applications.
Adapted from Wheeler CE. Comments on vaccines, August 1987. J Am Acad Dermatol. 1988;18(1 Pt 2):232-4.
90Y converts to 90Zr by beta decay. Which of the following elements exhibits chemical properties most similar to zirconium-90? (Note: Assume that 90Zr gains any needed valence electrons from its environment immediately after being formed by the nuclear conversion.)
A)90Nb
B)174Hf
C)46Sc
D)48Ca
Nuclear medicine uses radiopharmaceuticals for disease treatment and as tracers in medical imaging studies. Yttrium-90 (90Y) is a radiopharmaceutical agent used to treat overgrown joint lining known as pigmented villonodular synovitis, as well as some forms of liver cancer. It has a half-life of about 64 hours.Although the relatively short half-life of 90Y is optimal for use in medical applications, transportation and storage of the radiopharmaceutical are not feasible. As a result, strontium-90 (90Sr), with a half-life of 28.8 years, is the most common source of 90Y. 90Sr is created by a nuclear fission process that begins with uranium-235 (235U) in a nuclear reactor as shown in Reaction 1.235U → 90Sr + ZReaction 1Fission of 235U produces 90Sr as well as additional fission products (Z) including, but not limited to, technetium-99 (99Tc), iodine-129 (129I), and zirconium-93 (93Zr). Figure 1 shows the distribution of fission products.
 Figure 1 Distribution of 235U fission products by atomic weightTo create the final 90Y required for nuclear medicine studies, 90Sr decay is carried out in a controlled 90Y generator. 90Y is then separated from residual 90Sr for use in clinical applications.
Figure 1 Distribution of 235U fission products by atomic weightTo create the final 90Y required for nuclear medicine studies, 90Sr decay is carried out in a controlled 90Y generator. 90Y is then separated from residual 90Sr for use in clinical applications.Adapted from Wheeler CE. Comments on vaccines, August 1987. J Am Acad Dermatol. 1988;18(1 Pt 2):232-4.
90Y converts to 90Zr by beta decay. Which of the following elements exhibits chemical properties most similar to zirconium-90? (Note: Assume that 90Zr gains any needed valence electrons from its environment immediately after being formed by the nuclear conversion.)
A)90Nb
B)174Hf
C)46Sc
D)48Ca

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
21
Passage
In polluted urban environments, airborne proteins can undergo nitration reactions that increase their allergenic potential toward the human respiratory system. For example, upon exposure to ozone (O3) and nitrogen dioxide (NO2) under atmospheric conditions, the amino acid tyrosine is known to undergo a two-step irreversible reaction leading to the formation of nitrotyrosine. The first step involves the formation of a reactive oxygen intermediate (ROI) tyrosyl radical through oxidation of the phenolic site of tyrosine. Nitrotyrosine is then formed in the second step through addition of NO2 by a radical-radical termination reaction. The elementary steps for this reaction are shown in Scheme 1.
![<strong>Passage In polluted urban environments, airborne proteins can undergo nitration reactions that increase their allergenic potential toward the human respiratory system. For example, upon exposure to ozone (O<sub>3</sub>) and nitrogen dioxide (NO<sub>2</sub>) under atmospheric conditions, the amino acid tyrosine is known to undergo a two-step irreversible reaction leading to the formation of nitrotyrosine. The first step involves the formation of a reactive oxygen intermediate (ROI) tyrosyl radical through oxidation of the phenolic site of tyrosine. Nitrotyrosine is then formed in the second step through addition of NO<sub>2</sub> by a radical-radical termination reaction. The elementary steps for this reaction are shown in Scheme 1. <strong>Scheme 1</strong> Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies E<sub>a</sub> in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments. <strong>Figure 1</strong> Calculated activation energies for each step of the studied reactions in three environments<strong>Table 1</strong> Calculated Thermochemical Properties of the Studied Reactions (298.15 K) Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90. If the first step of the nitration of tyrosine in a lipid environment is slower than the second step, what is the rate law for the overall reaction?</strong> A)Rate = k<sub>1</sub>[tyrosine][O<sub>3</sub>] B)Rate = k<sub>1</sub>[tyrosine][O<sub>3</sub>][NO<sub>2</sub>] C)Rate = k<sub>2</sub>[ROI][NO<sub>2</sub>] D)Rate = k<sub>2</sub>[tyrosine][O<sub>3</sub>]](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1199_8092_a3bf_07c78ad73b88_MD0008_00.jpg) Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments.
Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments.
![<strong>Passage In polluted urban environments, airborne proteins can undergo nitration reactions that increase their allergenic potential toward the human respiratory system. For example, upon exposure to ozone (O<sub>3</sub>) and nitrogen dioxide (NO<sub>2</sub>) under atmospheric conditions, the amino acid tyrosine is known to undergo a two-step irreversible reaction leading to the formation of nitrotyrosine. The first step involves the formation of a reactive oxygen intermediate (ROI) tyrosyl radical through oxidation of the phenolic site of tyrosine. Nitrotyrosine is then formed in the second step through addition of NO<sub>2</sub> by a radical-radical termination reaction. The elementary steps for this reaction are shown in Scheme 1. <strong>Scheme 1</strong> Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies E<sub>a</sub> in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments. <strong>Figure 1</strong> Calculated activation energies for each step of the studied reactions in three environments<strong>Table 1</strong> Calculated Thermochemical Properties of the Studied Reactions (298.15 K) Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90. If the first step of the nitration of tyrosine in a lipid environment is slower than the second step, what is the rate law for the overall reaction?</strong> A)Rate = k<sub>1</sub>[tyrosine][O<sub>3</sub>] B)Rate = k<sub>1</sub>[tyrosine][O<sub>3</sub>][NO<sub>2</sub>] C)Rate = k<sub>2</sub>[ROI][NO<sub>2</sub>] D)Rate = k<sub>2</sub>[tyrosine][O<sub>3</sub>]](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1199_8093_a3bf_6dee19a8923a_MD0008_00.jpg) Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K)
Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K)
![<strong>Passage In polluted urban environments, airborne proteins can undergo nitration reactions that increase their allergenic potential toward the human respiratory system. For example, upon exposure to ozone (O<sub>3</sub>) and nitrogen dioxide (NO<sub>2</sub>) under atmospheric conditions, the amino acid tyrosine is known to undergo a two-step irreversible reaction leading to the formation of nitrotyrosine. The first step involves the formation of a reactive oxygen intermediate (ROI) tyrosyl radical through oxidation of the phenolic site of tyrosine. Nitrotyrosine is then formed in the second step through addition of NO<sub>2</sub> by a radical-radical termination reaction. The elementary steps for this reaction are shown in Scheme 1. <strong>Scheme 1</strong> Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies E<sub>a</sub> in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments. <strong>Figure 1</strong> Calculated activation energies for each step of the studied reactions in three environments<strong>Table 1</strong> Calculated Thermochemical Properties of the Studied Reactions (298.15 K) Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90. If the first step of the nitration of tyrosine in a lipid environment is slower than the second step, what is the rate law for the overall reaction?</strong> A)Rate = k<sub>1</sub>[tyrosine][O<sub>3</sub>] B)Rate = k<sub>1</sub>[tyrosine][O<sub>3</sub>][NO<sub>2</sub>] C)Rate = k<sub>2</sub>[ROI][NO<sub>2</sub>] D)Rate = k<sub>2</sub>[tyrosine][O<sub>3</sub>]](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1199_a7a4_a3bf_17e94e285108_MD0008_00.jpg) Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90.
Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90.
If the first step of the nitration of tyrosine in a lipid environment is slower than the second step, what is the rate law for the overall reaction?
A)Rate = k1[tyrosine][O3]
B)Rate = k1[tyrosine][O3][NO2]
C)Rate = k2[ROI][NO2]
D)Rate = k2[tyrosine][O3]
In polluted urban environments, airborne proteins can undergo nitration reactions that increase their allergenic potential toward the human respiratory system. For example, upon exposure to ozone (O3) and nitrogen dioxide (NO2) under atmospheric conditions, the amino acid tyrosine is known to undergo a two-step irreversible reaction leading to the formation of nitrotyrosine. The first step involves the formation of a reactive oxygen intermediate (ROI) tyrosyl radical through oxidation of the phenolic site of tyrosine. Nitrotyrosine is then formed in the second step through addition of NO2 by a radical-radical termination reaction. The elementary steps for this reaction are shown in Scheme 1.
![<strong>Passage In polluted urban environments, airborne proteins can undergo nitration reactions that increase their allergenic potential toward the human respiratory system. For example, upon exposure to ozone (O<sub>3</sub>) and nitrogen dioxide (NO<sub>2</sub>) under atmospheric conditions, the amino acid tyrosine is known to undergo a two-step irreversible reaction leading to the formation of nitrotyrosine. The first step involves the formation of a reactive oxygen intermediate (ROI) tyrosyl radical through oxidation of the phenolic site of tyrosine. Nitrotyrosine is then formed in the second step through addition of NO<sub>2</sub> by a radical-radical termination reaction. The elementary steps for this reaction are shown in Scheme 1. <strong>Scheme 1</strong> Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies E<sub>a</sub> in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments. <strong>Figure 1</strong> Calculated activation energies for each step of the studied reactions in three environments<strong>Table 1</strong> Calculated Thermochemical Properties of the Studied Reactions (298.15 K) Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90. If the first step of the nitration of tyrosine in a lipid environment is slower than the second step, what is the rate law for the overall reaction?</strong> A)Rate = k<sub>1</sub>[tyrosine][O<sub>3</sub>] B)Rate = k<sub>1</sub>[tyrosine][O<sub>3</sub>][NO<sub>2</sub>] C)Rate = k<sub>2</sub>[ROI][NO<sub>2</sub>] D)Rate = k<sub>2</sub>[tyrosine][O<sub>3</sub>]](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1199_8092_a3bf_07c78ad73b88_MD0008_00.jpg) Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments.
Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments.![<strong>Passage In polluted urban environments, airborne proteins can undergo nitration reactions that increase their allergenic potential toward the human respiratory system. For example, upon exposure to ozone (O<sub>3</sub>) and nitrogen dioxide (NO<sub>2</sub>) under atmospheric conditions, the amino acid tyrosine is known to undergo a two-step irreversible reaction leading to the formation of nitrotyrosine. The first step involves the formation of a reactive oxygen intermediate (ROI) tyrosyl radical through oxidation of the phenolic site of tyrosine. Nitrotyrosine is then formed in the second step through addition of NO<sub>2</sub> by a radical-radical termination reaction. The elementary steps for this reaction are shown in Scheme 1. <strong>Scheme 1</strong> Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies E<sub>a</sub> in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments. <strong>Figure 1</strong> Calculated activation energies for each step of the studied reactions in three environments<strong>Table 1</strong> Calculated Thermochemical Properties of the Studied Reactions (298.15 K) Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90. If the first step of the nitration of tyrosine in a lipid environment is slower than the second step, what is the rate law for the overall reaction?</strong> A)Rate = k<sub>1</sub>[tyrosine][O<sub>3</sub>] B)Rate = k<sub>1</sub>[tyrosine][O<sub>3</sub>][NO<sub>2</sub>] C)Rate = k<sub>2</sub>[ROI][NO<sub>2</sub>] D)Rate = k<sub>2</sub>[tyrosine][O<sub>3</sub>]](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1199_8093_a3bf_6dee19a8923a_MD0008_00.jpg) Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K)
Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K)![<strong>Passage In polluted urban environments, airborne proteins can undergo nitration reactions that increase their allergenic potential toward the human respiratory system. For example, upon exposure to ozone (O<sub>3</sub>) and nitrogen dioxide (NO<sub>2</sub>) under atmospheric conditions, the amino acid tyrosine is known to undergo a two-step irreversible reaction leading to the formation of nitrotyrosine. The first step involves the formation of a reactive oxygen intermediate (ROI) tyrosyl radical through oxidation of the phenolic site of tyrosine. Nitrotyrosine is then formed in the second step through addition of NO<sub>2</sub> by a radical-radical termination reaction. The elementary steps for this reaction are shown in Scheme 1. <strong>Scheme 1</strong> Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies E<sub>a</sub> in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments. <strong>Figure 1</strong> Calculated activation energies for each step of the studied reactions in three environments<strong>Table 1</strong> Calculated Thermochemical Properties of the Studied Reactions (298.15 K) Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90. If the first step of the nitration of tyrosine in a lipid environment is slower than the second step, what is the rate law for the overall reaction?</strong> A)Rate = k<sub>1</sub>[tyrosine][O<sub>3</sub>] B)Rate = k<sub>1</sub>[tyrosine][O<sub>3</sub>][NO<sub>2</sub>] C)Rate = k<sub>2</sub>[ROI][NO<sub>2</sub>] D)Rate = k<sub>2</sub>[tyrosine][O<sub>3</sub>]](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_1199_a7a4_a3bf_17e94e285108_MD0008_00.jpg) Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90.
Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90.If the first step of the nitration of tyrosine in a lipid environment is slower than the second step, what is the rate law for the overall reaction?
A)Rate = k1[tyrosine][O3]
B)Rate = k1[tyrosine][O3][NO2]
C)Rate = k2[ROI][NO2]
D)Rate = k2[tyrosine][O3]

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
22
Passage
Umami is one of five basic tastes that humans recognize, and it is triggered by L-amino acids as well as some synthetic ingredients and organic acids. Three common umami tastant molecules are shown in Figure 1.
 Figure 1 Three common tastant molecules known to trigger the umami taste sensationUmami taste signals are triggered upon binding of tastant molecules to the G protein-coupled receptor (GPCR) heterodimer T1R1-T1R3, which ultimately causes the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers the release of Ca2+ cations and the subsequent gating of the taste-transduction channels (Figure 2).
Figure 1 Three common tastant molecules known to trigger the umami taste sensationUmami taste signals are triggered upon binding of tastant molecules to the G protein-coupled receptor (GPCR) heterodimer T1R1-T1R3, which ultimately causes the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers the release of Ca2+ cations and the subsequent gating of the taste-transduction channels (Figure 2).
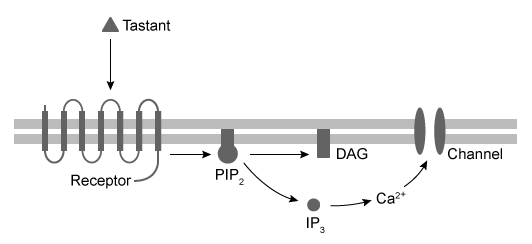 Figure 2 Signal pathway for transduction of umami taste sensationInosine monophosphate (IMP) has been suggested as enhancing (potentiating) the response of T1R1+T1R3 GPCRs to umami tastant molecules. Scientists tested this hypothesis by culturing receptors from human embryonic kidney (HEK) cells containing promiscuous G proteins. The HEK cells were then stimulated with umami tastants in the presence or absence of IMP. In each test, the cells were exposed to the same amounts of amino acid tastant and IMP. The number of cells that responded to each tastant was measured using a calcium indicator dye (FURA-2AM) in conjunction with fluorescence microscopy. By comparing the cellular response ratio of the stimulated to unstimulated HEK cells, the extent of potentiation can be assessed. The results are shown in Figure 3.
Figure 2 Signal pathway for transduction of umami taste sensationInosine monophosphate (IMP) has been suggested as enhancing (potentiating) the response of T1R1+T1R3 GPCRs to umami tastant molecules. Scientists tested this hypothesis by culturing receptors from human embryonic kidney (HEK) cells containing promiscuous G proteins. The HEK cells were then stimulated with umami tastants in the presence or absence of IMP. In each test, the cells were exposed to the same amounts of amino acid tastant and IMP. The number of cells that responded to each tastant was measured using a calcium indicator dye (FURA-2AM) in conjunction with fluorescence microscopy. By comparing the cellular response ratio of the stimulated to unstimulated HEK cells, the extent of potentiation can be assessed. The results are shown in Figure 3.
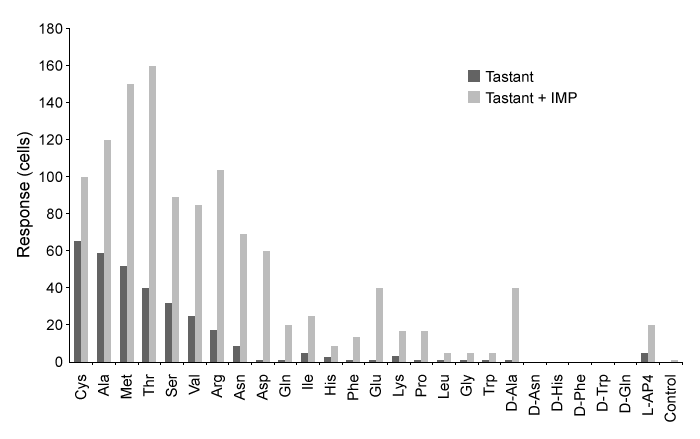 Figure 3 Quantification of amino-acid responses for T1R1+T1R3 with and without IMP
Figure 3 Quantification of amino-acid responses for T1R1+T1R3 with and without IMP
Adapted from Nelson G, Chandrashekar J, Hoon MA, et al. An amino-acid taste receptor. Nature. 2002;416(6877):199-202.
Which of the following statements is true regarding the bonds in the tastant molecule L-methionine?The pi bond in C=O has a smaller dissociation energy than the sigma bond.The C=O bond is stronger than the C-O bond.The pi bond in C=O is more stable than the sigma bond.
A)I only
B)I and II only
C)II and III only
D)I, II, and III
Umami is one of five basic tastes that humans recognize, and it is triggered by L-amino acids as well as some synthetic ingredients and organic acids. Three common umami tastant molecules are shown in Figure 1.
 Figure 1 Three common tastant molecules known to trigger the umami taste sensationUmami taste signals are triggered upon binding of tastant molecules to the G protein-coupled receptor (GPCR) heterodimer T1R1-T1R3, which ultimately causes the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers the release of Ca2+ cations and the subsequent gating of the taste-transduction channels (Figure 2).
Figure 1 Three common tastant molecules known to trigger the umami taste sensationUmami taste signals are triggered upon binding of tastant molecules to the G protein-coupled receptor (GPCR) heterodimer T1R1-T1R3, which ultimately causes the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers the release of Ca2+ cations and the subsequent gating of the taste-transduction channels (Figure 2). Figure 2 Signal pathway for transduction of umami taste sensationInosine monophosphate (IMP) has been suggested as enhancing (potentiating) the response of T1R1+T1R3 GPCRs to umami tastant molecules. Scientists tested this hypothesis by culturing receptors from human embryonic kidney (HEK) cells containing promiscuous G proteins. The HEK cells were then stimulated with umami tastants in the presence or absence of IMP. In each test, the cells were exposed to the same amounts of amino acid tastant and IMP. The number of cells that responded to each tastant was measured using a calcium indicator dye (FURA-2AM) in conjunction with fluorescence microscopy. By comparing the cellular response ratio of the stimulated to unstimulated HEK cells, the extent of potentiation can be assessed. The results are shown in Figure 3.
Figure 2 Signal pathway for transduction of umami taste sensationInosine monophosphate (IMP) has been suggested as enhancing (potentiating) the response of T1R1+T1R3 GPCRs to umami tastant molecules. Scientists tested this hypothesis by culturing receptors from human embryonic kidney (HEK) cells containing promiscuous G proteins. The HEK cells were then stimulated with umami tastants in the presence or absence of IMP. In each test, the cells were exposed to the same amounts of amino acid tastant and IMP. The number of cells that responded to each tastant was measured using a calcium indicator dye (FURA-2AM) in conjunction with fluorescence microscopy. By comparing the cellular response ratio of the stimulated to unstimulated HEK cells, the extent of potentiation can be assessed. The results are shown in Figure 3. Figure 3 Quantification of amino-acid responses for T1R1+T1R3 with and without IMP
Figure 3 Quantification of amino-acid responses for T1R1+T1R3 with and without IMPAdapted from Nelson G, Chandrashekar J, Hoon MA, et al. An amino-acid taste receptor. Nature. 2002;416(6877):199-202.
Which of the following statements is true regarding the bonds in the tastant molecule L-methionine?The pi bond in C=O has a smaller dissociation energy than the sigma bond.The C=O bond is stronger than the C-O bond.The pi bond in C=O is more stable than the sigma bond.
A)I only
B)I and II only
C)II and III only
D)I, II, and III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
23
Passage
Catabolism is an oxidative process in which electrons are transferred from lipids, proteins, and carbohydrates to generate high-energy electron carriers from nicotinamide adenine dinucleotide (NADH/NAD+) and flavin adenine dinucleotide (FADH2/FAD). Both are used in the electron transport chain (ETC) as electron providers in a series of reactions. The transfer of electrons provides energy that drives oxidative phosphorylation and the production of ATP. Reduction of NAD+ proceeds according to the following half-reaction:
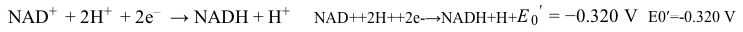 Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.
Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.
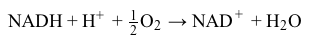 NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf), that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.
NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf), that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.
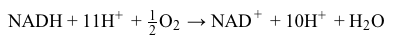 NADH+11H++12O2→NAD++10H++H2OReaction 3
NADH+11H++12O2→NAD++10H++H2OReaction 3
Adapted from Garrett R, Grisham CM, Sabat M. Biochemistry. Cengage Learning; 2011.
The reduction half-reaction for oxygen is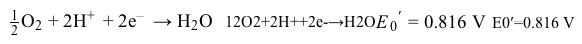 What is the overall reduction potential for the ETC?
What is the overall reduction potential for the ETC?
A)−1.14 V
B)−0.50 V
C)0.50 V
D)1.14 V
Catabolism is an oxidative process in which electrons are transferred from lipids, proteins, and carbohydrates to generate high-energy electron carriers from nicotinamide adenine dinucleotide (NADH/NAD+) and flavin adenine dinucleotide (FADH2/FAD). Both are used in the electron transport chain (ETC) as electron providers in a series of reactions. The transfer of electrons provides energy that drives oxidative phosphorylation and the production of ATP. Reduction of NAD+ proceeds according to the following half-reaction:
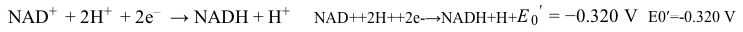 Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.
Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.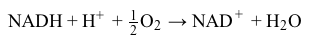 NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf), that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.
NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf), that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.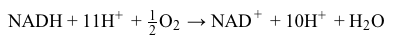 NADH+11H++12O2→NAD++10H++H2OReaction 3
NADH+11H++12O2→NAD++10H++H2OReaction 3Adapted from Garrett R, Grisham CM, Sabat M. Biochemistry. Cengage Learning; 2011.
The reduction half-reaction for oxygen is
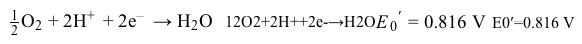 What is the overall reduction potential for the ETC?
What is the overall reduction potential for the ETC?A)−1.14 V
B)−0.50 V
C)0.50 V
D)1.14 V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
24
Passage
Catabolism is an oxidative process in which electrons are transferred from lipids, proteins, and carbohydrates to generate high-energy electron carriers from nicotinamide adenine dinucleotide (NADH/NAD+) and flavin adenine dinucleotide (FADH2/FAD). Both are used in the electron transport chain (ETC) as electron providers in a series of reactions. The transfer of electrons provides energy that drives oxidative phosphorylation and the production of ATP. Reduction of NAD+ proceeds according to the following half-reaction:
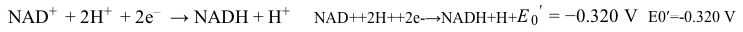 Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.
Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.
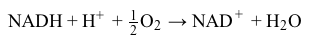 NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf), that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.
NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf), that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.
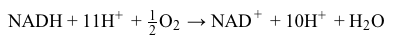 NADH+11H++12O2→NAD++10H++H2OReaction 3
NADH+11H++12O2→NAD++10H++H2OReaction 3
Adapted from Garrett R, Grisham CM, Sabat M. Biochemistry. Cengage Learning; 2011.
Researchers want to measure the reduction potential when NADH is oxidized by oxygen to compare the experimental potential with the calculated reduction potential in the mitochondria. They set up a galvanic cell, as shown in the following diagram.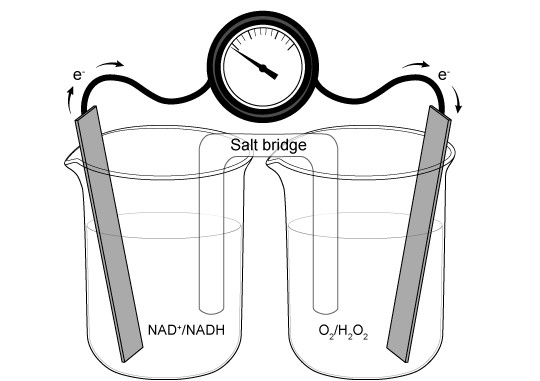 Which of the following statements is true about NAD+/NADH in this experiment?
Which of the following statements is true about NAD+/NADH in this experiment?
A)NAD+ becomes oxidized at the anode.
B)An applied potential causes NADH to lose electrons at the cathode.
C)NADH is oxidized at the anode, and NAD+ is reduced at the cathode.
D)An electrical potential exists because NADH loses electrons at the anode.
Catabolism is an oxidative process in which electrons are transferred from lipids, proteins, and carbohydrates to generate high-energy electron carriers from nicotinamide adenine dinucleotide (NADH/NAD+) and flavin adenine dinucleotide (FADH2/FAD). Both are used in the electron transport chain (ETC) as electron providers in a series of reactions. The transfer of electrons provides energy that drives oxidative phosphorylation and the production of ATP. Reduction of NAD+ proceeds according to the following half-reaction:
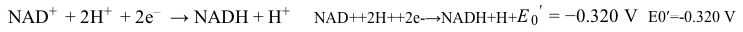 Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.
Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.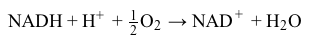 NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf), that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.
NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf), that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.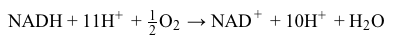 NADH+11H++12O2→NAD++10H++H2OReaction 3
NADH+11H++12O2→NAD++10H++H2OReaction 3Adapted from Garrett R, Grisham CM, Sabat M. Biochemistry. Cengage Learning; 2011.
Researchers want to measure the reduction potential when NADH is oxidized by oxygen to compare the experimental potential with the calculated reduction potential in the mitochondria. They set up a galvanic cell, as shown in the following diagram.
 Which of the following statements is true about NAD+/NADH in this experiment?
Which of the following statements is true about NAD+/NADH in this experiment?A)NAD+ becomes oxidized at the anode.
B)An applied potential causes NADH to lose electrons at the cathode.
C)NADH is oxidized at the anode, and NAD+ is reduced at the cathode.
D)An electrical potential exists because NADH loses electrons at the anode.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
25
Passage
In polluted urban environments, airborne proteins can undergo nitration reactions that increase their allergenic potential toward the human respiratory system. For example, upon exposure to ozone (O3) and nitrogen dioxide (NO2) under atmospheric conditions, the amino acid tyrosine is known to undergo a two-step irreversible reaction leading to the formation of nitrotyrosine. The first step involves the formation of a reactive oxygen intermediate (ROI) tyrosyl radical through oxidation of the phenolic site of tyrosine. Nitrotyrosine is then formed in the second step through addition of NO2 by a radical-radical termination reaction. The elementary steps for this reaction are shown in Scheme 1.
 Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments.
Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments.
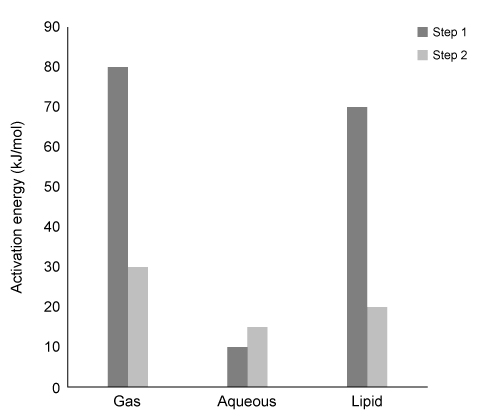 Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K)
Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K)
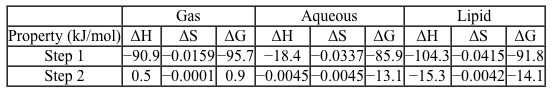 Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90.
Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90.
Because step 2 of the reaction in aqueous phase is rate-limiting, the formation of ROI from tyrosine and ozone in step 1 can be assumed to reach a state of equilibrium.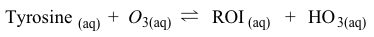 Tyrosine(aq)+ O3(aq)⇌ ROI(aq) + HO3(aq)Which of the following will occur if the temperature of this system is raised from 298.15 K to 350 K?
Tyrosine(aq)+ O3(aq)⇌ ROI(aq) + HO3(aq)Which of the following will occur if the temperature of this system is raised from 298.15 K to 350 K?
A)Keq will increase.
B)The reaction will be driven toward the reactants.
C)The reaction will be driven toward the products.
D)Keq will remain the same.
In polluted urban environments, airborne proteins can undergo nitration reactions that increase their allergenic potential toward the human respiratory system. For example, upon exposure to ozone (O3) and nitrogen dioxide (NO2) under atmospheric conditions, the amino acid tyrosine is known to undergo a two-step irreversible reaction leading to the formation of nitrotyrosine. The first step involves the formation of a reactive oxygen intermediate (ROI) tyrosyl radical through oxidation of the phenolic site of tyrosine. Nitrotyrosine is then formed in the second step through addition of NO2 by a radical-radical termination reaction. The elementary steps for this reaction are shown in Scheme 1.
 Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments.
Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments. Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K)
Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K) Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90.
Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90.Because step 2 of the reaction in aqueous phase is rate-limiting, the formation of ROI from tyrosine and ozone in step 1 can be assumed to reach a state of equilibrium.
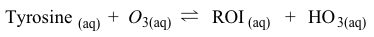 Tyrosine(aq)+ O3(aq)⇌ ROI(aq) + HO3(aq)Which of the following will occur if the temperature of this system is raised from 298.15 K to 350 K?
Tyrosine(aq)+ O3(aq)⇌ ROI(aq) + HO3(aq)Which of the following will occur if the temperature of this system is raised from 298.15 K to 350 K?A)Keq will increase.
B)The reaction will be driven toward the reactants.
C)The reaction will be driven toward the products.
D)Keq will remain the same.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
26
Passage
Catabolism is an oxidative process in which electrons are transferred from lipids, proteins, and carbohydrates to generate high-energy electron carriers from nicotinamide adenine dinucleotide (NADH/NAD+) and flavin adenine dinucleotide (FADH2/FAD). Both are used in the electron transport chain (ETC) as electron providers in a series of reactions. The transfer of electrons provides energy that drives oxidative phosphorylation and the production of ATP. Reduction of NAD+ proceeds according to the following half-reaction:
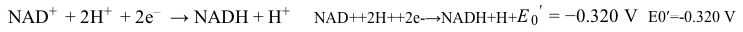 Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.
Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.
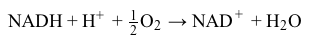 NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf), that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.
NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf), that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.
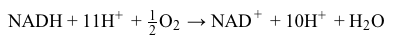 NADH+11H++12O2→NAD++10H++H2OReaction 3
NADH+11H++12O2→NAD++10H++H2OReaction 3
Adapted from Garrett R, Grisham CM, Sabat M. Biochemistry. Cengage Learning; 2011.
For biological processes, which of the following is true of oxidation-reduction reactions?Reduction often involves the formation of bonds to hydrogen.Oxidation often involves the formation of bonds to oxygen.Reducing agents undergo a change to a lower oxidation state.
A)I only
B)I and II only
C)II and III only
D)I, II, and III
Catabolism is an oxidative process in which electrons are transferred from lipids, proteins, and carbohydrates to generate high-energy electron carriers from nicotinamide adenine dinucleotide (NADH/NAD+) and flavin adenine dinucleotide (FADH2/FAD). Both are used in the electron transport chain (ETC) as electron providers in a series of reactions. The transfer of electrons provides energy that drives oxidative phosphorylation and the production of ATP. Reduction of NAD+ proceeds according to the following half-reaction:
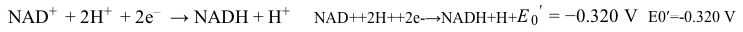 Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.
Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.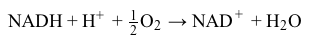 NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf), that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.
NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf), that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.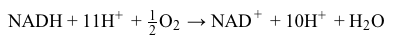 NADH+11H++12O2→NAD++10H++H2OReaction 3
NADH+11H++12O2→NAD++10H++H2OReaction 3Adapted from Garrett R, Grisham CM, Sabat M. Biochemistry. Cengage Learning; 2011.
For biological processes, which of the following is true of oxidation-reduction reactions?Reduction often involves the formation of bonds to hydrogen.Oxidation often involves the formation of bonds to oxygen.Reducing agents undergo a change to a lower oxidation state.
A)I only
B)I and II only
C)II and III only
D)I, II, and III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
27
Passage
In polluted urban environments, airborne proteins can undergo nitration reactions that increase their allergenic potential toward the human respiratory system. For example, upon exposure to ozone (O3) and nitrogen dioxide (NO2) under atmospheric conditions, the amino acid tyrosine is known to undergo a two-step irreversible reaction leading to the formation of nitrotyrosine. The first step involves the formation of a reactive oxygen intermediate (ROI) tyrosyl radical through oxidation of the phenolic site of tyrosine. Nitrotyrosine is then formed in the second step through addition of NO2 by a radical-radical termination reaction. The elementary steps for this reaction are shown in Scheme 1.
 Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments.
Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments.
 Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K)
Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K)
 Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90.
Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90.
Which of the following are true concerning the overall reaction in an aqueous environment?The rate constant increases exponentially with increasing temperature.The rate constant increases exponentially with decreasing activation energy.The reaction rate decreases exponentially with increasing activation energy.The rate constant of the catalyzed reaction will be smaller than the rate constant of the uncatalyzed reaction.
A)I and II only
B)I, II, and III only
C)II and IV only
D)I, III, and IV only
In polluted urban environments, airborne proteins can undergo nitration reactions that increase their allergenic potential toward the human respiratory system. For example, upon exposure to ozone (O3) and nitrogen dioxide (NO2) under atmospheric conditions, the amino acid tyrosine is known to undergo a two-step irreversible reaction leading to the formation of nitrotyrosine. The first step involves the formation of a reactive oxygen intermediate (ROI) tyrosyl radical through oxidation of the phenolic site of tyrosine. Nitrotyrosine is then formed in the second step through addition of NO2 by a radical-radical termination reaction. The elementary steps for this reaction are shown in Scheme 1.
 Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments.
Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments. Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K)
Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K) Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90.
Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90.Which of the following are true concerning the overall reaction in an aqueous environment?The rate constant increases exponentially with increasing temperature.The rate constant increases exponentially with decreasing activation energy.The reaction rate decreases exponentially with increasing activation energy.The rate constant of the catalyzed reaction will be smaller than the rate constant of the uncatalyzed reaction.
A)I and II only
B)I, II, and III only
C)II and IV only
D)I, III, and IV only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
28
Passage
Umami is one of five basic tastes that humans recognize, and it is triggered by L-amino acids as well as some synthetic ingredients and organic acids. Three common umami tastant molecules are shown in Figure 1.
 Figure 1 Three common tastant molecules known to trigger the umami taste sensationUmami taste signals are triggered upon binding of tastant molecules to the G protein-coupled receptor (GPCR) heterodimer T1R1-T1R3, which ultimately causes the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers the release of Ca2+ cations and the subsequent gating of the taste-transduction channels (Figure 2).
Figure 1 Three common tastant molecules known to trigger the umami taste sensationUmami taste signals are triggered upon binding of tastant molecules to the G protein-coupled receptor (GPCR) heterodimer T1R1-T1R3, which ultimately causes the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers the release of Ca2+ cations and the subsequent gating of the taste-transduction channels (Figure 2).
 Figure 2 Signal pathway for transduction of umami taste sensationInosine monophosphate (IMP) has been suggested as enhancing (potentiating) the response of T1R1+T1R3 GPCRs to umami tastant molecules. Scientists tested this hypothesis by culturing receptors from human embryonic kidney (HEK) cells containing promiscuous G proteins. The HEK cells were then stimulated with umami tastants in the presence or absence of IMP. In each test, the cells were exposed to the same amounts of amino acid tastant and IMP. The number of cells that responded to each tastant was measured using a calcium indicator dye (FURA-2AM) in conjunction with fluorescence microscopy. By comparing the cellular response ratio of the stimulated to unstimulated HEK cells, the extent of potentiation can be assessed. The results are shown in Figure 3.
Figure 2 Signal pathway for transduction of umami taste sensationInosine monophosphate (IMP) has been suggested as enhancing (potentiating) the response of T1R1+T1R3 GPCRs to umami tastant molecules. Scientists tested this hypothesis by culturing receptors from human embryonic kidney (HEK) cells containing promiscuous G proteins. The HEK cells were then stimulated with umami tastants in the presence or absence of IMP. In each test, the cells were exposed to the same amounts of amino acid tastant and IMP. The number of cells that responded to each tastant was measured using a calcium indicator dye (FURA-2AM) in conjunction with fluorescence microscopy. By comparing the cellular response ratio of the stimulated to unstimulated HEK cells, the extent of potentiation can be assessed. The results are shown in Figure 3.
 Figure 3 Quantification of amino-acid responses for T1R1+T1R3 with and without IMP
Figure 3 Quantification of amino-acid responses for T1R1+T1R3 with and without IMP
Adapted from Nelson G, Chandrashekar J, Hoon MA, et al. An amino-acid taste receptor. Nature. 2002;416(6877):199-202.
What are the shapes of the groups bonded to the central atoms 1, 2, and 3, respectively in the tastant molecule L-AP4 shown?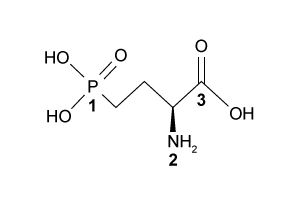
A)Tetrahedral, trigonal pyramidal, trigonal planar
B)Tetrahedral, trigonal planar, trigonal planar
C)Trigonal pyramidal, trigonal planar, bent
D)Trigonal pyramidal, trigonal pyramidal, trigonal pyramidal
Umami is one of five basic tastes that humans recognize, and it is triggered by L-amino acids as well as some synthetic ingredients and organic acids. Three common umami tastant molecules are shown in Figure 1.
 Figure 1 Three common tastant molecules known to trigger the umami taste sensationUmami taste signals are triggered upon binding of tastant molecules to the G protein-coupled receptor (GPCR) heterodimer T1R1-T1R3, which ultimately causes the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers the release of Ca2+ cations and the subsequent gating of the taste-transduction channels (Figure 2).
Figure 1 Three common tastant molecules known to trigger the umami taste sensationUmami taste signals are triggered upon binding of tastant molecules to the G protein-coupled receptor (GPCR) heterodimer T1R1-T1R3, which ultimately causes the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers the release of Ca2+ cations and the subsequent gating of the taste-transduction channels (Figure 2). Figure 2 Signal pathway for transduction of umami taste sensationInosine monophosphate (IMP) has been suggested as enhancing (potentiating) the response of T1R1+T1R3 GPCRs to umami tastant molecules. Scientists tested this hypothesis by culturing receptors from human embryonic kidney (HEK) cells containing promiscuous G proteins. The HEK cells were then stimulated with umami tastants in the presence or absence of IMP. In each test, the cells were exposed to the same amounts of amino acid tastant and IMP. The number of cells that responded to each tastant was measured using a calcium indicator dye (FURA-2AM) in conjunction with fluorescence microscopy. By comparing the cellular response ratio of the stimulated to unstimulated HEK cells, the extent of potentiation can be assessed. The results are shown in Figure 3.
Figure 2 Signal pathway for transduction of umami taste sensationInosine monophosphate (IMP) has been suggested as enhancing (potentiating) the response of T1R1+T1R3 GPCRs to umami tastant molecules. Scientists tested this hypothesis by culturing receptors from human embryonic kidney (HEK) cells containing promiscuous G proteins. The HEK cells were then stimulated with umami tastants in the presence or absence of IMP. In each test, the cells were exposed to the same amounts of amino acid tastant and IMP. The number of cells that responded to each tastant was measured using a calcium indicator dye (FURA-2AM) in conjunction with fluorescence microscopy. By comparing the cellular response ratio of the stimulated to unstimulated HEK cells, the extent of potentiation can be assessed. The results are shown in Figure 3. Figure 3 Quantification of amino-acid responses for T1R1+T1R3 with and without IMP
Figure 3 Quantification of amino-acid responses for T1R1+T1R3 with and without IMPAdapted from Nelson G, Chandrashekar J, Hoon MA, et al. An amino-acid taste receptor. Nature. 2002;416(6877):199-202.
What are the shapes of the groups bonded to the central atoms 1, 2, and 3, respectively in the tastant molecule L-AP4 shown?

A)Tetrahedral, trigonal pyramidal, trigonal planar
B)Tetrahedral, trigonal planar, trigonal planar
C)Trigonal pyramidal, trigonal planar, bent
D)Trigonal pyramidal, trigonal pyramidal, trigonal pyramidal

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
29
Passage
Umami is one of five basic tastes that humans recognize, and it is triggered by L-amino acids as well as some synthetic ingredients and organic acids. Three common umami tastant molecules are shown in Figure 1.
 Figure 1 Three common tastant molecules known to trigger the umami taste sensationUmami taste signals are triggered upon binding of tastant molecules to the G protein-coupled receptor (GPCR) heterodimer T1R1-T1R3, which ultimately causes the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers the release of Ca2+ cations and the subsequent gating of the taste-transduction channels (Figure 2).
Figure 1 Three common tastant molecules known to trigger the umami taste sensationUmami taste signals are triggered upon binding of tastant molecules to the G protein-coupled receptor (GPCR) heterodimer T1R1-T1R3, which ultimately causes the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers the release of Ca2+ cations and the subsequent gating of the taste-transduction channels (Figure 2).
 Figure 2 Signal pathway for transduction of umami taste sensationInosine monophosphate (IMP) has been suggested as enhancing (potentiating) the response of T1R1+T1R3 GPCRs to umami tastant molecules. Scientists tested this hypothesis by culturing receptors from human embryonic kidney (HEK) cells containing promiscuous G proteins. The HEK cells were then stimulated with umami tastants in the presence or absence of IMP. In each test, the cells were exposed to the same amounts of amino acid tastant and IMP. The number of cells that responded to each tastant was measured using a calcium indicator dye (FURA-2AM) in conjunction with fluorescence microscopy. By comparing the cellular response ratio of the stimulated to unstimulated HEK cells, the extent of potentiation can be assessed. The results are shown in Figure 3.
Figure 2 Signal pathway for transduction of umami taste sensationInosine monophosphate (IMP) has been suggested as enhancing (potentiating) the response of T1R1+T1R3 GPCRs to umami tastant molecules. Scientists tested this hypothesis by culturing receptors from human embryonic kidney (HEK) cells containing promiscuous G proteins. The HEK cells were then stimulated with umami tastants in the presence or absence of IMP. In each test, the cells were exposed to the same amounts of amino acid tastant and IMP. The number of cells that responded to each tastant was measured using a calcium indicator dye (FURA-2AM) in conjunction with fluorescence microscopy. By comparing the cellular response ratio of the stimulated to unstimulated HEK cells, the extent of potentiation can be assessed. The results are shown in Figure 3.
 Figure 3 Quantification of amino-acid responses for T1R1+T1R3 with and without IMP
Figure 3 Quantification of amino-acid responses for T1R1+T1R3 with and without IMP
Adapted from Nelson G, Chandrashekar J, Hoon MA, et al. An amino-acid taste receptor. Nature. 2002;416(6877):199-202.
Which of the following best explains the results regarding D-amino acids in Figure 3?
A)D-amino acids are not as abundant in HEK cells as L-amino acids.
B)D-amino acids do not fluoresce upon addition of FURA-2AM dye.
C)D-amino acids do not trigger the formation of DAG.
D)Most D-amino acids do not effectively bind to the GPCR heterodimer T1R1+T1R3.
Umami is one of five basic tastes that humans recognize, and it is triggered by L-amino acids as well as some synthetic ingredients and organic acids. Three common umami tastant molecules are shown in Figure 1.
 Figure 1 Three common tastant molecules known to trigger the umami taste sensationUmami taste signals are triggered upon binding of tastant molecules to the G protein-coupled receptor (GPCR) heterodimer T1R1-T1R3, which ultimately causes the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers the release of Ca2+ cations and the subsequent gating of the taste-transduction channels (Figure 2).
Figure 1 Three common tastant molecules known to trigger the umami taste sensationUmami taste signals are triggered upon binding of tastant molecules to the G protein-coupled receptor (GPCR) heterodimer T1R1-T1R3, which ultimately causes the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers the release of Ca2+ cations and the subsequent gating of the taste-transduction channels (Figure 2). Figure 2 Signal pathway for transduction of umami taste sensationInosine monophosphate (IMP) has been suggested as enhancing (potentiating) the response of T1R1+T1R3 GPCRs to umami tastant molecules. Scientists tested this hypothesis by culturing receptors from human embryonic kidney (HEK) cells containing promiscuous G proteins. The HEK cells were then stimulated with umami tastants in the presence or absence of IMP. In each test, the cells were exposed to the same amounts of amino acid tastant and IMP. The number of cells that responded to each tastant was measured using a calcium indicator dye (FURA-2AM) in conjunction with fluorescence microscopy. By comparing the cellular response ratio of the stimulated to unstimulated HEK cells, the extent of potentiation can be assessed. The results are shown in Figure 3.
Figure 2 Signal pathway for transduction of umami taste sensationInosine monophosphate (IMP) has been suggested as enhancing (potentiating) the response of T1R1+T1R3 GPCRs to umami tastant molecules. Scientists tested this hypothesis by culturing receptors from human embryonic kidney (HEK) cells containing promiscuous G proteins. The HEK cells were then stimulated with umami tastants in the presence or absence of IMP. In each test, the cells were exposed to the same amounts of amino acid tastant and IMP. The number of cells that responded to each tastant was measured using a calcium indicator dye (FURA-2AM) in conjunction with fluorescence microscopy. By comparing the cellular response ratio of the stimulated to unstimulated HEK cells, the extent of potentiation can be assessed. The results are shown in Figure 3. Figure 3 Quantification of amino-acid responses for T1R1+T1R3 with and without IMP
Figure 3 Quantification of amino-acid responses for T1R1+T1R3 with and without IMPAdapted from Nelson G, Chandrashekar J, Hoon MA, et al. An amino-acid taste receptor. Nature. 2002;416(6877):199-202.
Which of the following best explains the results regarding D-amino acids in Figure 3?
A)D-amino acids are not as abundant in HEK cells as L-amino acids.
B)D-amino acids do not fluoresce upon addition of FURA-2AM dye.
C)D-amino acids do not trigger the formation of DAG.
D)Most D-amino acids do not effectively bind to the GPCR heterodimer T1R1+T1R3.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
30
Passage
Depending on the active compound's specific method of action, sunscreens are broadly classified into two groups: organic and inorganic. Organic sunscreens typically contain aromatic compounds that absorb ultraviolet (UV) radiation. The absorbed energy from the UV radiation causes these compounds to enter electronically excited states which then relax back to the ground state and harmlessly re-emit the absorbed radiation as heat. Alternatively, inorganic sunscreens contain metallic nanoparticles that serve to reflect, scatter, and absorb incident radiation. The mechanism for UV protection of each group is shown in Figure 1.
 Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF). However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens
Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF). However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens
 In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested.
In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested.
 Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreens
Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreens
Adapted from Van der molen RG, Out-luiting C, Driller H, Claas FH, Koerten HK, Mommaas AM. Broad-spectrum sunscreens offer protection against urocanic acid photoisomerization by artificial ultraviolet radiation in human skin. J Invest Dermatol. 2000;115(3):421-6.
Which of the following is true concerning the energy difference ΔE between the ground state and the photo-excited state that occurs most often for the active compound in sunscreen B10?
A)ΔE=hc×297 nm
B)ΔE=hc×(250 nm−300 nm)
C)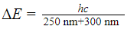
D)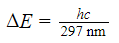
Depending on the active compound's specific method of action, sunscreens are broadly classified into two groups: organic and inorganic. Organic sunscreens typically contain aromatic compounds that absorb ultraviolet (UV) radiation. The absorbed energy from the UV radiation causes these compounds to enter electronically excited states which then relax back to the ground state and harmlessly re-emit the absorbed radiation as heat. Alternatively, inorganic sunscreens contain metallic nanoparticles that serve to reflect, scatter, and absorb incident radiation. The mechanism for UV protection of each group is shown in Figure 1.
 Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF). However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens
Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF). However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested.
In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested. Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreens
Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreensAdapted from Van der molen RG, Out-luiting C, Driller H, Claas FH, Koerten HK, Mommaas AM. Broad-spectrum sunscreens offer protection against urocanic acid photoisomerization by artificial ultraviolet radiation in human skin. J Invest Dermatol. 2000;115(3):421-6.
Which of the following is true concerning the energy difference ΔE between the ground state and the photo-excited state that occurs most often for the active compound in sunscreen B10?
A)ΔE=hc×297 nm
B)ΔE=hc×(250 nm−300 nm)
C)
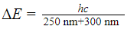
D)
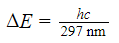

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
31
Passage
Catabolism is an oxidative process in which electrons are transferred from lipids, proteins, and carbohydrates to generate high-energy electron carriers from nicotinamide adenine dinucleotide (NADH/NAD+) and flavin adenine dinucleotide (FADH2/FAD). Both are used in the electron transport chain (ETC) as electron providers in a series of reactions. The transfer of electrons provides energy that drives oxidative phosphorylation and the production of ATP. Reduction of NAD+ proceeds according to the following half-reaction:
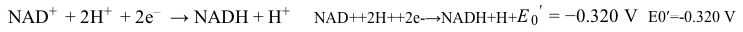 Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.
Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.
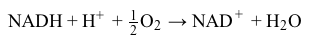 NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf), that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.
NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf), that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.
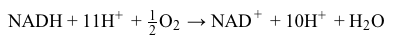 NADH+11H++12O2→NAD++10H++H2OReaction 3
NADH+11H++12O2→NAD++10H++H2OReaction 3
Adapted from Garrett R, Grisham CM, Sabat M. Biochemistry. Cengage Learning; 2011.
If the ETC is a spontaneous process, which of the following MUST be true about the redox reactions within each of the four complexes?
A)The electron-carrying molecules in the ETC have E0′ values greater than that of oxygen.
B)The overall free energy (ΔG°′) of the ETC is positive.
C)The E0′ values increase as electrons are passed from one carrier to the next.
D)The redox reactions in complex IV are faster than the reactions in complex I.
Catabolism is an oxidative process in which electrons are transferred from lipids, proteins, and carbohydrates to generate high-energy electron carriers from nicotinamide adenine dinucleotide (NADH/NAD+) and flavin adenine dinucleotide (FADH2/FAD). Both are used in the electron transport chain (ETC) as electron providers in a series of reactions. The transfer of electrons provides energy that drives oxidative phosphorylation and the production of ATP. Reduction of NAD+ proceeds according to the following half-reaction:
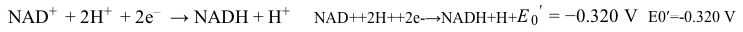 Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.
Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.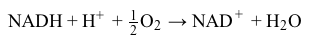 NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf), that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.
NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf), that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.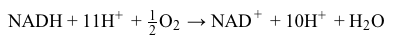 NADH+11H++12O2→NAD++10H++H2OReaction 3
NADH+11H++12O2→NAD++10H++H2OReaction 3Adapted from Garrett R, Grisham CM, Sabat M. Biochemistry. Cengage Learning; 2011.
If the ETC is a spontaneous process, which of the following MUST be true about the redox reactions within each of the four complexes?
A)The electron-carrying molecules in the ETC have E0′ values greater than that of oxygen.
B)The overall free energy (ΔG°′) of the ETC is positive.
C)The E0′ values increase as electrons are passed from one carrier to the next.
D)The redox reactions in complex IV are faster than the reactions in complex I.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
32
Passage
Depending on the active compound's specific method of action, sunscreens are broadly classified into two groups: organic and inorganic. Organic sunscreens typically contain aromatic compounds that absorb ultraviolet (UV) radiation. The absorbed energy from the UV radiation causes these compounds to enter electronically excited states which then relax back to the ground state and harmlessly re-emit the absorbed radiation as heat. Alternatively, inorganic sunscreens contain metallic nanoparticles that serve to reflect, scatter, and absorb incident radiation. The mechanism for UV protection of each group is shown in Figure 1.
 Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF). However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens
Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF). However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens
 In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested.
In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested.
 Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreens
Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreens
Adapted from Van der molen RG, Out-luiting C, Driller H, Claas FH, Koerten HK, Mommaas AM. Broad-spectrum sunscreens offer protection against urocanic acid photoisomerization by artificial ultraviolet radiation in human skin. J Invest Dermatol. 2000;115(3):421-6.
Zinc, titanium, iron, and aluminum are all ingredients found in inorganic sunscreens. Which of the following choices correctly lists the atoms in order of increasing atomic radius?
A)Al < Zn < Fe < Ti
B)Zn < Fe < Ti < Al
C)Ti < Fe < Zn < Al
D)Al < Ti < Fe < Zn
Depending on the active compound's specific method of action, sunscreens are broadly classified into two groups: organic and inorganic. Organic sunscreens typically contain aromatic compounds that absorb ultraviolet (UV) radiation. The absorbed energy from the UV radiation causes these compounds to enter electronically excited states which then relax back to the ground state and harmlessly re-emit the absorbed radiation as heat. Alternatively, inorganic sunscreens contain metallic nanoparticles that serve to reflect, scatter, and absorb incident radiation. The mechanism for UV protection of each group is shown in Figure 1.
 Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF). However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens
Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF). However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested.
In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested. Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreens
Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreensAdapted from Van der molen RG, Out-luiting C, Driller H, Claas FH, Koerten HK, Mommaas AM. Broad-spectrum sunscreens offer protection against urocanic acid photoisomerization by artificial ultraviolet radiation in human skin. J Invest Dermatol. 2000;115(3):421-6.
Zinc, titanium, iron, and aluminum are all ingredients found in inorganic sunscreens. Which of the following choices correctly lists the atoms in order of increasing atomic radius?
A)Al < Zn < Fe < Ti
B)Zn < Fe < Ti < Al
C)Ti < Fe < Zn < Al
D)Al < Ti < Fe < Zn

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
33
Passage
Umami is one of five basic tastes that humans recognize, and it is triggered by L-amino acids as well as some synthetic ingredients and organic acids. Three common umami tastant molecules are shown in Figure 1.
 Figure 1 Three common tastant molecules known to trigger the umami taste sensationUmami taste signals are triggered upon binding of tastant molecules to the G protein-coupled receptor (GPCR) heterodimer T1R1-T1R3, which ultimately causes the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers the release of Ca2+ cations and the subsequent gating of the taste-transduction channels (Figure 2).
Figure 1 Three common tastant molecules known to trigger the umami taste sensationUmami taste signals are triggered upon binding of tastant molecules to the G protein-coupled receptor (GPCR) heterodimer T1R1-T1R3, which ultimately causes the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers the release of Ca2+ cations and the subsequent gating of the taste-transduction channels (Figure 2).
 Figure 2 Signal pathway for transduction of umami taste sensationInosine monophosphate (IMP) has been suggested as enhancing (potentiating) the response of T1R1+T1R3 GPCRs to umami tastant molecules. Scientists tested this hypothesis by culturing receptors from human embryonic kidney (HEK) cells containing promiscuous G proteins. The HEK cells were then stimulated with umami tastants in the presence or absence of IMP. In each test, the cells were exposed to the same amounts of amino acid tastant and IMP. The number of cells that responded to each tastant was measured using a calcium indicator dye (FURA-2AM) in conjunction with fluorescence microscopy. By comparing the cellular response ratio of the stimulated to unstimulated HEK cells, the extent of potentiation can be assessed. The results are shown in Figure 3.
Figure 2 Signal pathway for transduction of umami taste sensationInosine monophosphate (IMP) has been suggested as enhancing (potentiating) the response of T1R1+T1R3 GPCRs to umami tastant molecules. Scientists tested this hypothesis by culturing receptors from human embryonic kidney (HEK) cells containing promiscuous G proteins. The HEK cells were then stimulated with umami tastants in the presence or absence of IMP. In each test, the cells were exposed to the same amounts of amino acid tastant and IMP. The number of cells that responded to each tastant was measured using a calcium indicator dye (FURA-2AM) in conjunction with fluorescence microscopy. By comparing the cellular response ratio of the stimulated to unstimulated HEK cells, the extent of potentiation can be assessed. The results are shown in Figure 3.
 Figure 3 Quantification of amino-acid responses for T1R1+T1R3 with and without IMP
Figure 3 Quantification of amino-acid responses for T1R1+T1R3 with and without IMP
Adapted from Nelson G, Chandrashekar J, Hoon MA, et al. An amino-acid taste receptor. Nature. 2002;416(6877):199-202.
What is the hybridization of the numbered atoms in the tastant molecule L-AP4 shown?
A)sp, sp3, sp, sp3
B)sp3, sp3d, sp3, sp2
C)sp3, sp4, sp3, sp3
D)sp3, sp3, sp3, sp2
Umami is one of five basic tastes that humans recognize, and it is triggered by L-amino acids as well as some synthetic ingredients and organic acids. Three common umami tastant molecules are shown in Figure 1.
 Figure 1 Three common tastant molecules known to trigger the umami taste sensationUmami taste signals are triggered upon binding of tastant molecules to the G protein-coupled receptor (GPCR) heterodimer T1R1-T1R3, which ultimately causes the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers the release of Ca2+ cations and the subsequent gating of the taste-transduction channels (Figure 2).
Figure 1 Three common tastant molecules known to trigger the umami taste sensationUmami taste signals are triggered upon binding of tastant molecules to the G protein-coupled receptor (GPCR) heterodimer T1R1-T1R3, which ultimately causes the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers the release of Ca2+ cations and the subsequent gating of the taste-transduction channels (Figure 2). Figure 2 Signal pathway for transduction of umami taste sensationInosine monophosphate (IMP) has been suggested as enhancing (potentiating) the response of T1R1+T1R3 GPCRs to umami tastant molecules. Scientists tested this hypothesis by culturing receptors from human embryonic kidney (HEK) cells containing promiscuous G proteins. The HEK cells were then stimulated with umami tastants in the presence or absence of IMP. In each test, the cells were exposed to the same amounts of amino acid tastant and IMP. The number of cells that responded to each tastant was measured using a calcium indicator dye (FURA-2AM) in conjunction with fluorescence microscopy. By comparing the cellular response ratio of the stimulated to unstimulated HEK cells, the extent of potentiation can be assessed. The results are shown in Figure 3.
Figure 2 Signal pathway for transduction of umami taste sensationInosine monophosphate (IMP) has been suggested as enhancing (potentiating) the response of T1R1+T1R3 GPCRs to umami tastant molecules. Scientists tested this hypothesis by culturing receptors from human embryonic kidney (HEK) cells containing promiscuous G proteins. The HEK cells were then stimulated with umami tastants in the presence or absence of IMP. In each test, the cells were exposed to the same amounts of amino acid tastant and IMP. The number of cells that responded to each tastant was measured using a calcium indicator dye (FURA-2AM) in conjunction with fluorescence microscopy. By comparing the cellular response ratio of the stimulated to unstimulated HEK cells, the extent of potentiation can be assessed. The results are shown in Figure 3. Figure 3 Quantification of amino-acid responses for T1R1+T1R3 with and without IMP
Figure 3 Quantification of amino-acid responses for T1R1+T1R3 with and without IMPAdapted from Nelson G, Chandrashekar J, Hoon MA, et al. An amino-acid taste receptor. Nature. 2002;416(6877):199-202.
What is the hybridization of the numbered atoms in the tastant molecule L-AP4 shown?

A)sp, sp3, sp, sp3
B)sp3, sp3d, sp3, sp2
C)sp3, sp4, sp3, sp3
D)sp3, sp3, sp3, sp2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
34
Passage
Catabolism is an oxidative process in which electrons are transferred from lipids, proteins, and carbohydrates to generate high-energy electron carriers from nicotinamide adenine dinucleotide (NADH/NAD+) and flavin adenine dinucleotide (FADH2/FAD). Both are used in the electron transport chain (ETC) as electron providers in a series of reactions. The transfer of electrons provides energy that drives oxidative phosphorylation and the production of ATP. Reduction of NAD+ proceeds according to the following half-reaction:
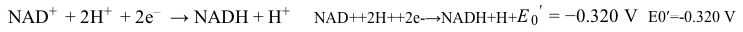 Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.
Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.
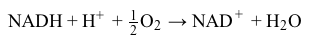 NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf), that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.
NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf), that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.
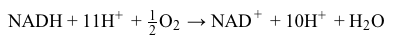 NADH+11H++12O2→NAD++10H++H2OReaction 3
NADH+11H++12O2→NAD++10H++H2OReaction 3
Adapted from Garrett R, Grisham CM, Sabat M. Biochemistry. Cengage Learning; 2011.
Which of the following is NOT true concerning catabolism and the function of the ETC?
A)The number of ATP molecules produced is dependent on the ΔG of the ETC.
B)ΔG of the ETC depends on the overall ΔE0 of the ETC.
C)Catabolism requires NADH to serve as an oxidizing agent.
D)Catabolism produces energy in the form of electron transduction.
Catabolism is an oxidative process in which electrons are transferred from lipids, proteins, and carbohydrates to generate high-energy electron carriers from nicotinamide adenine dinucleotide (NADH/NAD+) and flavin adenine dinucleotide (FADH2/FAD). Both are used in the electron transport chain (ETC) as electron providers in a series of reactions. The transfer of electrons provides energy that drives oxidative phosphorylation and the production of ATP. Reduction of NAD+ proceeds according to the following half-reaction:
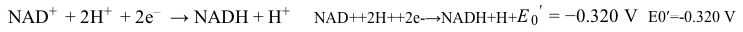 Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.
Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.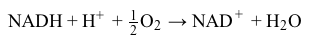 NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf), that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.
NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf), that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.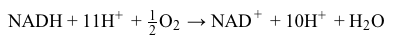 NADH+11H++12O2→NAD++10H++H2OReaction 3
NADH+11H++12O2→NAD++10H++H2OReaction 3Adapted from Garrett R, Grisham CM, Sabat M. Biochemistry. Cengage Learning; 2011.
Which of the following is NOT true concerning catabolism and the function of the ETC?
A)The number of ATP molecules produced is dependent on the ΔG of the ETC.
B)ΔG of the ETC depends on the overall ΔE0 of the ETC.
C)Catabolism requires NADH to serve as an oxidizing agent.
D)Catabolism produces energy in the form of electron transduction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
35
Passage
In polluted urban environments, airborne proteins can undergo nitration reactions that increase their allergenic potential toward the human respiratory system. For example, upon exposure to ozone (O3) and nitrogen dioxide (NO2) under atmospheric conditions, the amino acid tyrosine is known to undergo a two-step irreversible reaction leading to the formation of nitrotyrosine. The first step involves the formation of a reactive oxygen intermediate (ROI) tyrosyl radical through oxidation of the phenolic site of tyrosine. Nitrotyrosine is then formed in the second step through addition of NO2 by a radical-radical termination reaction. The elementary steps for this reaction are shown in Scheme 1.
 Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments.
Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments.
 Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K)
Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K)
 Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90.
Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90.
Suppose a chemist decides to conduct and monitor the reaction for 10 min at 298.15 K in the three environments described in the passage. Which of the three reactions is most likely to form the greatest amount of product?
A)The reaction in a lipid environment
B)The reaction in an aqueous environment
C)The reaction in a gaseous environment
D)Cannot be determined from the information given
In polluted urban environments, airborne proteins can undergo nitration reactions that increase their allergenic potential toward the human respiratory system. For example, upon exposure to ozone (O3) and nitrogen dioxide (NO2) under atmospheric conditions, the amino acid tyrosine is known to undergo a two-step irreversible reaction leading to the formation of nitrotyrosine. The first step involves the formation of a reactive oxygen intermediate (ROI) tyrosyl radical through oxidation of the phenolic site of tyrosine. Nitrotyrosine is then formed in the second step through addition of NO2 by a radical-radical termination reaction. The elementary steps for this reaction are shown in Scheme 1.
 Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments.
Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments. Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K)
Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K) Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90.
Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90.Suppose a chemist decides to conduct and monitor the reaction for 10 min at 298.15 K in the three environments described in the passage. Which of the three reactions is most likely to form the greatest amount of product?
A)The reaction in a lipid environment
B)The reaction in an aqueous environment
C)The reaction in a gaseous environment
D)Cannot be determined from the information given

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
36
Passage
Umami is one of five basic tastes that humans recognize, and it is triggered by L-amino acids as well as some synthetic ingredients and organic acids. Three common umami tastant molecules are shown in Figure 1.
 Figure 1 Three common tastant molecules known to trigger the umami taste sensationUmami taste signals are triggered upon binding of tastant molecules to the G protein-coupled receptor (GPCR) heterodimer T1R1-T1R3, which ultimately causes the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers the release of Ca2+ cations and the subsequent gating of the taste-transduction channels (Figure 2).
Figure 1 Three common tastant molecules known to trigger the umami taste sensationUmami taste signals are triggered upon binding of tastant molecules to the G protein-coupled receptor (GPCR) heterodimer T1R1-T1R3, which ultimately causes the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers the release of Ca2+ cations and the subsequent gating of the taste-transduction channels (Figure 2).
 Figure 2 Signal pathway for transduction of umami taste sensationInosine monophosphate (IMP) has been suggested as enhancing (potentiating) the response of T1R1+T1R3 GPCRs to umami tastant molecules. Scientists tested this hypothesis by culturing receptors from human embryonic kidney (HEK) cells containing promiscuous G proteins. The HEK cells were then stimulated with umami tastants in the presence or absence of IMP. In each test, the cells were exposed to the same amounts of amino acid tastant and IMP. The number of cells that responded to each tastant was measured using a calcium indicator dye (FURA-2AM) in conjunction with fluorescence microscopy. By comparing the cellular response ratio of the stimulated to unstimulated HEK cells, the extent of potentiation can be assessed. The results are shown in Figure 3.
Figure 2 Signal pathway for transduction of umami taste sensationInosine monophosphate (IMP) has been suggested as enhancing (potentiating) the response of T1R1+T1R3 GPCRs to umami tastant molecules. Scientists tested this hypothesis by culturing receptors from human embryonic kidney (HEK) cells containing promiscuous G proteins. The HEK cells were then stimulated with umami tastants in the presence or absence of IMP. In each test, the cells were exposed to the same amounts of amino acid tastant and IMP. The number of cells that responded to each tastant was measured using a calcium indicator dye (FURA-2AM) in conjunction with fluorescence microscopy. By comparing the cellular response ratio of the stimulated to unstimulated HEK cells, the extent of potentiation can be assessed. The results are shown in Figure 3.
 Figure 3 Quantification of amino-acid responses for T1R1+T1R3 with and without IMP
Figure 3 Quantification of amino-acid responses for T1R1+T1R3 with and without IMP
Adapted from Nelson G, Chandrashekar J, Hoon MA, et al. An amino-acid taste receptor. Nature. 2002;416(6877):199-202.
Which of the following is an acceptable resonance structure for the umami tastant molecule L-histidine shown in Figure 1?
A)
B)
C)
D)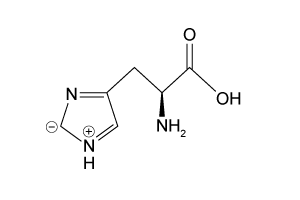
Umami is one of five basic tastes that humans recognize, and it is triggered by L-amino acids as well as some synthetic ingredients and organic acids. Three common umami tastant molecules are shown in Figure 1.
 Figure 1 Three common tastant molecules known to trigger the umami taste sensationUmami taste signals are triggered upon binding of tastant molecules to the G protein-coupled receptor (GPCR) heterodimer T1R1-T1R3, which ultimately causes the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers the release of Ca2+ cations and the subsequent gating of the taste-transduction channels (Figure 2).
Figure 1 Three common tastant molecules known to trigger the umami taste sensationUmami taste signals are triggered upon binding of tastant molecules to the G protein-coupled receptor (GPCR) heterodimer T1R1-T1R3, which ultimately causes the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers the release of Ca2+ cations and the subsequent gating of the taste-transduction channels (Figure 2). Figure 2 Signal pathway for transduction of umami taste sensationInosine monophosphate (IMP) has been suggested as enhancing (potentiating) the response of T1R1+T1R3 GPCRs to umami tastant molecules. Scientists tested this hypothesis by culturing receptors from human embryonic kidney (HEK) cells containing promiscuous G proteins. The HEK cells were then stimulated with umami tastants in the presence or absence of IMP. In each test, the cells were exposed to the same amounts of amino acid tastant and IMP. The number of cells that responded to each tastant was measured using a calcium indicator dye (FURA-2AM) in conjunction with fluorescence microscopy. By comparing the cellular response ratio of the stimulated to unstimulated HEK cells, the extent of potentiation can be assessed. The results are shown in Figure 3.
Figure 2 Signal pathway for transduction of umami taste sensationInosine monophosphate (IMP) has been suggested as enhancing (potentiating) the response of T1R1+T1R3 GPCRs to umami tastant molecules. Scientists tested this hypothesis by culturing receptors from human embryonic kidney (HEK) cells containing promiscuous G proteins. The HEK cells were then stimulated with umami tastants in the presence or absence of IMP. In each test, the cells were exposed to the same amounts of amino acid tastant and IMP. The number of cells that responded to each tastant was measured using a calcium indicator dye (FURA-2AM) in conjunction with fluorescence microscopy. By comparing the cellular response ratio of the stimulated to unstimulated HEK cells, the extent of potentiation can be assessed. The results are shown in Figure 3. Figure 3 Quantification of amino-acid responses for T1R1+T1R3 with and without IMP
Figure 3 Quantification of amino-acid responses for T1R1+T1R3 with and without IMPAdapted from Nelson G, Chandrashekar J, Hoon MA, et al. An amino-acid taste receptor. Nature. 2002;416(6877):199-202.
Which of the following is an acceptable resonance structure for the umami tastant molecule L-histidine shown in Figure 1?
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
37
Passage
In polluted urban environments, airborne proteins can undergo nitration reactions that increase their allergenic potential toward the human respiratory system. For example, upon exposure to ozone (O3) and nitrogen dioxide (NO2) under atmospheric conditions, the amino acid tyrosine is known to undergo a two-step irreversible reaction leading to the formation of nitrotyrosine. The first step involves the formation of a reactive oxygen intermediate (ROI) tyrosyl radical through oxidation of the phenolic site of tyrosine. Nitrotyrosine is then formed in the second step through addition of NO2 by a radical-radical termination reaction. The elementary steps for this reaction are shown in Scheme 1.
 Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments.
Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments.
 Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K)
Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K)
 Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90.
Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90.
Which of the following reaction coordinate diagrams best represents the two-step reaction involving tyrosine to nitrotyrosine in a lipid environment?
A)
B)
C)
D)
In polluted urban environments, airborne proteins can undergo nitration reactions that increase their allergenic potential toward the human respiratory system. For example, upon exposure to ozone (O3) and nitrogen dioxide (NO2) under atmospheric conditions, the amino acid tyrosine is known to undergo a two-step irreversible reaction leading to the formation of nitrotyrosine. The first step involves the formation of a reactive oxygen intermediate (ROI) tyrosyl radical through oxidation of the phenolic site of tyrosine. Nitrotyrosine is then formed in the second step through addition of NO2 by a radical-radical termination reaction. The elementary steps for this reaction are shown in Scheme 1.
 Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments.
Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments. Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K)
Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K) Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90.
Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90.Which of the following reaction coordinate diagrams best represents the two-step reaction involving tyrosine to nitrotyrosine in a lipid environment?
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
38
Passage
Depending on the active compound's specific method of action, sunscreens are broadly classified into two groups: organic and inorganic. Organic sunscreens typically contain aromatic compounds that absorb ultraviolet (UV) radiation. The absorbed energy from the UV radiation causes these compounds to enter electronically excited states which then relax back to the ground state and harmlessly re-emit the absorbed radiation as heat. Alternatively, inorganic sunscreens contain metallic nanoparticles that serve to reflect, scatter, and absorb incident radiation. The mechanism for UV protection of each group is shown in Figure 1.
 Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF). However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens
Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF). However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens
 In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested.
In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested.
 Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreens
Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreens
Adapted from Van der molen RG, Out-luiting C, Driller H, Claas FH, Koerten HK, Mommaas AM. Broad-spectrum sunscreens offer protection against urocanic acid photoisomerization by artificial ultraviolet radiation in human skin. J Invest Dermatol. 2000;115(3):421-6.
Based on the results from the experiment in Figure 2, which of the following conclusions can be drawn?
A)Acute exposure to UV radiation leads to greater immunosuppression than chronic exposure.
B)Without sunscreen, UV radiation causes one in three UCA molecules to become cis isomers.
C)T10 and B19 offer the best protection against acute and chronic UV exposure, respectively.
D)T5 offers the best overall protection from UV radiation.
Depending on the active compound's specific method of action, sunscreens are broadly classified into two groups: organic and inorganic. Organic sunscreens typically contain aromatic compounds that absorb ultraviolet (UV) radiation. The absorbed energy from the UV radiation causes these compounds to enter electronically excited states which then relax back to the ground state and harmlessly re-emit the absorbed radiation as heat. Alternatively, inorganic sunscreens contain metallic nanoparticles that serve to reflect, scatter, and absorb incident radiation. The mechanism for UV protection of each group is shown in Figure 1.
 Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF). However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens
Figure 1 Method of action for organic and inorganic sunscreensThe effectiveness of sunscreens is traditionally evaluated based on their ability to prevent reddening of the skin, as indicated by their sun protection factor (SPF). However, studies have shown that additional deleterious effects, such as the suppression of T cell-mediated immune responses, can occur at lower UV doses than erythema, indicating the need for new metrics to judge sunscreen efficacy. For this reason, researchers conducted a study to compare the protective ability of six sunscreens against photo-isomerization of the photoreceptor trans-urocanic acid (UCA) to its cis form, a process believed to be linked to UV-induced immunosuppression. Table 1 lists the sunscreens tested and their relevant characteristics.Table 1 Results from Tested Sunscreens In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested.
In vivo experiments were conducted on two groups of human volunteers. Participants in the acute exposure group received a single UV dose of 100 mJ/cm2 whereas participants in the chronic exposure group received the same dosage once daily over four consecutive days. Each sunscreen was applied to a different spot on the volunteers' back prior to irradiation. A portion of skin without sunscreen was also irradiated to serve as a control. After irradiation, the sunscreens were removed and the skin was wiped with filter papers. Filters were washed with potassium hydroxide, and the aqueous solution was analyzed for UCA using high-pressure liquid chromatography. Figure 2 shows the mean percentages of cis-UCA formed upon irradiation for each exposure type and sunscreen tested. Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreens
Figure 2 Percentage of UCA in cis configuration upon UV exposure with various sunscreensAdapted from Van der molen RG, Out-luiting C, Driller H, Claas FH, Koerten HK, Mommaas AM. Broad-spectrum sunscreens offer protection against urocanic acid photoisomerization by artificial ultraviolet radiation in human skin. J Invest Dermatol. 2000;115(3):421-6.
Based on the results from the experiment in Figure 2, which of the following conclusions can be drawn?
A)Acute exposure to UV radiation leads to greater immunosuppression than chronic exposure.
B)Without sunscreen, UV radiation causes one in three UCA molecules to become cis isomers.
C)T10 and B19 offer the best protection against acute and chronic UV exposure, respectively.
D)T5 offers the best overall protection from UV radiation.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
39
Passage
In polluted urban environments, airborne proteins can undergo nitration reactions that increase their allergenic potential toward the human respiratory system. For example, upon exposure to ozone (O3) and nitrogen dioxide (NO2) under atmospheric conditions, the amino acid tyrosine is known to undergo a two-step irreversible reaction leading to the formation of nitrotyrosine. The first step involves the formation of a reactive oxygen intermediate (ROI) tyrosyl radical through oxidation of the phenolic site of tyrosine. Nitrotyrosine is then formed in the second step through addition of NO2 by a radical-radical termination reaction. The elementary steps for this reaction are shown in Scheme 1.
 Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments.
Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments.
 Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K)
Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K)
 Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90.
Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90.
Given that entropy is negative (disorder decreases) in the reaction, which of the following statements is true regarding the formation of aqueous nitrotyrosine?
A)The formation of nitrotyrosine is less thermodynamically favorable than the formation of ROI at 298.15 K.
B)The overall reaction becomes more thermodynamically favorable at higher temperatures.
C)Neither the formation of ROI nor nitrotyrosine is thermodynamically favorable at 298.15 K.
D)The overall reaction becomes less thermodynamically favorable at higher temperatures.
In polluted urban environments, airborne proteins can undergo nitration reactions that increase their allergenic potential toward the human respiratory system. For example, upon exposure to ozone (O3) and nitrogen dioxide (NO2) under atmospheric conditions, the amino acid tyrosine is known to undergo a two-step irreversible reaction leading to the formation of nitrotyrosine. The first step involves the formation of a reactive oxygen intermediate (ROI) tyrosyl radical through oxidation of the phenolic site of tyrosine. Nitrotyrosine is then formed in the second step through addition of NO2 by a radical-radical termination reaction. The elementary steps for this reaction are shown in Scheme 1.
 Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments.
Scheme 1 Elementary steps of nitration of tyrosineAirborne protein particles can exist in a variety of environments including the aqueous phase, gaseous phase, or as conglomerates with other biomolecules such as lipids. Because the thermodynamic properties and kinetics of the nitration reaction vary based on the chemical environment in which it occurs, recent studies have focused on understanding the reaction under different conditions.Density functional theory is a powerful computational quantum-mechanical modeling method that can determine the properties of many-electron systems by producing approximate solutions to the Schrödinger equation. Researchers used density functional theory to model the geometries and potential energy surfaces of the reactants, intermediates, and products of the nitration reaction in gaseous, aqueous, and lipid-rich environments. From this modeling, the thermodynamic and kinetic properties were calculated. Figure 1 shows the calculated activation energies Ea in kJ/mol, and Table 1 lists the associated thermochemical properties for both steps of the reaction in all three environments. Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K)
Figure 1 Calculated activation energies for each step of the studied reactions in three environmentsTable 1 Calculated Thermochemical Properties of the Studied Reactions (298.15 K) Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90.
Adapted from Sandhiya L, Kolandaivel P, Senthilkumar K. Oxidation and nitration of tyrosine by ozone and nitrogen dioxide: reaction mechanisms and biological and atmospheric implications. J Phys Chem B. 2014;118(13):3479-90.Given that entropy is negative (disorder decreases) in the reaction, which of the following statements is true regarding the formation of aqueous nitrotyrosine?
A)The formation of nitrotyrosine is less thermodynamically favorable than the formation of ROI at 298.15 K.
B)The overall reaction becomes more thermodynamically favorable at higher temperatures.
C)Neither the formation of ROI nor nitrotyrosine is thermodynamically favorable at 298.15 K.
D)The overall reaction becomes less thermodynamically favorable at higher temperatures.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
40
Passage
Catabolism is an oxidative process in which electrons are transferred from lipids, proteins, and carbohydrates to generate high-energy electron carriers from nicotinamide adenine dinucleotide (NADH/NAD+) and flavin adenine dinucleotide (FADH2/FAD). Both are used in the electron transport chain (ETC) as electron providers in a series of reactions. The transfer of electrons provides energy that drives oxidative phosphorylation and the production of ATP. Reduction of NAD+ proceeds according to the following half-reaction:
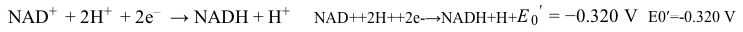 Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.
Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.
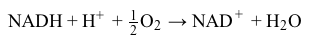 NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf), that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.
NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf), that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.
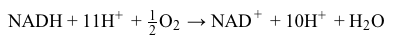 NADH+11H++12O2→NAD++10H++H2OReaction 3
NADH+11H++12O2→NAD++10H++H2OReaction 3
Adapted from Garrett R, Grisham CM, Sabat M. Biochemistry. Cengage Learning; 2011.
Barbiturates are known to inhibit complex I but do not affect complexes II-IV. Based on this fact, how will barbiturates affect the proton motive force?
A)The proton motive force increases.
B)The proton motive force decreases.
C)The proton motive force is unchanged.
D)The proton motive force becomes zero.
Catabolism is an oxidative process in which electrons are transferred from lipids, proteins, and carbohydrates to generate high-energy electron carriers from nicotinamide adenine dinucleotide (NADH/NAD+) and flavin adenine dinucleotide (FADH2/FAD). Both are used in the electron transport chain (ETC) as electron providers in a series of reactions. The transfer of electrons provides energy that drives oxidative phosphorylation and the production of ATP. Reduction of NAD+ proceeds according to the following half-reaction:
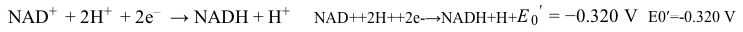 Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.
Reaction 1Reactions in the ETC occur in four protein complexes (I-IV) located in the inner mitochondrial membrane. Here high-energy electrons are shuttled from complexes I, II, and III to complex IV, where O2 serves as the final electron acceptor. The net result of NADH oxidation is described by Reaction 2.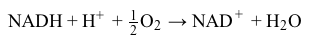 NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf), that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.
NADH+H++12O2→NAD++H2OReaction 2At complexes I, III, and IV, hydrogen ions move from the mitochondrial matrix to the intermembrane space and create the electrochemical gradient, or proton motive force (pmf), that drives ATP synthesis. Each NADH molecule drives the net pumping of 10 protons across the membrane, as per Reaction 3.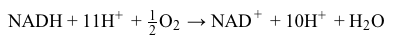 NADH+11H++12O2→NAD++10H++H2OReaction 3
NADH+11H++12O2→NAD++10H++H2OReaction 3Adapted from Garrett R, Grisham CM, Sabat M. Biochemistry. Cengage Learning; 2011.
Barbiturates are known to inhibit complex I but do not affect complexes II-IV. Based on this fact, how will barbiturates affect the proton motive force?
A)The proton motive force increases.
B)The proton motive force decreases.
C)The proton motive force is unchanged.
D)The proton motive force becomes zero.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
41
Passage
Water is unique in that all three of its phases exist over a small range of pressures and temperatures, as shown in Figure 1.
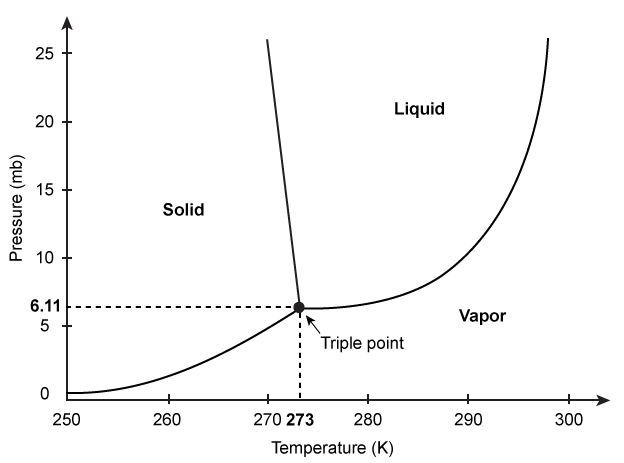 Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars
Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars
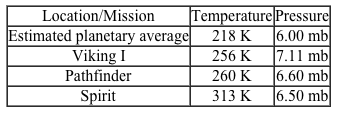 Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL), are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure).The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.
Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL), are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure).The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.
Which of the following molecules is unable to form hydrogen bonds with water?
A)C2H4Br2
B)C3H9N
C)C9H18O2
D)C2F4
Water is unique in that all three of its phases exist over a small range of pressures and temperatures, as shown in Figure 1.
 Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars
Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL), are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure).The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.
Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL), are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure).The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.Which of the following molecules is unable to form hydrogen bonds with water?
A)C2H4Br2
B)C3H9N
C)C9H18O2
D)C2F4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
42
Passage
Heme is found in several proteins, including hemoglobin in blood and myoglobin in muscle. It has a four-coordinate iron-bound porphyrin structure where ferrous iron (Fe2+) is bound to four nitrogen atoms through coordinate covalent bonds. The most common heme found in the body is heme b in hemoglobin (Figure 1).
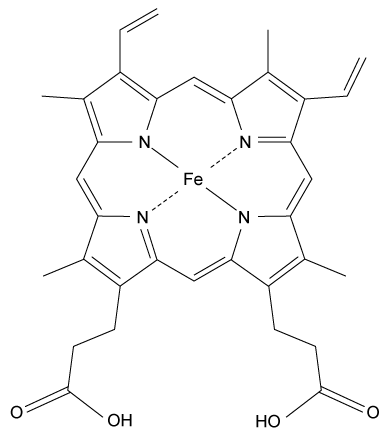 Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP). ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm.
Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP). ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm.
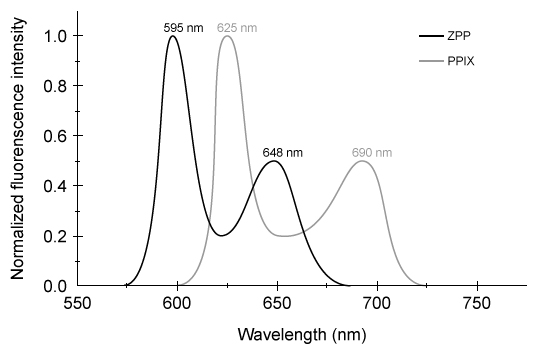 Figure 2 ZPP and PPIX fluorescence emission spectra
Figure 2 ZPP and PPIX fluorescence emission spectra
Adapted from Hennig G, Gruber C, Vogeser M, et al. Dual-wavelength excitation for fluorescence-based quantification of zinc protoporphyrin IX and protoporphyrin IX in whole blood. J Biophotonics. 2014;7(7):514-24.
Which of the following does NOT describe the iron-porphyrin complex formed between Fe2+ and the four nitrogen atoms in Figure 1?
A)Each nitrogen atom provides both bonding electrons in each coordinate bond.
B)The net charge of -the iron-porphyrin complex is +2.
C)Nitrogen's electrons interact with iron's d orbitals.
D)Iron's electron configuration determines coordinate bond strength.
Heme is found in several proteins, including hemoglobin in blood and myoglobin in muscle. It has a four-coordinate iron-bound porphyrin structure where ferrous iron (Fe2+) is bound to four nitrogen atoms through coordinate covalent bonds. The most common heme found in the body is heme b in hemoglobin (Figure 1).
 Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP). ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm.
Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP). ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm. Figure 2 ZPP and PPIX fluorescence emission spectra
Figure 2 ZPP and PPIX fluorescence emission spectraAdapted from Hennig G, Gruber C, Vogeser M, et al. Dual-wavelength excitation for fluorescence-based quantification of zinc protoporphyrin IX and protoporphyrin IX in whole blood. J Biophotonics. 2014;7(7):514-24.
Which of the following does NOT describe the iron-porphyrin complex formed between Fe2+ and the four nitrogen atoms in Figure 1?
A)Each nitrogen atom provides both bonding electrons in each coordinate bond.
B)The net charge of -the iron-porphyrin complex is +2.
C)Nitrogen's electrons interact with iron's d orbitals.
D)Iron's electron configuration determines coordinate bond strength.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
43
Passage
Water is unique in that all three of its phases exist over a small range of pressures and temperatures, as shown in Figure 1.
 Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars
Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars
 Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL), are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure).The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.
Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL), are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure).The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.
Which of the following conclusions can be made based on Figure 1 and the data in Table 1?
A)Liquid water initially at 280 K and 15.0 mb will remain liquid at the Viking I location.
B)A block of ice at the Pathfinder location will sublimate when heated to 274 K.
C)Water vapor released into Mars' average conditions will condense and then freeze.
D)Liquid water initially at 274 K and 6.50 mb will boil at the Spirit location.
Water is unique in that all three of its phases exist over a small range of pressures and temperatures, as shown in Figure 1.
 Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars
Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL), are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure).The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.
Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL), are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure).The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.Which of the following conclusions can be made based on Figure 1 and the data in Table 1?
A)Liquid water initially at 280 K and 15.0 mb will remain liquid at the Viking I location.
B)A block of ice at the Pathfinder location will sublimate when heated to 274 K.
C)Water vapor released into Mars' average conditions will condense and then freeze.
D)Liquid water initially at 274 K and 6.50 mb will boil at the Spirit location.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
44
Passage
Heme is found in several proteins, including hemoglobin in blood and myoglobin in muscle. It has a four-coordinate iron-bound porphyrin structure where ferrous iron (Fe2+) is bound to four nitrogen atoms through coordinate covalent bonds. The most common heme found in the body is heme b in hemoglobin (Figure 1).
 Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP). ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm.
Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP). ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm.
 Figure 2 ZPP and PPIX fluorescence emission spectra
Figure 2 ZPP and PPIX fluorescence emission spectra
Adapted from Hennig G, Gruber C, Vogeser M, et al. Dual-wavelength excitation for fluorescence-based quantification of zinc protoporphyrin IX and protoporphyrin IX in whole blood. J Biophotonics. 2014;7(7):514-24.
Which of the following depicts iron's coordination geometry when oxygen is bound to heme b in hemoglobin?
A)
B)
C)
D)
Heme is found in several proteins, including hemoglobin in blood and myoglobin in muscle. It has a four-coordinate iron-bound porphyrin structure where ferrous iron (Fe2+) is bound to four nitrogen atoms through coordinate covalent bonds. The most common heme found in the body is heme b in hemoglobin (Figure 1).
 Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP). ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm.
Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP). ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm. Figure 2 ZPP and PPIX fluorescence emission spectra
Figure 2 ZPP and PPIX fluorescence emission spectraAdapted from Hennig G, Gruber C, Vogeser M, et al. Dual-wavelength excitation for fluorescence-based quantification of zinc protoporphyrin IX and protoporphyrin IX in whole blood. J Biophotonics. 2014;7(7):514-24.
Which of the following depicts iron's coordination geometry when oxygen is bound to heme b in hemoglobin?
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
45
Passage
Bone growth and remodeling during fracture healing are often slow and have potentially poor outcomes, including malunion or nonunion at the fracture site. Magnesium bone implants can aid in bone formation and safely biodegrade in the body according to the following reaction:Mg(s) + 2H2O(l) → Mg(OH)2(s) + H2(g)Reaction 1 Corrosion of magnesium metal in aqueous mediumHowever, magnesium metal tends to degrade too quickly and forms hydrogen gas pockets in the body. Corrosion is slowed as the metal naturally forms a protective layer of magnesium hydroxide in a process called passivation. Magnesium hydroxide is less soluble than magnesium metal, and its dissolution is described by the endothermic reaction:
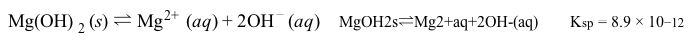 Reaction 2 Solubility equilibrium of Mg(OH)2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C
Reaction 2 Solubility equilibrium of Mg(OH)2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C
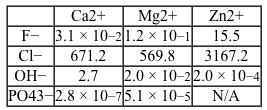 Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35.
Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35.
Throughout the experiment, researchers monitored the amount of magnesium that dissolved at various time points. Which of the following is NOT a valid test for the rate of magnesium corrosion?
A)Periodically measure the volume of H2 gas evolved from the reaction.
B)Periodically measure the mass of the magnesium disks.
C)Periodically measure Mg2+ concentration in the SBF using elemental analysis techniques.
D)Periodically measure the amount of OH− produced by titrating the beaker contents with HCl.
Bone growth and remodeling during fracture healing are often slow and have potentially poor outcomes, including malunion or nonunion at the fracture site. Magnesium bone implants can aid in bone formation and safely biodegrade in the body according to the following reaction:Mg(s) + 2H2O(l) → Mg(OH)2(s) + H2(g)Reaction 1 Corrosion of magnesium metal in aqueous mediumHowever, magnesium metal tends to degrade too quickly and forms hydrogen gas pockets in the body. Corrosion is slowed as the metal naturally forms a protective layer of magnesium hydroxide in a process called passivation. Magnesium hydroxide is less soluble than magnesium metal, and its dissolution is described by the endothermic reaction:
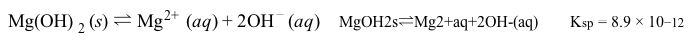 Reaction 2 Solubility equilibrium of Mg(OH)2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C
Reaction 2 Solubility equilibrium of Mg(OH)2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35.
Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35.Throughout the experiment, researchers monitored the amount of magnesium that dissolved at various time points. Which of the following is NOT a valid test for the rate of magnesium corrosion?
A)Periodically measure the volume of H2 gas evolved from the reaction.
B)Periodically measure the mass of the magnesium disks.
C)Periodically measure Mg2+ concentration in the SBF using elemental analysis techniques.
D)Periodically measure the amount of OH− produced by titrating the beaker contents with HCl.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
46
Passage
Water is unique in that all three of its phases exist over a small range of pressures and temperatures, as shown in Figure 1.
 Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars
Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars
 Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL), are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure).The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.
Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL), are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure).The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.
Which of the following does not explain water's ability to act as a solvent?
A)Water has the geometry of a bent molecule.
B)Hydrogen is less electronegative than oxygen.
C)Water is a relatively small molecule.
D)Water has a relatively high surface tension.
Water is unique in that all three of its phases exist over a small range of pressures and temperatures, as shown in Figure 1.
 Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars
Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL), are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure).The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.
Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL), are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure).The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.Which of the following does not explain water's ability to act as a solvent?
A)Water has the geometry of a bent molecule.
B)Hydrogen is less electronegative than oxygen.
C)Water is a relatively small molecule.
D)Water has a relatively high surface tension.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
47
Passage
Household cleaners commonly contain either ammonia (NH3) or bleach (NaOCl) as the principal active ingredient. Mixing ammonia-based and bleach-based cleaners can be hazardous, as the resulting reactions can form several toxic products. One of these reactions forms the irritant dichloramine (NHCl2) as follows:
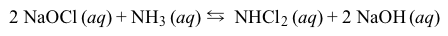 2 NaOClaq+NH3aq⇆ NHCl2aq+2 NaOHaqReaction 1The overall reaction has a standard enthalpy change of −55 kJ/mol and a standard entropy change of −150 J/mol·K. Partial thermodynamic data for formation of Reaction 1 reagents from their elements are shown in Table 1.Table 1 Partial Data for Enthalpies, Entropies, and Free Energies of Formation of Several Compounds at 25 °C
2 NaOClaq+NH3aq⇆ NHCl2aq+2 NaOHaqReaction 1The overall reaction has a standard enthalpy change of −55 kJ/mol and a standard entropy change of −150 J/mol·K. Partial thermodynamic data for formation of Reaction 1 reagents from their elements are shown in Table 1.Table 1 Partial Data for Enthalpies, Entropies, and Free Energies of Formation of Several Compounds at 25 °C
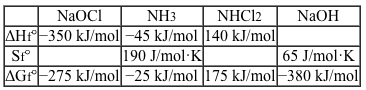 After it is formed, dichloramine readily enters the gas phase, where it may be inhaled. In the lungs, dichloramine reacts with water to produce hydrochloric acid and ammonia, as well as reactive oxygen species. In small doses, these products cause mild irritation, but inhalation of large amounts of chloramines can lead to pneumonitis (inflammation of the lungs) and may require medical intervention.
After it is formed, dichloramine readily enters the gas phase, where it may be inhaled. In the lungs, dichloramine reacts with water to produce hydrochloric acid and ammonia, as well as reactive oxygen species. In small doses, these products cause mild irritation, but inhalation of large amounts of chloramines can lead to pneumonitis (inflammation of the lungs) and may require medical intervention.
Adapted from Trogolo D, Arey JS. Benchmark thermochemistry of chloramines, bromamines, and bromochloramines: halogen oxidants stabilized by electron correlation. Phys Chem Chem Phys. 2015;17(5):3584-98.
Which of the following graphs depicts the expected change in temperature of the reaction mixture over the course of the reaction described in the passage?
A)
B)
C)
D)
Household cleaners commonly contain either ammonia (NH3) or bleach (NaOCl) as the principal active ingredient. Mixing ammonia-based and bleach-based cleaners can be hazardous, as the resulting reactions can form several toxic products. One of these reactions forms the irritant dichloramine (NHCl2) as follows:
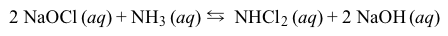 2 NaOClaq+NH3aq⇆ NHCl2aq+2 NaOHaqReaction 1The overall reaction has a standard enthalpy change of −55 kJ/mol and a standard entropy change of −150 J/mol·K. Partial thermodynamic data for formation of Reaction 1 reagents from their elements are shown in Table 1.Table 1 Partial Data for Enthalpies, Entropies, and Free Energies of Formation of Several Compounds at 25 °C
2 NaOClaq+NH3aq⇆ NHCl2aq+2 NaOHaqReaction 1The overall reaction has a standard enthalpy change of −55 kJ/mol and a standard entropy change of −150 J/mol·K. Partial thermodynamic data for formation of Reaction 1 reagents from their elements are shown in Table 1.Table 1 Partial Data for Enthalpies, Entropies, and Free Energies of Formation of Several Compounds at 25 °C After it is formed, dichloramine readily enters the gas phase, where it may be inhaled. In the lungs, dichloramine reacts with water to produce hydrochloric acid and ammonia, as well as reactive oxygen species. In small doses, these products cause mild irritation, but inhalation of large amounts of chloramines can lead to pneumonitis (inflammation of the lungs) and may require medical intervention.
After it is formed, dichloramine readily enters the gas phase, where it may be inhaled. In the lungs, dichloramine reacts with water to produce hydrochloric acid and ammonia, as well as reactive oxygen species. In small doses, these products cause mild irritation, but inhalation of large amounts of chloramines can lead to pneumonitis (inflammation of the lungs) and may require medical intervention.Adapted from Trogolo D, Arey JS. Benchmark thermochemistry of chloramines, bromamines, and bromochloramines: halogen oxidants stabilized by electron correlation. Phys Chem Chem Phys. 2015;17(5):3584-98.
Which of the following graphs depicts the expected change in temperature of the reaction mixture over the course of the reaction described in the passage?
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
48
Passage
Bone growth and remodeling during fracture healing are often slow and have potentially poor outcomes, including malunion or nonunion at the fracture site. Magnesium bone implants can aid in bone formation and safely biodegrade in the body according to the following reaction:Mg(s) + 2H2O(l) → Mg(OH)2(s) + H2(g)Reaction 1 Corrosion of magnesium metal in aqueous mediumHowever, magnesium metal tends to degrade too quickly and forms hydrogen gas pockets in the body. Corrosion is slowed as the metal naturally forms a protective layer of magnesium hydroxide in a process called passivation. Magnesium hydroxide is less soluble than magnesium metal, and its dissolution is described by the endothermic reaction:
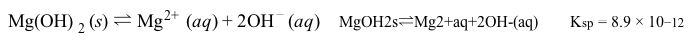 Reaction 2 Solubility equilibrium of Mg(OH)2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C
Reaction 2 Solubility equilibrium of Mg(OH)2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C
 Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35.
Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35.
Magnesium corrodes quickly in the body because Mg2+ combines with anions other than OH− to form soluble salts. Based on the solubility data given in Table 1, which of the following salts, if present, will cause the magnesium implant to corrode most quickly?
A)Ca3(PO4)2
B)Ca(OH)2
C)NaCl
D)NaF
Bone growth and remodeling during fracture healing are often slow and have potentially poor outcomes, including malunion or nonunion at the fracture site. Magnesium bone implants can aid in bone formation and safely biodegrade in the body according to the following reaction:Mg(s) + 2H2O(l) → Mg(OH)2(s) + H2(g)Reaction 1 Corrosion of magnesium metal in aqueous mediumHowever, magnesium metal tends to degrade too quickly and forms hydrogen gas pockets in the body. Corrosion is slowed as the metal naturally forms a protective layer of magnesium hydroxide in a process called passivation. Magnesium hydroxide is less soluble than magnesium metal, and its dissolution is described by the endothermic reaction:
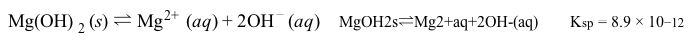 Reaction 2 Solubility equilibrium of Mg(OH)2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C
Reaction 2 Solubility equilibrium of Mg(OH)2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35.
Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35.Magnesium corrodes quickly in the body because Mg2+ combines with anions other than OH− to form soluble salts. Based on the solubility data given in Table 1, which of the following salts, if present, will cause the magnesium implant to corrode most quickly?
A)Ca3(PO4)2
B)Ca(OH)2
C)NaCl
D)NaF

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
49
Passage
Heme is found in several proteins, including hemoglobin in blood and myoglobin in muscle. It has a four-coordinate iron-bound porphyrin structure where ferrous iron (Fe2+) is bound to four nitrogen atoms through coordinate covalent bonds. The most common heme found in the body is heme b in hemoglobin (Figure 1).
 Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP). ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm.
Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP). ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm.
 Figure 2 ZPP and PPIX fluorescence emission spectra
Figure 2 ZPP and PPIX fluorescence emission spectra
Adapted from Hennig G, Gruber C, Vogeser M, et al. Dual-wavelength excitation for fluorescence-based quantification of zinc protoporphyrin IX and protoporphyrin IX in whole blood. J Biophotonics. 2014;7(7):514-24.
For the electron configuration of iron, Fe(0), do electrons fill the 4s orbitals before the 3d orbitals are filled?
A)Yes, because electrons fill the s and p orbitals before they fill the d orbitals, even if they are in different energy levels.
B)No, because 3d orbitals are lower in energy than 4s orbitals.
C)Yes, because according to the Aufbau principle, electrons fill lower energy levels first.
D)No, because orbitals are filled based on the order of the principal quantum number, and 3d comes before 4s.
Heme is found in several proteins, including hemoglobin in blood and myoglobin in muscle. It has a four-coordinate iron-bound porphyrin structure where ferrous iron (Fe2+) is bound to four nitrogen atoms through coordinate covalent bonds. The most common heme found in the body is heme b in hemoglobin (Figure 1).
 Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP). ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm.
Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP). ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm. Figure 2 ZPP and PPIX fluorescence emission spectra
Figure 2 ZPP and PPIX fluorescence emission spectraAdapted from Hennig G, Gruber C, Vogeser M, et al. Dual-wavelength excitation for fluorescence-based quantification of zinc protoporphyrin IX and protoporphyrin IX in whole blood. J Biophotonics. 2014;7(7):514-24.
For the electron configuration of iron, Fe(0), do electrons fill the 4s orbitals before the 3d orbitals are filled?
A)Yes, because electrons fill the s and p orbitals before they fill the d orbitals, even if they are in different energy levels.
B)No, because 3d orbitals are lower in energy than 4s orbitals.
C)Yes, because according to the Aufbau principle, electrons fill lower energy levels first.
D)No, because orbitals are filled based on the order of the principal quantum number, and 3d comes before 4s.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
50
Passage
Bone growth and remodeling during fracture healing are often slow and have potentially poor outcomes, including malunion or nonunion at the fracture site. Magnesium bone implants can aid in bone formation and safely biodegrade in the body according to the following reaction:Mg(s) + 2H2O(l) → Mg(OH)2(s) + H2(g)Reaction 1 Corrosion of magnesium metal in aqueous mediumHowever, magnesium metal tends to degrade too quickly and forms hydrogen gas pockets in the body. Corrosion is slowed as the metal naturally forms a protective layer of magnesium hydroxide in a process called passivation. Magnesium hydroxide is less soluble than magnesium metal, and its dissolution is described by the endothermic reaction:
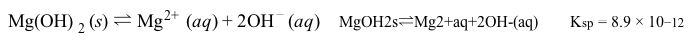 Reaction 2 Solubility equilibrium of Mg(OH)2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C
Reaction 2 Solubility equilibrium of Mg(OH)2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C
 Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35.
Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35.
The experiment was originally performed at physiological pH (7.4). If the pH were raised to 12, how would the corrosion rate of the magnesium metal change?
A)The magnesium disks will corrode more quickly.
B)The magnesium disks will corrode more slowly.
C)The corrosion rate will not change.
D)The magnesium disks will not corrode.
Bone growth and remodeling during fracture healing are often slow and have potentially poor outcomes, including malunion or nonunion at the fracture site. Magnesium bone implants can aid in bone formation and safely biodegrade in the body according to the following reaction:Mg(s) + 2H2O(l) → Mg(OH)2(s) + H2(g)Reaction 1 Corrosion of magnesium metal in aqueous mediumHowever, magnesium metal tends to degrade too quickly and forms hydrogen gas pockets in the body. Corrosion is slowed as the metal naturally forms a protective layer of magnesium hydroxide in a process called passivation. Magnesium hydroxide is less soluble than magnesium metal, and its dissolution is described by the endothermic reaction:
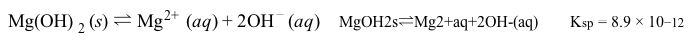 Reaction 2 Solubility equilibrium of Mg(OH)2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C
Reaction 2 Solubility equilibrium of Mg(OH)2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35.
Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35.The experiment was originally performed at physiological pH (7.4). If the pH were raised to 12, how would the corrosion rate of the magnesium metal change?
A)The magnesium disks will corrode more quickly.
B)The magnesium disks will corrode more slowly.
C)The corrosion rate will not change.
D)The magnesium disks will not corrode.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
51
Passage
Bone growth and remodeling during fracture healing are often slow and have potentially poor outcomes, including malunion or nonunion at the fracture site. Magnesium bone implants can aid in bone formation and safely biodegrade in the body according to the following reaction:Mg(s) + 2H2O(l) → Mg(OH)2(s) + H2(g)Reaction 1 Corrosion of magnesium metal in aqueous mediumHowever, magnesium metal tends to degrade too quickly and forms hydrogen gas pockets in the body. Corrosion is slowed as the metal naturally forms a protective layer of magnesium hydroxide in a process called passivation. Magnesium hydroxide is less soluble than magnesium metal, and its dissolution is described by the endothermic reaction:
![<strong>Passage Bone growth and remodeling during fracture healing are often slow and have potentially poor outcomes, including malunion or nonunion at the fracture site. Magnesium bone implants can aid in bone formation and safely biodegrade in the body according to the following reaction:Mg(s) + 2H<sub>2</sub>O(l) → Mg(OH)<sub>2</sub>(s) + H<sub>2</sub>(g)<strong>Reaction 1</strong> Corrosion of magnesium metal in aqueous mediumHowever, magnesium metal tends to degrade too quickly and forms hydrogen gas pockets in the body. Corrosion is slowed as the metal naturally forms a protective layer of magnesium hydroxide in a process called passivation. Magnesium hydroxide is less soluble than magnesium metal, and its dissolution is described by the endothermic reaction: <strong>Reaction 2</strong> Solubility equilibrium of Mg(OH)<sub>2</sub> in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.<strong>Table 1</strong> Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35. Magnesium hydroxide begins to precipitate onto the magnesium metal surface when which of the following expressions is true?</strong> A)[Mg<sup>2+</sup>][OH<sup>−</sup>]<sup>2</sup> > 8.9 × 10<sup>−12</sup> B)[OH<sup>−</sup>] < 1 × 10<sup>−7</sup> C)[Mg<sup>2+</sup>] = 8.9 × 10<sup>−12</sup> D)[OH<sup>−</sup>] = [Mg<sup>2+</sup>]](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_11a7_b19a_a3bf_bb71127efd28_MD0008_00.jpg) Reaction 2 Solubility equilibrium of Mg(OH)2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C
Reaction 2 Solubility equilibrium of Mg(OH)2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C
![<strong>Passage Bone growth and remodeling during fracture healing are often slow and have potentially poor outcomes, including malunion or nonunion at the fracture site. Magnesium bone implants can aid in bone formation and safely biodegrade in the body according to the following reaction:Mg(s) + 2H<sub>2</sub>O(l) → Mg(OH)<sub>2</sub>(s) + H<sub>2</sub>(g)<strong>Reaction 1</strong> Corrosion of magnesium metal in aqueous mediumHowever, magnesium metal tends to degrade too quickly and forms hydrogen gas pockets in the body. Corrosion is slowed as the metal naturally forms a protective layer of magnesium hydroxide in a process called passivation. Magnesium hydroxide is less soluble than magnesium metal, and its dissolution is described by the endothermic reaction: <strong>Reaction 2</strong> Solubility equilibrium of Mg(OH)<sub>2</sub> in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.<strong>Table 1</strong> Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35. Magnesium hydroxide begins to precipitate onto the magnesium metal surface when which of the following expressions is true?</strong> A)[Mg<sup>2+</sup>][OH<sup>−</sup>]<sup>2</sup> > 8.9 × 10<sup>−12</sup> B)[OH<sup>−</sup>] < 1 × 10<sup>−7</sup> C)[Mg<sup>2+</sup>] = 8.9 × 10<sup>−12</sup> D)[OH<sup>−</sup>] = [Mg<sup>2+</sup>]](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_11a7_b19b_a3bf_7f725503e77a_MD0008_00.jpg) Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35.
Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35.
Magnesium hydroxide begins to precipitate onto the magnesium metal surface when which of the following expressions is true?
A)[Mg2+][OH−]2 > 8.9 × 10−12
B)[OH−] < 1 × 10−7
C)[Mg2+] = 8.9 × 10−12
D)[OH−] = [Mg2+]
Bone growth and remodeling during fracture healing are often slow and have potentially poor outcomes, including malunion or nonunion at the fracture site. Magnesium bone implants can aid in bone formation and safely biodegrade in the body according to the following reaction:Mg(s) + 2H2O(l) → Mg(OH)2(s) + H2(g)Reaction 1 Corrosion of magnesium metal in aqueous mediumHowever, magnesium metal tends to degrade too quickly and forms hydrogen gas pockets in the body. Corrosion is slowed as the metal naturally forms a protective layer of magnesium hydroxide in a process called passivation. Magnesium hydroxide is less soluble than magnesium metal, and its dissolution is described by the endothermic reaction:
![<strong>Passage Bone growth and remodeling during fracture healing are often slow and have potentially poor outcomes, including malunion or nonunion at the fracture site. Magnesium bone implants can aid in bone formation and safely biodegrade in the body according to the following reaction:Mg(s) + 2H<sub>2</sub>O(l) → Mg(OH)<sub>2</sub>(s) + H<sub>2</sub>(g)<strong>Reaction 1</strong> Corrosion of magnesium metal in aqueous mediumHowever, magnesium metal tends to degrade too quickly and forms hydrogen gas pockets in the body. Corrosion is slowed as the metal naturally forms a protective layer of magnesium hydroxide in a process called passivation. Magnesium hydroxide is less soluble than magnesium metal, and its dissolution is described by the endothermic reaction: <strong>Reaction 2</strong> Solubility equilibrium of Mg(OH)<sub>2</sub> in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.<strong>Table 1</strong> Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35. Magnesium hydroxide begins to precipitate onto the magnesium metal surface when which of the following expressions is true?</strong> A)[Mg<sup>2+</sup>][OH<sup>−</sup>]<sup>2</sup> > 8.9 × 10<sup>−12</sup> B)[OH<sup>−</sup>] < 1 × 10<sup>−7</sup> C)[Mg<sup>2+</sup>] = 8.9 × 10<sup>−12</sup> D)[OH<sup>−</sup>] = [Mg<sup>2+</sup>]](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_11a7_b19a_a3bf_bb71127efd28_MD0008_00.jpg) Reaction 2 Solubility equilibrium of Mg(OH)2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C
Reaction 2 Solubility equilibrium of Mg(OH)2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C![<strong>Passage Bone growth and remodeling during fracture healing are often slow and have potentially poor outcomes, including malunion or nonunion at the fracture site. Magnesium bone implants can aid in bone formation and safely biodegrade in the body according to the following reaction:Mg(s) + 2H<sub>2</sub>O(l) → Mg(OH)<sub>2</sub>(s) + H<sub>2</sub>(g)<strong>Reaction 1</strong> Corrosion of magnesium metal in aqueous mediumHowever, magnesium metal tends to degrade too quickly and forms hydrogen gas pockets in the body. Corrosion is slowed as the metal naturally forms a protective layer of magnesium hydroxide in a process called passivation. Magnesium hydroxide is less soluble than magnesium metal, and its dissolution is described by the endothermic reaction: <strong>Reaction 2</strong> Solubility equilibrium of Mg(OH)<sub>2</sub> in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.<strong>Table 1</strong> Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35. Magnesium hydroxide begins to precipitate onto the magnesium metal surface when which of the following expressions is true?</strong> A)[Mg<sup>2+</sup>][OH<sup>−</sup>]<sup>2</sup> > 8.9 × 10<sup>−12</sup> B)[OH<sup>−</sup>] < 1 × 10<sup>−7</sup> C)[Mg<sup>2+</sup>] = 8.9 × 10<sup>−12</sup> D)[OH<sup>−</sup>] = [Mg<sup>2+</sup>]](https://d2lvgg3v3hfg70.cloudfront.net/MD0008/11ecba2d_11a7_b19b_a3bf_7f725503e77a_MD0008_00.jpg) Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35.
Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35.Magnesium hydroxide begins to precipitate onto the magnesium metal surface when which of the following expressions is true?
A)[Mg2+][OH−]2 > 8.9 × 10−12
B)[OH−] < 1 × 10−7
C)[Mg2+] = 8.9 × 10−12
D)[OH−] = [Mg2+]

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
52
Passage
Heme is found in several proteins, including hemoglobin in blood and myoglobin in muscle. It has a four-coordinate iron-bound porphyrin structure where ferrous iron (Fe2+) is bound to four nitrogen atoms through coordinate covalent bonds. The most common heme found in the body is heme b in hemoglobin (Figure 1).
 Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP). ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm.
Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP). ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm.
 Figure 2 ZPP and PPIX fluorescence emission spectra
Figure 2 ZPP and PPIX fluorescence emission spectra
Adapted from Hennig G, Gruber C, Vogeser M, et al. Dual-wavelength excitation for fluorescence-based quantification of zinc protoporphyrin IX and protoporphyrin IX in whole blood. J Biophotonics. 2014;7(7):514-24.
Comparing the peak excitation wavelengths stated in the passage and the highest-intensity fluorescence peaks in Figure 2, which of the following is true about ZPP detection in blood samples?
A)ZPP and PPIX have peak photon emissions that are equal in energy.
B)ZPP requires less energy to excite its electrons from the ground state than does PPIX.
C)The photons used to excite ZPP and PPIX were of equal energy, but the photons absorbed by each molecule were of different energy.
D)The photons absorbed by ZPP and PPIX were of equal energy, but the resulting electronic transitions in the molecules were of different energy.
Heme is found in several proteins, including hemoglobin in blood and myoglobin in muscle. It has a four-coordinate iron-bound porphyrin structure where ferrous iron (Fe2+) is bound to four nitrogen atoms through coordinate covalent bonds. The most common heme found in the body is heme b in hemoglobin (Figure 1).
 Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP). ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm.
Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP). ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm. Figure 2 ZPP and PPIX fluorescence emission spectra
Figure 2 ZPP and PPIX fluorescence emission spectraAdapted from Hennig G, Gruber C, Vogeser M, et al. Dual-wavelength excitation for fluorescence-based quantification of zinc protoporphyrin IX and protoporphyrin IX in whole blood. J Biophotonics. 2014;7(7):514-24.
Comparing the peak excitation wavelengths stated in the passage and the highest-intensity fluorescence peaks in Figure 2, which of the following is true about ZPP detection in blood samples?
A)ZPP and PPIX have peak photon emissions that are equal in energy.
B)ZPP requires less energy to excite its electrons from the ground state than does PPIX.
C)The photons used to excite ZPP and PPIX were of equal energy, but the photons absorbed by each molecule were of different energy.
D)The photons absorbed by ZPP and PPIX were of equal energy, but the resulting electronic transitions in the molecules were of different energy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
53
Passage
Umami is one of five basic tastes that humans recognize, and it is triggered by L-amino acids as well as some synthetic ingredients and organic acids. Three common umami tastant molecules are shown in Figure 1.
 Figure 1 Three common tastant molecules known to trigger the umami taste sensationUmami taste signals are triggered upon binding of tastant molecules to the G protein-coupled receptor (GPCR) heterodimer T1R1-T1R3, which ultimately causes the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers the release of Ca2+ cations and the subsequent gating of the taste-transduction channels (Figure 2).
Figure 1 Three common tastant molecules known to trigger the umami taste sensationUmami taste signals are triggered upon binding of tastant molecules to the G protein-coupled receptor (GPCR) heterodimer T1R1-T1R3, which ultimately causes the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers the release of Ca2+ cations and the subsequent gating of the taste-transduction channels (Figure 2).
 Figure 2 Signal pathway for transduction of umami taste sensationInosine monophosphate (IMP) has been suggested as enhancing (potentiating) the response of T1R1+T1R3 GPCRs to umami tastant molecules. Scientists tested this hypothesis by culturing receptors from human embryonic kidney (HEK) cells containing promiscuous G proteins. The HEK cells were then stimulated with umami tastants in the presence or absence of IMP. In each test, the cells were exposed to the same amounts of amino acid tastant and IMP. The number of cells that responded to each tastant was measured using a calcium indicator dye (FURA-2AM) in conjunction with fluorescence microscopy. By comparing the cellular response ratio of the stimulated to unstimulated HEK cells, the extent of potentiation can be assessed. The results are shown in Figure 3.
Figure 2 Signal pathway for transduction of umami taste sensationInosine monophosphate (IMP) has been suggested as enhancing (potentiating) the response of T1R1+T1R3 GPCRs to umami tastant molecules. Scientists tested this hypothesis by culturing receptors from human embryonic kidney (HEK) cells containing promiscuous G proteins. The HEK cells were then stimulated with umami tastants in the presence or absence of IMP. In each test, the cells were exposed to the same amounts of amino acid tastant and IMP. The number of cells that responded to each tastant was measured using a calcium indicator dye (FURA-2AM) in conjunction with fluorescence microscopy. By comparing the cellular response ratio of the stimulated to unstimulated HEK cells, the extent of potentiation can be assessed. The results are shown in Figure 3.
 Figure 3 Quantification of amino-acid responses for T1R1+T1R3 with and without IMP
Figure 3 Quantification of amino-acid responses for T1R1+T1R3 with and without IMP
Adapted from Nelson G, Chandrashekar J, Hoon MA, et al. An amino-acid taste receptor. Nature. 2002;416(6877):199-202.
Which of the following statements is true concerning the results shown in Figure 3?
A)For the compounds potentiated by IMP, the extent of potentiation is five-fold.
B)For the compounds potentiated by IMP, the extent of potentiation is equal.
C)IMP most effectively potentiates Thr.
D)IMP most effectively potentiates Asp.
Umami is one of five basic tastes that humans recognize, and it is triggered by L-amino acids as well as some synthetic ingredients and organic acids. Three common umami tastant molecules are shown in Figure 1.
 Figure 1 Three common tastant molecules known to trigger the umami taste sensationUmami taste signals are triggered upon binding of tastant molecules to the G protein-coupled receptor (GPCR) heterodimer T1R1-T1R3, which ultimately causes the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers the release of Ca2+ cations and the subsequent gating of the taste-transduction channels (Figure 2).
Figure 1 Three common tastant molecules known to trigger the umami taste sensationUmami taste signals are triggered upon binding of tastant molecules to the G protein-coupled receptor (GPCR) heterodimer T1R1-T1R3, which ultimately causes the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) into the messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers the release of Ca2+ cations and the subsequent gating of the taste-transduction channels (Figure 2). Figure 2 Signal pathway for transduction of umami taste sensationInosine monophosphate (IMP) has been suggested as enhancing (potentiating) the response of T1R1+T1R3 GPCRs to umami tastant molecules. Scientists tested this hypothesis by culturing receptors from human embryonic kidney (HEK) cells containing promiscuous G proteins. The HEK cells were then stimulated with umami tastants in the presence or absence of IMP. In each test, the cells were exposed to the same amounts of amino acid tastant and IMP. The number of cells that responded to each tastant was measured using a calcium indicator dye (FURA-2AM) in conjunction with fluorescence microscopy. By comparing the cellular response ratio of the stimulated to unstimulated HEK cells, the extent of potentiation can be assessed. The results are shown in Figure 3.
Figure 2 Signal pathway for transduction of umami taste sensationInosine monophosphate (IMP) has been suggested as enhancing (potentiating) the response of T1R1+T1R3 GPCRs to umami tastant molecules. Scientists tested this hypothesis by culturing receptors from human embryonic kidney (HEK) cells containing promiscuous G proteins. The HEK cells were then stimulated with umami tastants in the presence or absence of IMP. In each test, the cells were exposed to the same amounts of amino acid tastant and IMP. The number of cells that responded to each tastant was measured using a calcium indicator dye (FURA-2AM) in conjunction with fluorescence microscopy. By comparing the cellular response ratio of the stimulated to unstimulated HEK cells, the extent of potentiation can be assessed. The results are shown in Figure 3. Figure 3 Quantification of amino-acid responses for T1R1+T1R3 with and without IMP
Figure 3 Quantification of amino-acid responses for T1R1+T1R3 with and without IMPAdapted from Nelson G, Chandrashekar J, Hoon MA, et al. An amino-acid taste receptor. Nature. 2002;416(6877):199-202.
Which of the following statements is true concerning the results shown in Figure 3?
A)For the compounds potentiated by IMP, the extent of potentiation is five-fold.
B)For the compounds potentiated by IMP, the extent of potentiation is equal.
C)IMP most effectively potentiates Thr.
D)IMP most effectively potentiates Asp.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
54
Passage
Water is unique in that all three of its phases exist over a small range of pressures and temperatures, as shown in Figure 1.
 Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars
Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars
 Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL), are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure).The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.
Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL), are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure).The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.
Compared to the boiling point of a sample of pure water on Mars, the boiling point of a sample of briny water under the same atmospheric conditions would be:
A)lower, because the vapor pressure of briny water is higher than the vapor pressure of pure water.
B)higher, because the vapor pressure of briny water is lower than the vapor pressure of pure water.
C)lower, because the vapor pressure of briny water is lower than the vapor pressure of pure water.
D)higher, because the vapor pressure of briny water is higher than the vapor pressure of pure water.
Water is unique in that all three of its phases exist over a small range of pressures and temperatures, as shown in Figure 1.
 Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars
Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL), are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure).The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.
Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL), are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure).The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.Compared to the boiling point of a sample of pure water on Mars, the boiling point of a sample of briny water under the same atmospheric conditions would be:
A)lower, because the vapor pressure of briny water is higher than the vapor pressure of pure water.
B)higher, because the vapor pressure of briny water is lower than the vapor pressure of pure water.
C)lower, because the vapor pressure of briny water is lower than the vapor pressure of pure water.
D)higher, because the vapor pressure of briny water is higher than the vapor pressure of pure water.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
55
Passage
Bone growth and remodeling during fracture healing are often slow and have potentially poor outcomes, including malunion or nonunion at the fracture site. Magnesium bone implants can aid in bone formation and safely biodegrade in the body according to the following reaction:Mg(s) + 2H2O(l) → Mg(OH)2(s) + H2(g)Reaction 1 Corrosion of magnesium metal in aqueous mediumHowever, magnesium metal tends to degrade too quickly and forms hydrogen gas pockets in the body. Corrosion is slowed as the metal naturally forms a protective layer of magnesium hydroxide in a process called passivation. Magnesium hydroxide is less soluble than magnesium metal, and its dissolution is described by the endothermic reaction:
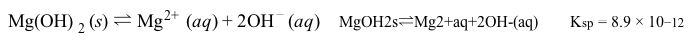 Reaction 2 Solubility equilibrium of Mg(OH)2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C
Reaction 2 Solubility equilibrium of Mg(OH)2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C
 Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35.
Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35.
If researchers wanted to use a coating material that is more soluble in pure water than Mg(OH)2 but less soluble than MgF2, which of the following would be a good option?
A)Ca(OH)2
B)CaF2
C)Zn(OH)2
D)Ca3(PO4)2
Bone growth and remodeling during fracture healing are often slow and have potentially poor outcomes, including malunion or nonunion at the fracture site. Magnesium bone implants can aid in bone formation and safely biodegrade in the body according to the following reaction:Mg(s) + 2H2O(l) → Mg(OH)2(s) + H2(g)Reaction 1 Corrosion of magnesium metal in aqueous mediumHowever, magnesium metal tends to degrade too quickly and forms hydrogen gas pockets in the body. Corrosion is slowed as the metal naturally forms a protective layer of magnesium hydroxide in a process called passivation. Magnesium hydroxide is less soluble than magnesium metal, and its dissolution is described by the endothermic reaction:
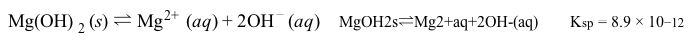 Reaction 2 Solubility equilibrium of Mg(OH)2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C
Reaction 2 Solubility equilibrium of Mg(OH)2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35.
Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35.If researchers wanted to use a coating material that is more soluble in pure water than Mg(OH)2 but less soluble than MgF2, which of the following would be a good option?
A)Ca(OH)2
B)CaF2
C)Zn(OH)2
D)Ca3(PO4)2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
56
Passage
Water is unique in that all three of its phases exist over a small range of pressures and temperatures, as shown in Figure 1.
 Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars
Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars
 Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL), are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure).The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.
Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL), are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure).The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.
Which of the following affects the freezing point of an ideal solution?The strength of intermolecular forcesThe strength of intramolecular forcesThe reactivity of the solute
A)I only
B)II only
C)I and III only
D)II and III only
Water is unique in that all three of its phases exist over a small range of pressures and temperatures, as shown in Figure 1.
 Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars
Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL), are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure).The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.
Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL), are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure).The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.Which of the following affects the freezing point of an ideal solution?The strength of intermolecular forcesThe strength of intramolecular forcesThe reactivity of the solute
A)I only
B)II only
C)I and III only
D)II and III only

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
57
Passage
Bone growth and remodeling during fracture healing are often slow and have potentially poor outcomes, including malunion or nonunion at the fracture site. Magnesium bone implants can aid in bone formation and safely biodegrade in the body according to the following reaction:Mg(s) + 2H2O(l) → Mg(OH)2(s) + H2(g)Reaction 1 Corrosion of magnesium metal in aqueous mediumHowever, magnesium metal tends to degrade too quickly and forms hydrogen gas pockets in the body. Corrosion is slowed as the metal naturally forms a protective layer of magnesium hydroxide in a process called passivation. Magnesium hydroxide is less soluble than magnesium metal, and its dissolution is described by the endothermic reaction:
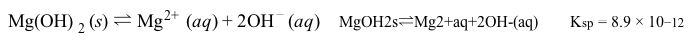 Reaction 2 Solubility equilibrium of Mg(OH)2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C
Reaction 2 Solubility equilibrium of Mg(OH)2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C
 Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35.
Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35.
If researchers wanted to replicate physiological conditions by increasing the SBF temperature to 37°C, how would this change affect the solubility product constant and equilibrium pH of Mg(OH)2 dissolution?
A)Ksp will decrease and equilibrium pH will increase.
B)Ksp and equilibrium pH will both increase.
C)Ksp will stay the same and equilibrium pH will increase.
D)Ksp will stay the same and equilibrium pH will decrease.
Bone growth and remodeling during fracture healing are often slow and have potentially poor outcomes, including malunion or nonunion at the fracture site. Magnesium bone implants can aid in bone formation and safely biodegrade in the body according to the following reaction:Mg(s) + 2H2O(l) → Mg(OH)2(s) + H2(g)Reaction 1 Corrosion of magnesium metal in aqueous mediumHowever, magnesium metal tends to degrade too quickly and forms hydrogen gas pockets in the body. Corrosion is slowed as the metal naturally forms a protective layer of magnesium hydroxide in a process called passivation. Magnesium hydroxide is less soluble than magnesium metal, and its dissolution is described by the endothermic reaction:
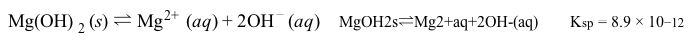 Reaction 2 Solubility equilibrium of Mg(OH)2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C
Reaction 2 Solubility equilibrium of Mg(OH)2 in water at 25°CAlthough passivation slows down the corrosion rate, it must be slowed even further to adequately facilitate bone reformation.Researchers are interested in the differences in the corrosion rates of magnesium metal protected by different biocompatible coatings. They compared 10-mm magnesium disks coated with compounds of various cation and anion combinations, with solubility results shown in Table 1. The coated disks were immersed in simulated body fluid (SBF) at room temperature for 50 hours.Table 1 Solubility for Biocompatible Inorganic Salts Given in mmol/100mL of Pure Water at 25°C Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35.
Adapted from Huan ZG, Leeflang MA, Zhou J, Fratila-apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623-35.If researchers wanted to replicate physiological conditions by increasing the SBF temperature to 37°C, how would this change affect the solubility product constant and equilibrium pH of Mg(OH)2 dissolution?
A)Ksp will decrease and equilibrium pH will increase.
B)Ksp and equilibrium pH will both increase.
C)Ksp will stay the same and equilibrium pH will increase.
D)Ksp will stay the same and equilibrium pH will decrease.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
58
Passage
Heme is found in several proteins, including hemoglobin in blood and myoglobin in muscle. It has a four-coordinate iron-bound porphyrin structure where ferrous iron (Fe2+) is bound to four nitrogen atoms through coordinate covalent bonds. The most common heme found in the body is heme b in hemoglobin (Figure 1).
 Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP). ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm.
Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP). ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm.
 Figure 2 ZPP and PPIX fluorescence emission spectra
Figure 2 ZPP and PPIX fluorescence emission spectra
Adapted from Hennig G, Gruber C, Vogeser M, et al. Dual-wavelength excitation for fluorescence-based quantification of zinc protoporphyrin IX and protoporphyrin IX in whole blood. J Biophotonics. 2014;7(7):514-24.
Which of the following statements best explains why hemoglobin is red when it binds to O2?
A)O2 interacts with iron's d orbitals.
B)O2 is a nonpolar ligand.
C)O2 has lone pairs of electrons.
D)O2 changes the surrounding protein structure.
Heme is found in several proteins, including hemoglobin in blood and myoglobin in muscle. It has a four-coordinate iron-bound porphyrin structure where ferrous iron (Fe2+) is bound to four nitrogen atoms through coordinate covalent bonds. The most common heme found in the body is heme b in hemoglobin (Figure 1).
 Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP). ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm.
Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP). ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm. Figure 2 ZPP and PPIX fluorescence emission spectra
Figure 2 ZPP and PPIX fluorescence emission spectraAdapted from Hennig G, Gruber C, Vogeser M, et al. Dual-wavelength excitation for fluorescence-based quantification of zinc protoporphyrin IX and protoporphyrin IX in whole blood. J Biophotonics. 2014;7(7):514-24.
Which of the following statements best explains why hemoglobin is red when it binds to O2?
A)O2 interacts with iron's d orbitals.
B)O2 is a nonpolar ligand.
C)O2 has lone pairs of electrons.
D)O2 changes the surrounding protein structure.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
59
Passage
Heme is found in several proteins, including hemoglobin in blood and myoglobin in muscle. It has a four-coordinate iron-bound porphyrin structure where ferrous iron (Fe2+) is bound to four nitrogen atoms through coordinate covalent bonds. The most common heme found in the body is heme b in hemoglobin (Figure 1).
 Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP). ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm.
Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP). ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm.
 Figure 2 ZPP and PPIX fluorescence emission spectra
Figure 2 ZPP and PPIX fluorescence emission spectra
Adapted from Hennig G, Gruber C, Vogeser M, et al. Dual-wavelength excitation for fluorescence-based quantification of zinc protoporphyrin IX and protoporphyrin IX in whole blood. J Biophotonics. 2014;7(7):514-24.
The figure below shows Zn2+ in place of Fe2+ to form ZPP, which cannot bind to His F8 or O2. Based on the structure of ZPP, which of the following is the coordination number for Zn2+?
A)2
B)4
C)6
D)The coordination number cannot be determined from the information given.
Heme is found in several proteins, including hemoglobin in blood and myoglobin in muscle. It has a four-coordinate iron-bound porphyrin structure where ferrous iron (Fe2+) is bound to four nitrogen atoms through coordinate covalent bonds. The most common heme found in the body is heme b in hemoglobin (Figure 1).
 Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP). ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm.
Figure 1 Structure of heme b found in hemoglobinAlong with the four nitrogen ligands in the porphyrin structure, His F8 also binds to Fe2+ in the axial position, leaving the other axial position available to bind oxygen. In this setting, iron's atomic d orbitals are no longer degenerate. The energetic difference between the d orbitals depends on the ligands.In deoxygenated heme, iron sits out of the plane formed by the nitrogen ligands in the porphyrin because its d orbitals make it slightly too large to fit in the porphyrin ring. However, when O2 binds to heme, iron's electrons re-arrange themselves. The d orbitals become smaller, and iron sits within the plane of the nitrogen atoms.When iron is scarce in the body, Zn2+ will coordinate to the ligands in the protoporphyrin structure through a nonenzymatic process, forming Zn-protoporphyrin IX (ZPP). ZPP and protoporphyrin IX (PPIX) have distinct emission spectra when excited in the blue range whereas heme does not. Hematofluorometry can be used as a simple, inexpensive indicator for iron deficiency in whole blood by placing a blood sample in a fluorometer and measuring the amount of ZPP relative to the amount of heme. Figure 2 illustrates the fluorescence emission spectra for ZPP and protoporphyrin IX measured from a blood sample when ZPP is excited at 425 nm and PPIX is excited at 407 nm. Figure 2 ZPP and PPIX fluorescence emission spectra
Figure 2 ZPP and PPIX fluorescence emission spectraAdapted from Hennig G, Gruber C, Vogeser M, et al. Dual-wavelength excitation for fluorescence-based quantification of zinc protoporphyrin IX and protoporphyrin IX in whole blood. J Biophotonics. 2014;7(7):514-24.
The figure below shows Zn2+ in place of Fe2+ to form ZPP, which cannot bind to His F8 or O2. Based on the structure of ZPP, which of the following is the coordination number for Zn2+?

A)2
B)4
C)6
D)The coordination number cannot be determined from the information given.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
60
Passage
Water is unique in that all three of its phases exist over a small range of pressures and temperatures, as shown in Figure 1.
 Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars
Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars
 Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL), are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure).The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.
Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL), are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure).The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.
Which of the following phase diagrams best shows the change from pure water's phase boundaries to the phase boundaries of the briny water found on Mars?
A)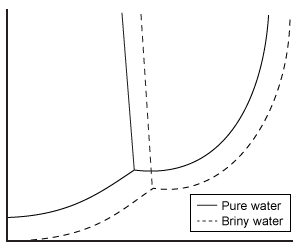
B)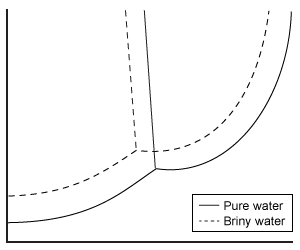
C)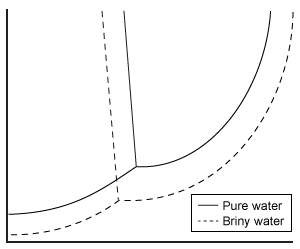
D)
Water is unique in that all three of its phases exist over a small range of pressures and temperatures, as shown in Figure 1.
 Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars
Figure 1 Phase diagram for waterIn the liquid phase, water dissolves and transports molecules, and aids in the biochemical reactions necessary to support life. For this reason, scientists believe liquid-phase water is essential to the existence of extraterrestrial life. Although it has been known for some time that water is present in both the gas and ice phases on Mars, the question remains as to whether there are regions where liquid-phase water can stably exist. Estimates of Mars' surface temperature and pressure generated from satellite readings and global climate models, as well as observations made by Mars rovers, reveal that surface conditions on the planet can vary greatly.Table 1 Temperature and Pressure of Various Locations on Mars Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL), are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure).The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.
Despite the extremely low pressures and temperatures on Mars, satellite images have repeatedly found evidence of flowing liquid water in several regions during the late Martian summer season. These regions, called recurring slope lineae (RSL), are hypothesized to be thermodynamically stable under certain Martian conditions due to the presence of dissolved salts. A simple understanding of this hypothesis is afforded by Raoult law, which states that the vapor pressure and freezing point of an ideal solution are lowered as the number of moles of dissolved solute increases. Moreover, this effect also impacts the solution boiling point (the point at which the vapor pressure of the solution becomes equal to the ambient pressure).The briny-water hypothesis was confirmed in 2015 when spectral data from the Compact Reconnaissance Imaging Spectrometer onboard the Mars Reconnaissance Orbiter revealed the presence of hydrated perchlorates in the same regions and times that RSL were observed. Studies are already underway to determine how common such briny water flows are and whether the conditions within them are suitable for microbial life.Which of the following phase diagrams best shows the change from pure water's phase boundaries to the phase boundaries of the briny water found on Mars?
A)

B)

C)

D)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
61
Passage
Household cleaners commonly contain either ammonia (NH3) or bleach (NaOCl) as the principal active ingredient. Mixing ammonia-based and bleach-based cleaners can be hazardous, as the resulting reactions can form several toxic products. One of these reactions forms the irritant dichloramine (NHCl2) as follows:
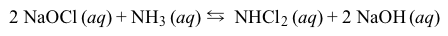 2 NaOClaq+NH3aq⇆ NHCl2aq+2 NaOHaqReaction 1The overall reaction has a standard enthalpy change of −55 kJ/mol and a standard entropy change of −150 J/mol·K. Partial thermodynamic data for formation of Reaction 1 reagents from their elements are shown in Table 1.Table 1 Partial Data for Enthalpies, Entropies, and Free Energies of Formation of Several Compounds at 25 °C
2 NaOClaq+NH3aq⇆ NHCl2aq+2 NaOHaqReaction 1The overall reaction has a standard enthalpy change of −55 kJ/mol and a standard entropy change of −150 J/mol·K. Partial thermodynamic data for formation of Reaction 1 reagents from their elements are shown in Table 1.Table 1 Partial Data for Enthalpies, Entropies, and Free Energies of Formation of Several Compounds at 25 °C
 After it is formed, dichloramine readily enters the gas phase, where it may be inhaled. In the lungs, dichloramine reacts with water to produce hydrochloric acid and ammonia, as well as reactive oxygen species. In small doses, these products cause mild irritation, but inhalation of large amounts of chloramines can lead to pneumonitis (inflammation of the lungs) and may require medical intervention.
After it is formed, dichloramine readily enters the gas phase, where it may be inhaled. In the lungs, dichloramine reacts with water to produce hydrochloric acid and ammonia, as well as reactive oxygen species. In small doses, these products cause mild irritation, but inhalation of large amounts of chloramines can lead to pneumonitis (inflammation of the lungs) and may require medical intervention.
Adapted from Trogolo D, Arey JS. Benchmark thermochemistry of chloramines, bromamines, and bromochloramines: halogen oxidants stabilized by electron correlation. Phys Chem Chem Phys. 2015;17(5):3584-98.
If dichloramine formation is spontaneous under standard conditions, the equilibrium constant must be:
A)< 0.
B)> 0 and < 1.
C)= 1.
D)> 1.
Household cleaners commonly contain either ammonia (NH3) or bleach (NaOCl) as the principal active ingredient. Mixing ammonia-based and bleach-based cleaners can be hazardous, as the resulting reactions can form several toxic products. One of these reactions forms the irritant dichloramine (NHCl2) as follows:
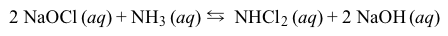 2 NaOClaq+NH3aq⇆ NHCl2aq+2 NaOHaqReaction 1The overall reaction has a standard enthalpy change of −55 kJ/mol and a standard entropy change of −150 J/mol·K. Partial thermodynamic data for formation of Reaction 1 reagents from their elements are shown in Table 1.Table 1 Partial Data for Enthalpies, Entropies, and Free Energies of Formation of Several Compounds at 25 °C
2 NaOClaq+NH3aq⇆ NHCl2aq+2 NaOHaqReaction 1The overall reaction has a standard enthalpy change of −55 kJ/mol and a standard entropy change of −150 J/mol·K. Partial thermodynamic data for formation of Reaction 1 reagents from their elements are shown in Table 1.Table 1 Partial Data for Enthalpies, Entropies, and Free Energies of Formation of Several Compounds at 25 °C After it is formed, dichloramine readily enters the gas phase, where it may be inhaled. In the lungs, dichloramine reacts with water to produce hydrochloric acid and ammonia, as well as reactive oxygen species. In small doses, these products cause mild irritation, but inhalation of large amounts of chloramines can lead to pneumonitis (inflammation of the lungs) and may require medical intervention.
After it is formed, dichloramine readily enters the gas phase, where it may be inhaled. In the lungs, dichloramine reacts with water to produce hydrochloric acid and ammonia, as well as reactive oxygen species. In small doses, these products cause mild irritation, but inhalation of large amounts of chloramines can lead to pneumonitis (inflammation of the lungs) and may require medical intervention.Adapted from Trogolo D, Arey JS. Benchmark thermochemistry of chloramines, bromamines, and bromochloramines: halogen oxidants stabilized by electron correlation. Phys Chem Chem Phys. 2015;17(5):3584-98.
If dichloramine formation is spontaneous under standard conditions, the equilibrium constant must be:
A)< 0.
B)> 0 and < 1.
C)= 1.
D)> 1.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
62
Passage
Household cleaners commonly contain either ammonia (NH3) or bleach (NaOCl) as the principal active ingredient. Mixing ammonia-based and bleach-based cleaners can be hazardous, as the resulting reactions can form several toxic products. One of these reactions forms the irritant dichloramine (NHCl2) as follows:
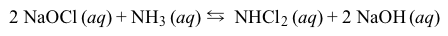 2 NaOClaq+NH3aq⇆ NHCl2aq+2 NaOHaqReaction 1The overall reaction has a standard enthalpy change of −55 kJ/mol and a standard entropy change of −150 J/mol·K. Partial thermodynamic data for formation of Reaction 1 reagents from their elements are shown in Table 1.Table 1 Partial Data for Enthalpies, Entropies, and Free Energies of Formation of Several Compounds at 25 °C
2 NaOClaq+NH3aq⇆ NHCl2aq+2 NaOHaqReaction 1The overall reaction has a standard enthalpy change of −55 kJ/mol and a standard entropy change of −150 J/mol·K. Partial thermodynamic data for formation of Reaction 1 reagents from their elements are shown in Table 1.Table 1 Partial Data for Enthalpies, Entropies, and Free Energies of Formation of Several Compounds at 25 °C
 After it is formed, dichloramine readily enters the gas phase, where it may be inhaled. In the lungs, dichloramine reacts with water to produce hydrochloric acid and ammonia, as well as reactive oxygen species. In small doses, these products cause mild irritation, but inhalation of large amounts of chloramines can lead to pneumonitis (inflammation of the lungs) and may require medical intervention.
After it is formed, dichloramine readily enters the gas phase, where it may be inhaled. In the lungs, dichloramine reacts with water to produce hydrochloric acid and ammonia, as well as reactive oxygen species. In small doses, these products cause mild irritation, but inhalation of large amounts of chloramines can lead to pneumonitis (inflammation of the lungs) and may require medical intervention.
Adapted from Trogolo D, Arey JS. Benchmark thermochemistry of chloramines, bromamines, and bromochloramines: halogen oxidants stabilized by electron correlation. Phys Chem Chem Phys. 2015;17(5):3584-98.
If large amounts of ammonia and bleach were mixed, emergency responders could help stop the reaction by doing which of the following?
A)Mixing sodium hydroxide into the solution
B)Adding ammonia to the solution
C)Removing dichloramine from the solution
D)Evaporating water from the solution
Household cleaners commonly contain either ammonia (NH3) or bleach (NaOCl) as the principal active ingredient. Mixing ammonia-based and bleach-based cleaners can be hazardous, as the resulting reactions can form several toxic products. One of these reactions forms the irritant dichloramine (NHCl2) as follows:
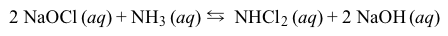 2 NaOClaq+NH3aq⇆ NHCl2aq+2 NaOHaqReaction 1The overall reaction has a standard enthalpy change of −55 kJ/mol and a standard entropy change of −150 J/mol·K. Partial thermodynamic data for formation of Reaction 1 reagents from their elements are shown in Table 1.Table 1 Partial Data for Enthalpies, Entropies, and Free Energies of Formation of Several Compounds at 25 °C
2 NaOClaq+NH3aq⇆ NHCl2aq+2 NaOHaqReaction 1The overall reaction has a standard enthalpy change of −55 kJ/mol and a standard entropy change of −150 J/mol·K. Partial thermodynamic data for formation of Reaction 1 reagents from their elements are shown in Table 1.Table 1 Partial Data for Enthalpies, Entropies, and Free Energies of Formation of Several Compounds at 25 °C After it is formed, dichloramine readily enters the gas phase, where it may be inhaled. In the lungs, dichloramine reacts with water to produce hydrochloric acid and ammonia, as well as reactive oxygen species. In small doses, these products cause mild irritation, but inhalation of large amounts of chloramines can lead to pneumonitis (inflammation of the lungs) and may require medical intervention.
After it is formed, dichloramine readily enters the gas phase, where it may be inhaled. In the lungs, dichloramine reacts with water to produce hydrochloric acid and ammonia, as well as reactive oxygen species. In small doses, these products cause mild irritation, but inhalation of large amounts of chloramines can lead to pneumonitis (inflammation of the lungs) and may require medical intervention.Adapted from Trogolo D, Arey JS. Benchmark thermochemistry of chloramines, bromamines, and bromochloramines: halogen oxidants stabilized by electron correlation. Phys Chem Chem Phys. 2015;17(5):3584-98.
If large amounts of ammonia and bleach were mixed, emergency responders could help stop the reaction by doing which of the following?
A)Mixing sodium hydroxide into the solution
B)Adding ammonia to the solution
C)Removing dichloramine from the solution
D)Evaporating water from the solution

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
63
Passage
Combustion occurs when an oxidation-reduction reaction takes place between a reduced fuel source and an oxidizer, most frequently oxygen. The reaction between hydrogen and oxygen forms water (Reaction 1) and is highly exothermic and thermodynamically favorable. At the completion of hydrogen combustion, essentially all H2 molecules have been converted to water. Nevertheless, at room temperature and atmospheric pressure, molecular hydrogen and oxygen can coexist quite stably.2 H2 + O2 → 2 H2OReaction 1A group of researchers designed two prototype internal combustion engines, each of which could inject a hydrogen/oxygen mixture into a 5-mL combustion chamber at a total pressure of 1 atm and could repeat this process for several cycles. In one engine design, a spark was generated to initiate combustion as hydrogen and oxygen were injected. In another method, the combustion chamber was coated with a small amount of platinum powder. Platinum dramatically increases the reaction rate at room temperature without the need for a spark. The platinum itself is not altered by the reaction and can be reused. Researchers measured the maximum power output of both engine designs under identical injection and temperature conditions (Figure 1).
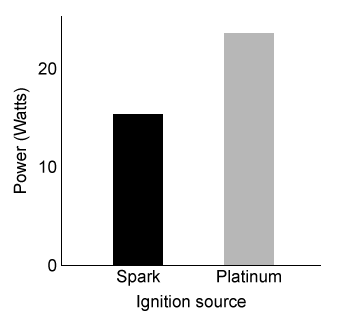 Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2.
Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2.
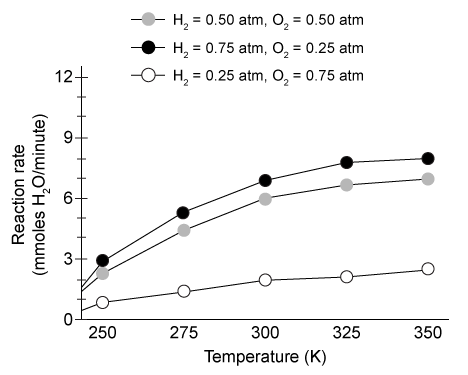 Figure 2 Rate of combustion in platinum-coated engines under various conditions
Figure 2 Rate of combustion in platinum-coated engines under various conditions
Adapted from Schultze M, Mantzaras J. Hetero-/homogeneous combustion of hydrogen/air mixtures over platinum: Fuel-lean versus fuel-rich combustion modes doi: 10.1016/j.ijhydene.2013.06.069
When the platinum-based engine is run continuously at 300 K for 1 hour with 0.5 atm of H2 and 0.5 atm of O2 injected for each reaction, approximately what mass of water will be produced?
A)0.36 g
B)6.5 g
C)360 g
D)6,500 g
Combustion occurs when an oxidation-reduction reaction takes place between a reduced fuel source and an oxidizer, most frequently oxygen. The reaction between hydrogen and oxygen forms water (Reaction 1) and is highly exothermic and thermodynamically favorable. At the completion of hydrogen combustion, essentially all H2 molecules have been converted to water. Nevertheless, at room temperature and atmospheric pressure, molecular hydrogen and oxygen can coexist quite stably.2 H2 + O2 → 2 H2OReaction 1A group of researchers designed two prototype internal combustion engines, each of which could inject a hydrogen/oxygen mixture into a 5-mL combustion chamber at a total pressure of 1 atm and could repeat this process for several cycles. In one engine design, a spark was generated to initiate combustion as hydrogen and oxygen were injected. In another method, the combustion chamber was coated with a small amount of platinum powder. Platinum dramatically increases the reaction rate at room temperature without the need for a spark. The platinum itself is not altered by the reaction and can be reused. Researchers measured the maximum power output of both engine designs under identical injection and temperature conditions (Figure 1).
 Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2.
Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2. Figure 2 Rate of combustion in platinum-coated engines under various conditions
Figure 2 Rate of combustion in platinum-coated engines under various conditionsAdapted from Schultze M, Mantzaras J. Hetero-/homogeneous combustion of hydrogen/air mixtures over platinum: Fuel-lean versus fuel-rich combustion modes doi: 10.1016/j.ijhydene.2013.06.069
When the platinum-based engine is run continuously at 300 K for 1 hour with 0.5 atm of H2 and 0.5 atm of O2 injected for each reaction, approximately what mass of water will be produced?
A)0.36 g
B)6.5 g
C)360 g
D)6,500 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
64
Passage
An automated external defibrillator (AED) is a medical device used to send an electric shock to the heart after cardiac arrest. A key component of the AED is the power source, or battery. Batteries used in AEDs need to have a good charge-to-weight ratio; they must be safe and reliable as well as rechargeable.Two types of rechargeable batteries used in early models of AEDs are the lead storage (also called lead-acid) battery and the nickel-cadmium (NiCd) battery. These batteries consist of multiple electrochemical cells that are connected in series to deliver a potential between 9 V and 18 V. A capacitor allows the AED to accumulate charge so that it can deliver between 300 V and 1,000 V.Some AEDs use a sealed lead storage battery. Lead storage batteries are robust and hold a charge for a long time. However, they have a low energy-to-weight ratio. Each lead storage cell delivers approximately 2.0 V, and a battery of four cells weighing 1000 g can provide 30 W∙h of energy.
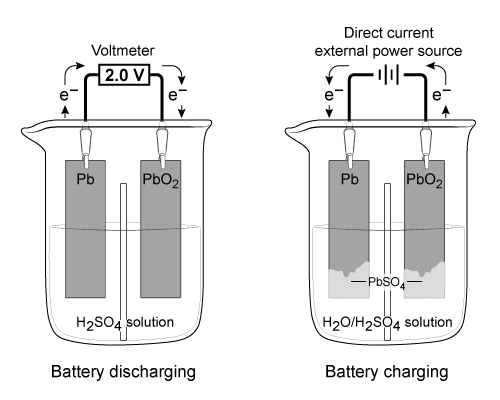 Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2:
Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2:
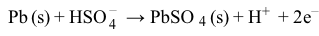 Reaction 1
Reaction 1
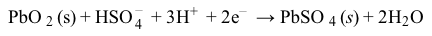 Reaction 2
Reaction 2
NiCd batteries have a higher energy-to-weight ratio than lead storage batteries but cannot hold as much charge. Each NiCd cell delivers approximately 1.3 V, and a single-cell battery weighing 120 g can provide 7.2 W∙h of energy.The half reactions at the anode and cathode for a NiCd battery in KOH are shown in Reactions 3 and 4:
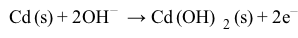 Reaction 3
Reaction 3
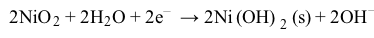 Reaction 4
Reaction 4
Would the concentration of H2SO4 remain constant as a lead storage battery is discharged?
A)No, because water is one of the products produced when the battery is discharged.
B)Yes, because PbSO4 increases as the battery is discharged.
C)No, because the SO42- ions are oxidized in the reaction.
D)Yes, because the H+ ions are not oxidized or reduced in the reaction.
An automated external defibrillator (AED) is a medical device used to send an electric shock to the heart after cardiac arrest. A key component of the AED is the power source, or battery. Batteries used in AEDs need to have a good charge-to-weight ratio; they must be safe and reliable as well as rechargeable.Two types of rechargeable batteries used in early models of AEDs are the lead storage (also called lead-acid) battery and the nickel-cadmium (NiCd) battery. These batteries consist of multiple electrochemical cells that are connected in series to deliver a potential between 9 V and 18 V. A capacitor allows the AED to accumulate charge so that it can deliver between 300 V and 1,000 V.Some AEDs use a sealed lead storage battery. Lead storage batteries are robust and hold a charge for a long time. However, they have a low energy-to-weight ratio. Each lead storage cell delivers approximately 2.0 V, and a battery of four cells weighing 1000 g can provide 30 W∙h of energy.
 Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2:
Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2: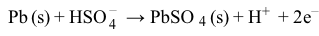 Reaction 1
Reaction 1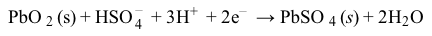 Reaction 2
Reaction 2NiCd batteries have a higher energy-to-weight ratio than lead storage batteries but cannot hold as much charge. Each NiCd cell delivers approximately 1.3 V, and a single-cell battery weighing 120 g can provide 7.2 W∙h of energy.The half reactions at the anode and cathode for a NiCd battery in KOH are shown in Reactions 3 and 4:
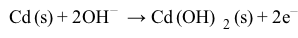 Reaction 3
Reaction 3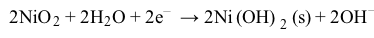 Reaction 4
Reaction 4Would the concentration of H2SO4 remain constant as a lead storage battery is discharged?
A)No, because water is one of the products produced when the battery is discharged.
B)Yes, because PbSO4 increases as the battery is discharged.
C)No, because the SO42- ions are oxidized in the reaction.
D)Yes, because the H+ ions are not oxidized or reduced in the reaction.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
65
Passage
An automated external defibrillator (AED) is a medical device used to send an electric shock to the heart after cardiac arrest. A key component of the AED is the power source, or battery. Batteries used in AEDs need to have a good charge-to-weight ratio; they must be safe and reliable as well as rechargeable.Two types of rechargeable batteries used in early models of AEDs are the lead storage (also called lead-acid) battery and the nickel-cadmium (NiCd) battery. These batteries consist of multiple electrochemical cells that are connected in series to deliver a potential between 9 V and 18 V. A capacitor allows the AED to accumulate charge so that it can deliver between 300 V and 1,000 V.Some AEDs use a sealed lead storage battery. Lead storage batteries are robust and hold a charge for a long time. However, they have a low energy-to-weight ratio. Each lead storage cell delivers approximately 2.0 V, and a battery of four cells weighing 1000 g can provide 30 W∙h of energy.
 Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2:
Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2:
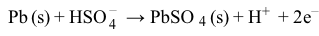 Reaction 1
Reaction 1
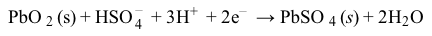 Reaction 2
Reaction 2
NiCd batteries have a higher energy-to-weight ratio than lead storage batteries but cannot hold as much charge. Each NiCd cell delivers approximately 1.3 V, and a single-cell battery weighing 120 g can provide 7.2 W∙h of energy.The half reactions at the anode and cathode for a NiCd battery in KOH are shown in Reactions 3 and 4:
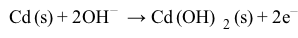 Reaction 3
Reaction 3
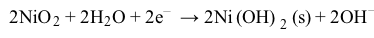 Reaction 4
Reaction 4
Assume that a NiCd battery charges for 30 minutes (t) with an applied current (I) of 5.0 A. Which expression could be used to calculate the number of moles of Cd produced on the cathode from electrolytic plating?
A)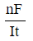
B)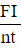
C)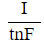
D)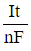
An automated external defibrillator (AED) is a medical device used to send an electric shock to the heart after cardiac arrest. A key component of the AED is the power source, or battery. Batteries used in AEDs need to have a good charge-to-weight ratio; they must be safe and reliable as well as rechargeable.Two types of rechargeable batteries used in early models of AEDs are the lead storage (also called lead-acid) battery and the nickel-cadmium (NiCd) battery. These batteries consist of multiple electrochemical cells that are connected in series to deliver a potential between 9 V and 18 V. A capacitor allows the AED to accumulate charge so that it can deliver between 300 V and 1,000 V.Some AEDs use a sealed lead storage battery. Lead storage batteries are robust and hold a charge for a long time. However, they have a low energy-to-weight ratio. Each lead storage cell delivers approximately 2.0 V, and a battery of four cells weighing 1000 g can provide 30 W∙h of energy.
 Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2:
Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2: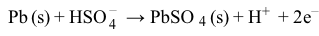 Reaction 1
Reaction 1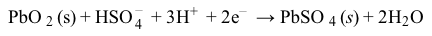 Reaction 2
Reaction 2NiCd batteries have a higher energy-to-weight ratio than lead storage batteries but cannot hold as much charge. Each NiCd cell delivers approximately 1.3 V, and a single-cell battery weighing 120 g can provide 7.2 W∙h of energy.The half reactions at the anode and cathode for a NiCd battery in KOH are shown in Reactions 3 and 4:
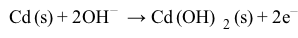 Reaction 3
Reaction 3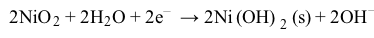 Reaction 4
Reaction 4Assume that a NiCd battery charges for 30 minutes (t) with an applied current (I) of 5.0 A. Which expression could be used to calculate the number of moles of Cd produced on the cathode from electrolytic plating?
A)
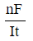
B)
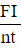
C)
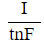
D)
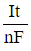

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
66
Passage
An automated external defibrillator (AED) is a medical device used to send an electric shock to the heart after cardiac arrest. A key component of the AED is the power source, or battery. Batteries used in AEDs need to have a good charge-to-weight ratio; they must be safe and reliable as well as rechargeable.Two types of rechargeable batteries used in early models of AEDs are the lead storage (also called lead-acid) battery and the nickel-cadmium (NiCd) battery. These batteries consist of multiple electrochemical cells that are connected in series to deliver a potential between 9 V and 18 V. A capacitor allows the AED to accumulate charge so that it can deliver between 300 V and 1,000 V.Some AEDs use a sealed lead storage battery. Lead storage batteries are robust and hold a charge for a long time. However, they have a low energy-to-weight ratio. Each lead storage cell delivers approximately 2.0 V, and a battery of four cells weighing 1000 g can provide 30 W∙h of energy.
 Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2:
Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2:
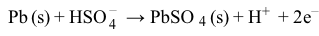 Reaction 1
Reaction 1
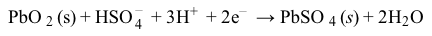 Reaction 2
Reaction 2
NiCd batteries have a higher energy-to-weight ratio than lead storage batteries but cannot hold as much charge. Each NiCd cell delivers approximately 1.3 V, and a single-cell battery weighing 120 g can provide 7.2 W∙h of energy.The half reactions at the anode and cathode for a NiCd battery in KOH are shown in Reactions 3 and 4:
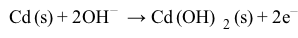 Reaction 3
Reaction 3
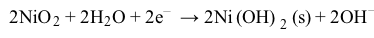 Reaction 4
Reaction 4
Which statement correctly describes the Pb electrode when the lead storage battery is charging as shown in Figure 1?
A)It is the anode and PbSO4 is oxidized at its surface.
B)It is the anode and H2SO4 is reduced at its surface.
C)It is the cathode and PbSO4 is reduced at its surface.
D)It is the cathode and H2SO4 is oxidized at its surface.
An automated external defibrillator (AED) is a medical device used to send an electric shock to the heart after cardiac arrest. A key component of the AED is the power source, or battery. Batteries used in AEDs need to have a good charge-to-weight ratio; they must be safe and reliable as well as rechargeable.Two types of rechargeable batteries used in early models of AEDs are the lead storage (also called lead-acid) battery and the nickel-cadmium (NiCd) battery. These batteries consist of multiple electrochemical cells that are connected in series to deliver a potential between 9 V and 18 V. A capacitor allows the AED to accumulate charge so that it can deliver between 300 V and 1,000 V.Some AEDs use a sealed lead storage battery. Lead storage batteries are robust and hold a charge for a long time. However, they have a low energy-to-weight ratio. Each lead storage cell delivers approximately 2.0 V, and a battery of four cells weighing 1000 g can provide 30 W∙h of energy.
 Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2:
Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2: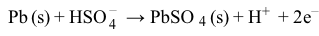 Reaction 1
Reaction 1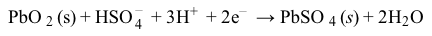 Reaction 2
Reaction 2NiCd batteries have a higher energy-to-weight ratio than lead storage batteries but cannot hold as much charge. Each NiCd cell delivers approximately 1.3 V, and a single-cell battery weighing 120 g can provide 7.2 W∙h of energy.The half reactions at the anode and cathode for a NiCd battery in KOH are shown in Reactions 3 and 4:
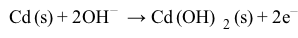 Reaction 3
Reaction 3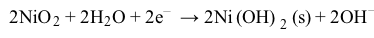 Reaction 4
Reaction 4Which statement correctly describes the Pb electrode when the lead storage battery is charging as shown in Figure 1?
A)It is the anode and PbSO4 is oxidized at its surface.
B)It is the anode and H2SO4 is reduced at its surface.
C)It is the cathode and PbSO4 is reduced at its surface.
D)It is the cathode and H2SO4 is oxidized at its surface.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
67
Passage
An automated external defibrillator (AED) is a medical device used to send an electric shock to the heart after cardiac arrest. A key component of the AED is the power source, or battery. Batteries used in AEDs need to have a good charge-to-weight ratio; they must be safe and reliable as well as rechargeable.Two types of rechargeable batteries used in early models of AEDs are the lead storage (also called lead-acid) battery and the nickel-cadmium (NiCd) battery. These batteries consist of multiple electrochemical cells that are connected in series to deliver a potential between 9 V and 18 V. A capacitor allows the AED to accumulate charge so that it can deliver between 300 V and 1,000 V.Some AEDs use a sealed lead storage battery. Lead storage batteries are robust and hold a charge for a long time. However, they have a low energy-to-weight ratio. Each lead storage cell delivers approximately 2.0 V, and a battery of four cells weighing 1000 g can provide 30 W∙h of energy.
 Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2:
Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2:
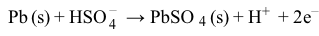 Reaction 1
Reaction 1
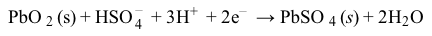 Reaction 2
Reaction 2
NiCd batteries have a higher energy-to-weight ratio than lead storage batteries but cannot hold as much charge. Each NiCd cell delivers approximately 1.3 V, and a single-cell battery weighing 120 g can provide 7.2 W∙h of energy.The half reactions at the anode and cathode for a NiCd battery in KOH are shown in Reactions 3 and 4:
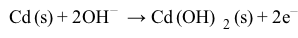 Reaction 3
Reaction 3
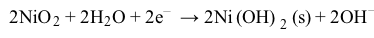 Reaction 4
Reaction 4
If energy density is the amount of energy per unit mass, which of the following statements must be true?
A)NiCd batteries have a higher energy density than lead storage batteries.
B)Lead storage batteries produce less energy than NiCd batteries.
C)NiCd batteries produce more electrons per mole of metal than lead storage batteries.
D)Both lead storage and NiCd batteries have the same energy density.
An automated external defibrillator (AED) is a medical device used to send an electric shock to the heart after cardiac arrest. A key component of the AED is the power source, or battery. Batteries used in AEDs need to have a good charge-to-weight ratio; they must be safe and reliable as well as rechargeable.Two types of rechargeable batteries used in early models of AEDs are the lead storage (also called lead-acid) battery and the nickel-cadmium (NiCd) battery. These batteries consist of multiple electrochemical cells that are connected in series to deliver a potential between 9 V and 18 V. A capacitor allows the AED to accumulate charge so that it can deliver between 300 V and 1,000 V.Some AEDs use a sealed lead storage battery. Lead storage batteries are robust and hold a charge for a long time. However, they have a low energy-to-weight ratio. Each lead storage cell delivers approximately 2.0 V, and a battery of four cells weighing 1000 g can provide 30 W∙h of energy.
 Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2:
Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2: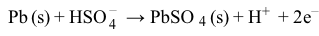 Reaction 1
Reaction 1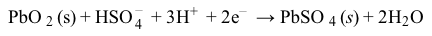 Reaction 2
Reaction 2NiCd batteries have a higher energy-to-weight ratio than lead storage batteries but cannot hold as much charge. Each NiCd cell delivers approximately 1.3 V, and a single-cell battery weighing 120 g can provide 7.2 W∙h of energy.The half reactions at the anode and cathode for a NiCd battery in KOH are shown in Reactions 3 and 4:
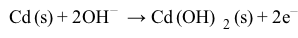 Reaction 3
Reaction 3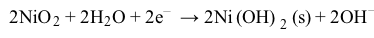 Reaction 4
Reaction 4If energy density is the amount of energy per unit mass, which of the following statements must be true?
A)NiCd batteries have a higher energy density than lead storage batteries.
B)Lead storage batteries produce less energy than NiCd batteries.
C)NiCd batteries produce more electrons per mole of metal than lead storage batteries.
D)Both lead storage and NiCd batteries have the same energy density.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
68
Passage
Combustion occurs when an oxidation-reduction reaction takes place between a reduced fuel source and an oxidizer, most frequently oxygen. The reaction between hydrogen and oxygen forms water (Reaction 1) and is highly exothermic and thermodynamically favorable. At the completion of hydrogen combustion, essentially all H2 molecules have been converted to water. Nevertheless, at room temperature and atmospheric pressure, molecular hydrogen and oxygen can coexist quite stably.2 H2 + O2 → 2 H2OReaction 1A group of researchers designed two prototype internal combustion engines, each of which could inject a hydrogen/oxygen mixture into a 5-mL combustion chamber at a total pressure of 1 atm and could repeat this process for several cycles. In one engine design, a spark was generated to initiate combustion as hydrogen and oxygen were injected. In another method, the combustion chamber was coated with a small amount of platinum powder. Platinum dramatically increases the reaction rate at room temperature without the need for a spark. The platinum itself is not altered by the reaction and can be reused. Researchers measured the maximum power output of both engine designs under identical injection and temperature conditions (Figure 1).
 Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2.
Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2.
 Figure 2 Rate of combustion in platinum-coated engines under various conditions
Figure 2 Rate of combustion in platinum-coated engines under various conditions
Adapted from Schultze M, Mantzaras J. Hetero-/homogeneous combustion of hydrogen/air mixtures over platinum: Fuel-lean versus fuel-rich combustion modes doi: 10.1016/j.ijhydene.2013.06.069
Platinum most likely increases the reaction rate by stabilizing:
A)the reactants.
B)the products.
C)the intermediate step.
D)the transition state.
Combustion occurs when an oxidation-reduction reaction takes place between a reduced fuel source and an oxidizer, most frequently oxygen. The reaction between hydrogen and oxygen forms water (Reaction 1) and is highly exothermic and thermodynamically favorable. At the completion of hydrogen combustion, essentially all H2 molecules have been converted to water. Nevertheless, at room temperature and atmospheric pressure, molecular hydrogen and oxygen can coexist quite stably.2 H2 + O2 → 2 H2OReaction 1A group of researchers designed two prototype internal combustion engines, each of which could inject a hydrogen/oxygen mixture into a 5-mL combustion chamber at a total pressure of 1 atm and could repeat this process for several cycles. In one engine design, a spark was generated to initiate combustion as hydrogen and oxygen were injected. In another method, the combustion chamber was coated with a small amount of platinum powder. Platinum dramatically increases the reaction rate at room temperature without the need for a spark. The platinum itself is not altered by the reaction and can be reused. Researchers measured the maximum power output of both engine designs under identical injection and temperature conditions (Figure 1).
 Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2.
Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2. Figure 2 Rate of combustion in platinum-coated engines under various conditions
Figure 2 Rate of combustion in platinum-coated engines under various conditionsAdapted from Schultze M, Mantzaras J. Hetero-/homogeneous combustion of hydrogen/air mixtures over platinum: Fuel-lean versus fuel-rich combustion modes doi: 10.1016/j.ijhydene.2013.06.069
Platinum most likely increases the reaction rate by stabilizing:
A)the reactants.
B)the products.
C)the intermediate step.
D)the transition state.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
69
Passage
Kidney stones are a common ailment affecting approximately 10% of adults in the United States. They form when solutes precipitate out of solution as crystals in the urinary tract, and they can cause severe pain in the side, back, abdomen, and groin. Individuals who have been previously diagnosed with kidney stones have an increased probability of developing new stones relative to unaffected individuals. Different measures may help prevent the formation of different kinds of stones, so analysis of the composition of stones that have been passed or removed can aid in preventing recurrence. Stones can be ground into fine powders, dissolved in a small amount of solvent, and analyzed by infrared (IR) spectroscopy, as shown in Figure 1.
 Figure 1 Schematic of kidney stone analysis by IR spectroscopyIR analysis of kidney stones from 50 individuals revealed the percentage of stones that contain each component, shown in Table 1 along with solubility data.Table 1 Kidney Stone Composition Parameters
Figure 1 Schematic of kidney stone analysis by IR spectroscopyIR analysis of kidney stones from 50 individuals revealed the percentage of stones that contain each component, shown in Table 1 along with solubility data.Table 1 Kidney Stone Composition Parameters
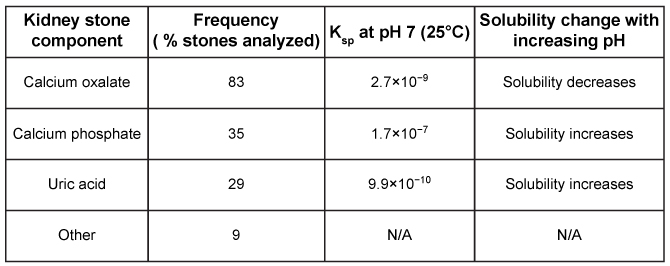 Some studies indicate that potassium citrate, taken orally, may prevent the formation of calcium oxalate crystals, the most abundant component of kidney stones. Oxalic acid, shown in Figure 2, is significantly more soluble than calcium oxalate.
Some studies indicate that potassium citrate, taken orally, may prevent the formation of calcium oxalate crystals, the most abundant component of kidney stones. Oxalic acid, shown in Figure 2, is significantly more soluble than calcium oxalate.
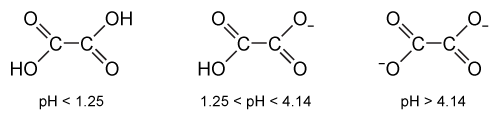 Figure 2 Structure of oxalic acid and its associated anions with increasing pHPotassium citrate alkalinizes the urine, potentially causing a decrease in oxalate solubility and the formation of more crystals. However, potassium citrate can also react with calcium oxalate according to the unbalanced equation shown in Reaction 1:CaC2O4 + K3(C6H5O7) → Ca3(C6H5O7)2 + K2C2O4Reaction 1Calcium citrate and potassium oxalate are both hundreds of times more soluble than calcium oxalate, so the presence of citrate and potassium ions can help maintain calcium and oxalate ions in solution. This effect may be sufficient to overcome the decreased solubility that occurs at higher pH levels.
Figure 2 Structure of oxalic acid and its associated anions with increasing pHPotassium citrate alkalinizes the urine, potentially causing a decrease in oxalate solubility and the formation of more crystals. However, potassium citrate can also react with calcium oxalate according to the unbalanced equation shown in Reaction 1:CaC2O4 + K3(C6H5O7) → Ca3(C6H5O7)2 + K2C2O4Reaction 1Calcium citrate and potassium oxalate are both hundreds of times more soluble than calcium oxalate, so the presence of citrate and potassium ions can help maintain calcium and oxalate ions in solution. This effect may be sufficient to overcome the decreased solubility that occurs at higher pH levels.
Adapted from Primiano A, Persichilli S, Gambaro G, et al. FT-IR analysis of urinary stones: a helpful tool for clinician comparison with the chemical spot test. Dis Markers. 2014;2014:176165.
Which of the following bond types are found in the calcium phosphate present in 35% of kidney stones?IonicPolar covalentNonpolar covalent
A)I and II only
B)I and III only
C)II and III only
D)I, II, and III
Kidney stones are a common ailment affecting approximately 10% of adults in the United States. They form when solutes precipitate out of solution as crystals in the urinary tract, and they can cause severe pain in the side, back, abdomen, and groin. Individuals who have been previously diagnosed with kidney stones have an increased probability of developing new stones relative to unaffected individuals. Different measures may help prevent the formation of different kinds of stones, so analysis of the composition of stones that have been passed or removed can aid in preventing recurrence. Stones can be ground into fine powders, dissolved in a small amount of solvent, and analyzed by infrared (IR) spectroscopy, as shown in Figure 1.
 Figure 1 Schematic of kidney stone analysis by IR spectroscopyIR analysis of kidney stones from 50 individuals revealed the percentage of stones that contain each component, shown in Table 1 along with solubility data.Table 1 Kidney Stone Composition Parameters
Figure 1 Schematic of kidney stone analysis by IR spectroscopyIR analysis of kidney stones from 50 individuals revealed the percentage of stones that contain each component, shown in Table 1 along with solubility data.Table 1 Kidney Stone Composition Parameters Some studies indicate that potassium citrate, taken orally, may prevent the formation of calcium oxalate crystals, the most abundant component of kidney stones. Oxalic acid, shown in Figure 2, is significantly more soluble than calcium oxalate.
Some studies indicate that potassium citrate, taken orally, may prevent the formation of calcium oxalate crystals, the most abundant component of kidney stones. Oxalic acid, shown in Figure 2, is significantly more soluble than calcium oxalate. Figure 2 Structure of oxalic acid and its associated anions with increasing pHPotassium citrate alkalinizes the urine, potentially causing a decrease in oxalate solubility and the formation of more crystals. However, potassium citrate can also react with calcium oxalate according to the unbalanced equation shown in Reaction 1:CaC2O4 + K3(C6H5O7) → Ca3(C6H5O7)2 + K2C2O4Reaction 1Calcium citrate and potassium oxalate are both hundreds of times more soluble than calcium oxalate, so the presence of citrate and potassium ions can help maintain calcium and oxalate ions in solution. This effect may be sufficient to overcome the decreased solubility that occurs at higher pH levels.
Figure 2 Structure of oxalic acid and its associated anions with increasing pHPotassium citrate alkalinizes the urine, potentially causing a decrease in oxalate solubility and the formation of more crystals. However, potassium citrate can also react with calcium oxalate according to the unbalanced equation shown in Reaction 1:CaC2O4 + K3(C6H5O7) → Ca3(C6H5O7)2 + K2C2O4Reaction 1Calcium citrate and potassium oxalate are both hundreds of times more soluble than calcium oxalate, so the presence of citrate and potassium ions can help maintain calcium and oxalate ions in solution. This effect may be sufficient to overcome the decreased solubility that occurs at higher pH levels.Adapted from Primiano A, Persichilli S, Gambaro G, et al. FT-IR analysis of urinary stones: a helpful tool for clinician comparison with the chemical spot test. Dis Markers. 2014;2014:176165.
Which of the following bond types are found in the calcium phosphate present in 35% of kidney stones?IonicPolar covalentNonpolar covalent
A)I and II only
B)I and III only
C)II and III only
D)I, II, and III

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
70
Passage
Household cleaners commonly contain either ammonia (NH3) or bleach (NaOCl) as the principal active ingredient. Mixing ammonia-based and bleach-based cleaners can be hazardous, as the resulting reactions can form several toxic products. One of these reactions forms the irritant dichloramine (NHCl2) as follows:
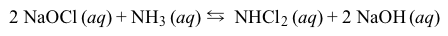 2 NaOClaq+NH3aq⇆ NHCl2aq+2 NaOHaqReaction 1The overall reaction has a standard enthalpy change of −55 kJ/mol and a standard entropy change of −150 J/mol·K. Partial thermodynamic data for formation of Reaction 1 reagents from their elements are shown in Table 1.Table 1 Partial Data for Enthalpies, Entropies, and Free Energies of Formation of Several Compounds at 25 °C
2 NaOClaq+NH3aq⇆ NHCl2aq+2 NaOHaqReaction 1The overall reaction has a standard enthalpy change of −55 kJ/mol and a standard entropy change of −150 J/mol·K. Partial thermodynamic data for formation of Reaction 1 reagents from their elements are shown in Table 1.Table 1 Partial Data for Enthalpies, Entropies, and Free Energies of Formation of Several Compounds at 25 °C
 After it is formed, dichloramine readily enters the gas phase, where it may be inhaled. In the lungs, dichloramine reacts with water to produce hydrochloric acid and ammonia, as well as reactive oxygen species. In small doses, these products cause mild irritation, but inhalation of large amounts of chloramines can lead to pneumonitis (inflammation of the lungs) and may require medical intervention.
After it is formed, dichloramine readily enters the gas phase, where it may be inhaled. In the lungs, dichloramine reacts with water to produce hydrochloric acid and ammonia, as well as reactive oxygen species. In small doses, these products cause mild irritation, but inhalation of large amounts of chloramines can lead to pneumonitis (inflammation of the lungs) and may require medical intervention.
Adapted from Trogolo D, Arey JS. Benchmark thermochemistry of chloramines, bromamines, and bromochloramines: halogen oxidants stabilized by electron correlation. Phys Chem Chem Phys. 2015;17(5):3584-98.
Based on the information given in the passage, which of the following is the standard molar enthalpy of formation for sodium hydroxide?
A)−590 kJ/mol
B)−470 kJ/mol
C)−940 kJ/mol
D)275 kJ/mol
Household cleaners commonly contain either ammonia (NH3) or bleach (NaOCl) as the principal active ingredient. Mixing ammonia-based and bleach-based cleaners can be hazardous, as the resulting reactions can form several toxic products. One of these reactions forms the irritant dichloramine (NHCl2) as follows:
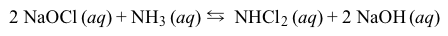 2 NaOClaq+NH3aq⇆ NHCl2aq+2 NaOHaqReaction 1The overall reaction has a standard enthalpy change of −55 kJ/mol and a standard entropy change of −150 J/mol·K. Partial thermodynamic data for formation of Reaction 1 reagents from their elements are shown in Table 1.Table 1 Partial Data for Enthalpies, Entropies, and Free Energies of Formation of Several Compounds at 25 °C
2 NaOClaq+NH3aq⇆ NHCl2aq+2 NaOHaqReaction 1The overall reaction has a standard enthalpy change of −55 kJ/mol and a standard entropy change of −150 J/mol·K. Partial thermodynamic data for formation of Reaction 1 reagents from their elements are shown in Table 1.Table 1 Partial Data for Enthalpies, Entropies, and Free Energies of Formation of Several Compounds at 25 °C After it is formed, dichloramine readily enters the gas phase, where it may be inhaled. In the lungs, dichloramine reacts with water to produce hydrochloric acid and ammonia, as well as reactive oxygen species. In small doses, these products cause mild irritation, but inhalation of large amounts of chloramines can lead to pneumonitis (inflammation of the lungs) and may require medical intervention.
After it is formed, dichloramine readily enters the gas phase, where it may be inhaled. In the lungs, dichloramine reacts with water to produce hydrochloric acid and ammonia, as well as reactive oxygen species. In small doses, these products cause mild irritation, but inhalation of large amounts of chloramines can lead to pneumonitis (inflammation of the lungs) and may require medical intervention.Adapted from Trogolo D, Arey JS. Benchmark thermochemistry of chloramines, bromamines, and bromochloramines: halogen oxidants stabilized by electron correlation. Phys Chem Chem Phys. 2015;17(5):3584-98.
Based on the information given in the passage, which of the following is the standard molar enthalpy of formation for sodium hydroxide?
A)−590 kJ/mol
B)−470 kJ/mol
C)−940 kJ/mol
D)275 kJ/mol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
71
Passage
An automated external defibrillator (AED) is a medical device used to send an electric shock to the heart after cardiac arrest. A key component of the AED is the power source, or battery. Batteries used in AEDs need to have a good charge-to-weight ratio; they must be safe and reliable as well as rechargeable.Two types of rechargeable batteries used in early models of AEDs are the lead storage (also called lead-acid) battery and the nickel-cadmium (NiCd) battery. These batteries consist of multiple electrochemical cells that are connected in series to deliver a potential between 9 V and 18 V. A capacitor allows the AED to accumulate charge so that it can deliver between 300 V and 1,000 V.Some AEDs use a sealed lead storage battery. Lead storage batteries are robust and hold a charge for a long time. However, they have a low energy-to-weight ratio. Each lead storage cell delivers approximately 2.0 V, and a battery of four cells weighing 1000 g can provide 30 W∙h of energy.
 Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2:
Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2:
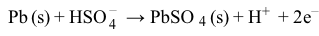 Reaction 1
Reaction 1
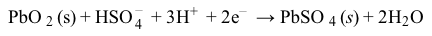 Reaction 2
Reaction 2
NiCd batteries have a higher energy-to-weight ratio than lead storage batteries but cannot hold as much charge. Each NiCd cell delivers approximately 1.3 V, and a single-cell battery weighing 120 g can provide 7.2 W∙h of energy.The half reactions at the anode and cathode for a NiCd battery in KOH are shown in Reactions 3 and 4:
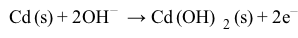 Reaction 3
Reaction 3
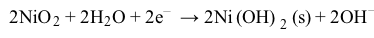 Reaction 4
Reaction 4
If the electromotive force of the battery in an AED is found to be −2.0 V while it is charging, the battery is functioning as a:
A)galvanic cell.
B)electrolytic cell.
C)concentration cell.
D)fuel cell.
An automated external defibrillator (AED) is a medical device used to send an electric shock to the heart after cardiac arrest. A key component of the AED is the power source, or battery. Batteries used in AEDs need to have a good charge-to-weight ratio; they must be safe and reliable as well as rechargeable.Two types of rechargeable batteries used in early models of AEDs are the lead storage (also called lead-acid) battery and the nickel-cadmium (NiCd) battery. These batteries consist of multiple electrochemical cells that are connected in series to deliver a potential between 9 V and 18 V. A capacitor allows the AED to accumulate charge so that it can deliver between 300 V and 1,000 V.Some AEDs use a sealed lead storage battery. Lead storage batteries are robust and hold a charge for a long time. However, they have a low energy-to-weight ratio. Each lead storage cell delivers approximately 2.0 V, and a battery of four cells weighing 1000 g can provide 30 W∙h of energy.
 Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2:
Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2: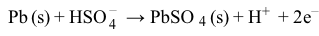 Reaction 1
Reaction 1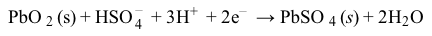 Reaction 2
Reaction 2NiCd batteries have a higher energy-to-weight ratio than lead storage batteries but cannot hold as much charge. Each NiCd cell delivers approximately 1.3 V, and a single-cell battery weighing 120 g can provide 7.2 W∙h of energy.The half reactions at the anode and cathode for a NiCd battery in KOH are shown in Reactions 3 and 4:
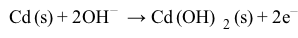 Reaction 3
Reaction 3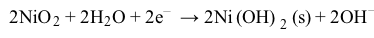 Reaction 4
Reaction 4If the electromotive force of the battery in an AED is found to be −2.0 V while it is charging, the battery is functioning as a:
A)galvanic cell.
B)electrolytic cell.
C)concentration cell.
D)fuel cell.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
72
Passage
Kidney stones are a common ailment affecting approximately 10% of adults in the United States. They form when solutes precipitate out of solution as crystals in the urinary tract, and they can cause severe pain in the side, back, abdomen, and groin. Individuals who have been previously diagnosed with kidney stones have an increased probability of developing new stones relative to unaffected individuals. Different measures may help prevent the formation of different kinds of stones, so analysis of the composition of stones that have been passed or removed can aid in preventing recurrence. Stones can be ground into fine powders, dissolved in a small amount of solvent, and analyzed by infrared (IR) spectroscopy, as shown in Figure 1.
 Figure 1 Schematic of kidney stone analysis by IR spectroscopyIR analysis of kidney stones from 50 individuals revealed the percentage of stones that contain each component, shown in Table 1 along with solubility data.Table 1 Kidney Stone Composition Parameters
Figure 1 Schematic of kidney stone analysis by IR spectroscopyIR analysis of kidney stones from 50 individuals revealed the percentage of stones that contain each component, shown in Table 1 along with solubility data.Table 1 Kidney Stone Composition Parameters
 Some studies indicate that potassium citrate, taken orally, may prevent the formation of calcium oxalate crystals, the most abundant component of kidney stones. Oxalic acid, shown in Figure 2, is significantly more soluble than calcium oxalate.
Some studies indicate that potassium citrate, taken orally, may prevent the formation of calcium oxalate crystals, the most abundant component of kidney stones. Oxalic acid, shown in Figure 2, is significantly more soluble than calcium oxalate.
 Figure 2 Structure of oxalic acid and its associated anions with increasing pHPotassium citrate alkalinizes the urine, potentially causing a decrease in oxalate solubility and the formation of more crystals. However, potassium citrate can also react with calcium oxalate according to the unbalanced equation shown in Reaction 1:CaC2O4 + K3(C6H5O7) → Ca3(C6H5O7)2 + K2C2O4Reaction 1Calcium citrate and potassium oxalate are both hundreds of times more soluble than calcium oxalate, so the presence of citrate and potassium ions can help maintain calcium and oxalate ions in solution. This effect may be sufficient to overcome the decreased solubility that occurs at higher pH levels.
Figure 2 Structure of oxalic acid and its associated anions with increasing pHPotassium citrate alkalinizes the urine, potentially causing a decrease in oxalate solubility and the formation of more crystals. However, potassium citrate can also react with calcium oxalate according to the unbalanced equation shown in Reaction 1:CaC2O4 + K3(C6H5O7) → Ca3(C6H5O7)2 + K2C2O4Reaction 1Calcium citrate and potassium oxalate are both hundreds of times more soluble than calcium oxalate, so the presence of citrate and potassium ions can help maintain calcium and oxalate ions in solution. This effect may be sufficient to overcome the decreased solubility that occurs at higher pH levels.
Adapted from Primiano A, Persichilli S, Gambaro G, et al. FT-IR analysis of urinary stones: a helpful tool for clinician comparison with the chemical spot test. Dis Markers. 2014;2014:176165.
Consider the trends in atomic radii on the periodic table. The bond in citrate that is the longest and weakest with the lowest bond dissociation energy is the:
A)C-H bond.
B)C=O bond.
C)O-H bond.
D)C-C bond.
Kidney stones are a common ailment affecting approximately 10% of adults in the United States. They form when solutes precipitate out of solution as crystals in the urinary tract, and they can cause severe pain in the side, back, abdomen, and groin. Individuals who have been previously diagnosed with kidney stones have an increased probability of developing new stones relative to unaffected individuals. Different measures may help prevent the formation of different kinds of stones, so analysis of the composition of stones that have been passed or removed can aid in preventing recurrence. Stones can be ground into fine powders, dissolved in a small amount of solvent, and analyzed by infrared (IR) spectroscopy, as shown in Figure 1.
 Figure 1 Schematic of kidney stone analysis by IR spectroscopyIR analysis of kidney stones from 50 individuals revealed the percentage of stones that contain each component, shown in Table 1 along with solubility data.Table 1 Kidney Stone Composition Parameters
Figure 1 Schematic of kidney stone analysis by IR spectroscopyIR analysis of kidney stones from 50 individuals revealed the percentage of stones that contain each component, shown in Table 1 along with solubility data.Table 1 Kidney Stone Composition Parameters Some studies indicate that potassium citrate, taken orally, may prevent the formation of calcium oxalate crystals, the most abundant component of kidney stones. Oxalic acid, shown in Figure 2, is significantly more soluble than calcium oxalate.
Some studies indicate that potassium citrate, taken orally, may prevent the formation of calcium oxalate crystals, the most abundant component of kidney stones. Oxalic acid, shown in Figure 2, is significantly more soluble than calcium oxalate. Figure 2 Structure of oxalic acid and its associated anions with increasing pHPotassium citrate alkalinizes the urine, potentially causing a decrease in oxalate solubility and the formation of more crystals. However, potassium citrate can also react with calcium oxalate according to the unbalanced equation shown in Reaction 1:CaC2O4 + K3(C6H5O7) → Ca3(C6H5O7)2 + K2C2O4Reaction 1Calcium citrate and potassium oxalate are both hundreds of times more soluble than calcium oxalate, so the presence of citrate and potassium ions can help maintain calcium and oxalate ions in solution. This effect may be sufficient to overcome the decreased solubility that occurs at higher pH levels.
Figure 2 Structure of oxalic acid and its associated anions with increasing pHPotassium citrate alkalinizes the urine, potentially causing a decrease in oxalate solubility and the formation of more crystals. However, potassium citrate can also react with calcium oxalate according to the unbalanced equation shown in Reaction 1:CaC2O4 + K3(C6H5O7) → Ca3(C6H5O7)2 + K2C2O4Reaction 1Calcium citrate and potassium oxalate are both hundreds of times more soluble than calcium oxalate, so the presence of citrate and potassium ions can help maintain calcium and oxalate ions in solution. This effect may be sufficient to overcome the decreased solubility that occurs at higher pH levels.Adapted from Primiano A, Persichilli S, Gambaro G, et al. FT-IR analysis of urinary stones: a helpful tool for clinician comparison with the chemical spot test. Dis Markers. 2014;2014:176165.
Consider the trends in atomic radii on the periodic table. The bond in citrate that is the longest and weakest with the lowest bond dissociation energy is the:
A)C-H bond.
B)C=O bond.
C)O-H bond.
D)C-C bond.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
73
Passage
Household cleaners commonly contain either ammonia (NH3) or bleach (NaOCl) as the principal active ingredient. Mixing ammonia-based and bleach-based cleaners can be hazardous, as the resulting reactions can form several toxic products. One of these reactions forms the irritant dichloramine (NHCl2) as follows:
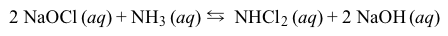 2 NaOClaq+NH3aq⇆ NHCl2aq+2 NaOHaqReaction 1The overall reaction has a standard enthalpy change of −55 kJ/mol and a standard entropy change of −150 J/mol·K. Partial thermodynamic data for formation of Reaction 1 reagents from their elements are shown in Table 1.Table 1 Partial Data for Enthalpies, Entropies, and Free Energies of Formation of Several Compounds at 25 °C
2 NaOClaq+NH3aq⇆ NHCl2aq+2 NaOHaqReaction 1The overall reaction has a standard enthalpy change of −55 kJ/mol and a standard entropy change of −150 J/mol·K. Partial thermodynamic data for formation of Reaction 1 reagents from their elements are shown in Table 1.Table 1 Partial Data for Enthalpies, Entropies, and Free Energies of Formation of Several Compounds at 25 °C
 After it is formed, dichloramine readily enters the gas phase, where it may be inhaled. In the lungs, dichloramine reacts with water to produce hydrochloric acid and ammonia, as well as reactive oxygen species. In small doses, these products cause mild irritation, but inhalation of large amounts of chloramines can lead to pneumonitis (inflammation of the lungs) and may require medical intervention.
After it is formed, dichloramine readily enters the gas phase, where it may be inhaled. In the lungs, dichloramine reacts with water to produce hydrochloric acid and ammonia, as well as reactive oxygen species. In small doses, these products cause mild irritation, but inhalation of large amounts of chloramines can lead to pneumonitis (inflammation of the lungs) and may require medical intervention.
Adapted from Trogolo D, Arey JS. Benchmark thermochemistry of chloramines, bromamines, and bromochloramines: halogen oxidants stabilized by electron correlation. Phys Chem Chem Phys. 2015;17(5):3584-98.
During the formation of dichloramine and NaOH, the order (organization) of the system:
A)decreases because entropy is always increasing.
B)stays the same because the number of moles stays the same.
C)increases because the change in entropy is negative.
D)does not change because the system is at equilibrium.
Household cleaners commonly contain either ammonia (NH3) or bleach (NaOCl) as the principal active ingredient. Mixing ammonia-based and bleach-based cleaners can be hazardous, as the resulting reactions can form several toxic products. One of these reactions forms the irritant dichloramine (NHCl2) as follows:
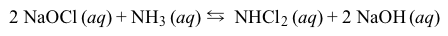 2 NaOClaq+NH3aq⇆ NHCl2aq+2 NaOHaqReaction 1The overall reaction has a standard enthalpy change of −55 kJ/mol and a standard entropy change of −150 J/mol·K. Partial thermodynamic data for formation of Reaction 1 reagents from their elements are shown in Table 1.Table 1 Partial Data for Enthalpies, Entropies, and Free Energies of Formation of Several Compounds at 25 °C
2 NaOClaq+NH3aq⇆ NHCl2aq+2 NaOHaqReaction 1The overall reaction has a standard enthalpy change of −55 kJ/mol and a standard entropy change of −150 J/mol·K. Partial thermodynamic data for formation of Reaction 1 reagents from their elements are shown in Table 1.Table 1 Partial Data for Enthalpies, Entropies, and Free Energies of Formation of Several Compounds at 25 °C After it is formed, dichloramine readily enters the gas phase, where it may be inhaled. In the lungs, dichloramine reacts with water to produce hydrochloric acid and ammonia, as well as reactive oxygen species. In small doses, these products cause mild irritation, but inhalation of large amounts of chloramines can lead to pneumonitis (inflammation of the lungs) and may require medical intervention.
After it is formed, dichloramine readily enters the gas phase, where it may be inhaled. In the lungs, dichloramine reacts with water to produce hydrochloric acid and ammonia, as well as reactive oxygen species. In small doses, these products cause mild irritation, but inhalation of large amounts of chloramines can lead to pneumonitis (inflammation of the lungs) and may require medical intervention.Adapted from Trogolo D, Arey JS. Benchmark thermochemistry of chloramines, bromamines, and bromochloramines: halogen oxidants stabilized by electron correlation. Phys Chem Chem Phys. 2015;17(5):3584-98.
During the formation of dichloramine and NaOH, the order (organization) of the system:
A)decreases because entropy is always increasing.
B)stays the same because the number of moles stays the same.
C)increases because the change in entropy is negative.
D)does not change because the system is at equilibrium.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
74
Passage
Combustion occurs when an oxidation-reduction reaction takes place between a reduced fuel source and an oxidizer, most frequently oxygen. The reaction between hydrogen and oxygen forms water (Reaction 1) and is highly exothermic and thermodynamically favorable. At the completion of hydrogen combustion, essentially all H2 molecules have been converted to water. Nevertheless, at room temperature and atmospheric pressure, molecular hydrogen and oxygen can coexist quite stably.2 H2 + O2 → 2 H2OReaction 1A group of researchers designed two prototype internal combustion engines, each of which could inject a hydrogen/oxygen mixture into a 5-mL combustion chamber at a total pressure of 1 atm and could repeat this process for several cycles. In one engine design, a spark was generated to initiate combustion as hydrogen and oxygen were injected. In another method, the combustion chamber was coated with a small amount of platinum powder. Platinum dramatically increases the reaction rate at room temperature without the need for a spark. The platinum itself is not altered by the reaction and can be reused. Researchers measured the maximum power output of both engine designs under identical injection and temperature conditions (Figure 1).
 Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2.
Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2.
 Figure 2 Rate of combustion in platinum-coated engines under various conditions
Figure 2 Rate of combustion in platinum-coated engines under various conditions
Adapted from Schultze M, Mantzaras J. Hetero-/homogeneous combustion of hydrogen/air mixtures over platinum: Fuel-lean versus fuel-rich combustion modes doi: 10.1016/j.ijhydene.2013.06.069
At a given temperature and injection composition, if researchers allowed each engine to carry out only one complete combustion reaction, which engine would produce more water?
A)The spark engine would produce more water because it has a lower power output.
B)The platinum engine would produce more water because it has a higher rate of combustion.
C)Both engines would produce the same amount because the equilibrium constant is the same for both.
D)Cannot be determined with the available information.
Combustion occurs when an oxidation-reduction reaction takes place between a reduced fuel source and an oxidizer, most frequently oxygen. The reaction between hydrogen and oxygen forms water (Reaction 1) and is highly exothermic and thermodynamically favorable. At the completion of hydrogen combustion, essentially all H2 molecules have been converted to water. Nevertheless, at room temperature and atmospheric pressure, molecular hydrogen and oxygen can coexist quite stably.2 H2 + O2 → 2 H2OReaction 1A group of researchers designed two prototype internal combustion engines, each of which could inject a hydrogen/oxygen mixture into a 5-mL combustion chamber at a total pressure of 1 atm and could repeat this process for several cycles. In one engine design, a spark was generated to initiate combustion as hydrogen and oxygen were injected. In another method, the combustion chamber was coated with a small amount of platinum powder. Platinum dramatically increases the reaction rate at room temperature without the need for a spark. The platinum itself is not altered by the reaction and can be reused. Researchers measured the maximum power output of both engine designs under identical injection and temperature conditions (Figure 1).
 Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2.
Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2. Figure 2 Rate of combustion in platinum-coated engines under various conditions
Figure 2 Rate of combustion in platinum-coated engines under various conditionsAdapted from Schultze M, Mantzaras J. Hetero-/homogeneous combustion of hydrogen/air mixtures over platinum: Fuel-lean versus fuel-rich combustion modes doi: 10.1016/j.ijhydene.2013.06.069
At a given temperature and injection composition, if researchers allowed each engine to carry out only one complete combustion reaction, which engine would produce more water?
A)The spark engine would produce more water because it has a lower power output.
B)The platinum engine would produce more water because it has a higher rate of combustion.
C)Both engines would produce the same amount because the equilibrium constant is the same for both.
D)Cannot be determined with the available information.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
75
Passage
Household cleaners commonly contain either ammonia (NH3) or bleach (NaOCl) as the principal active ingredient. Mixing ammonia-based and bleach-based cleaners can be hazardous, as the resulting reactions can form several toxic products. One of these reactions forms the irritant dichloramine (NHCl2) as follows:
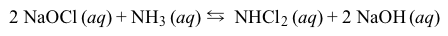 2 NaOClaq+NH3aq⇆ NHCl2aq+2 NaOHaqReaction 1The overall reaction has a standard enthalpy change of −55 kJ/mol and a standard entropy change of −150 J/mol·K. Partial thermodynamic data for formation of Reaction 1 reagents from their elements are shown in Table 1.Table 1 Partial Data for Enthalpies, Entropies, and Free Energies of Formation of Several Compounds at 25 °C
2 NaOClaq+NH3aq⇆ NHCl2aq+2 NaOHaqReaction 1The overall reaction has a standard enthalpy change of −55 kJ/mol and a standard entropy change of −150 J/mol·K. Partial thermodynamic data for formation of Reaction 1 reagents from their elements are shown in Table 1.Table 1 Partial Data for Enthalpies, Entropies, and Free Energies of Formation of Several Compounds at 25 °C
 After it is formed, dichloramine readily enters the gas phase, where it may be inhaled. In the lungs, dichloramine reacts with water to produce hydrochloric acid and ammonia, as well as reactive oxygen species. In small doses, these products cause mild irritation, but inhalation of large amounts of chloramines can lead to pneumonitis (inflammation of the lungs) and may require medical intervention.
After it is formed, dichloramine readily enters the gas phase, where it may be inhaled. In the lungs, dichloramine reacts with water to produce hydrochloric acid and ammonia, as well as reactive oxygen species. In small doses, these products cause mild irritation, but inhalation of large amounts of chloramines can lead to pneumonitis (inflammation of the lungs) and may require medical intervention.
Adapted from Trogolo D, Arey JS. Benchmark thermochemistry of chloramines, bromamines, and bromochloramines: halogen oxidants stabilized by electron correlation. Phys Chem Chem Phys. 2015;17(5):3584-98.
Assuming ΔH° and ΔS° are constant at all temperatures, at approximately which temperature (in degrees Celsius) will ΔG° of Reaction 1 equal zero (ie, switch from spontaneous to nonspontaneous)?
A)95°C
B)370°C
C)−270°C
D)−640°C
Household cleaners commonly contain either ammonia (NH3) or bleach (NaOCl) as the principal active ingredient. Mixing ammonia-based and bleach-based cleaners can be hazardous, as the resulting reactions can form several toxic products. One of these reactions forms the irritant dichloramine (NHCl2) as follows:
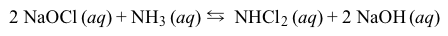 2 NaOClaq+NH3aq⇆ NHCl2aq+2 NaOHaqReaction 1The overall reaction has a standard enthalpy change of −55 kJ/mol and a standard entropy change of −150 J/mol·K. Partial thermodynamic data for formation of Reaction 1 reagents from their elements are shown in Table 1.Table 1 Partial Data for Enthalpies, Entropies, and Free Energies of Formation of Several Compounds at 25 °C
2 NaOClaq+NH3aq⇆ NHCl2aq+2 NaOHaqReaction 1The overall reaction has a standard enthalpy change of −55 kJ/mol and a standard entropy change of −150 J/mol·K. Partial thermodynamic data for formation of Reaction 1 reagents from their elements are shown in Table 1.Table 1 Partial Data for Enthalpies, Entropies, and Free Energies of Formation of Several Compounds at 25 °C After it is formed, dichloramine readily enters the gas phase, where it may be inhaled. In the lungs, dichloramine reacts with water to produce hydrochloric acid and ammonia, as well as reactive oxygen species. In small doses, these products cause mild irritation, but inhalation of large amounts of chloramines can lead to pneumonitis (inflammation of the lungs) and may require medical intervention.
After it is formed, dichloramine readily enters the gas phase, where it may be inhaled. In the lungs, dichloramine reacts with water to produce hydrochloric acid and ammonia, as well as reactive oxygen species. In small doses, these products cause mild irritation, but inhalation of large amounts of chloramines can lead to pneumonitis (inflammation of the lungs) and may require medical intervention.Adapted from Trogolo D, Arey JS. Benchmark thermochemistry of chloramines, bromamines, and bromochloramines: halogen oxidants stabilized by electron correlation. Phys Chem Chem Phys. 2015;17(5):3584-98.
Assuming ΔH° and ΔS° are constant at all temperatures, at approximately which temperature (in degrees Celsius) will ΔG° of Reaction 1 equal zero (ie, switch from spontaneous to nonspontaneous)?
A)95°C
B)370°C
C)−270°C
D)−640°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
76
Passage
Kidney stones are a common ailment affecting approximately 10% of adults in the United States. They form when solutes precipitate out of solution as crystals in the urinary tract, and they can cause severe pain in the side, back, abdomen, and groin. Individuals who have been previously diagnosed with kidney stones have an increased probability of developing new stones relative to unaffected individuals. Different measures may help prevent the formation of different kinds of stones, so analysis of the composition of stones that have been passed or removed can aid in preventing recurrence. Stones can be ground into fine powders, dissolved in a small amount of solvent, and analyzed by infrared (IR) spectroscopy, as shown in Figure 1.
 Figure 1 Schematic of kidney stone analysis by IR spectroscopyIR analysis of kidney stones from 50 individuals revealed the percentage of stones that contain each component, shown in Table 1 along with solubility data.Table 1 Kidney Stone Composition Parameters
Figure 1 Schematic of kidney stone analysis by IR spectroscopyIR analysis of kidney stones from 50 individuals revealed the percentage of stones that contain each component, shown in Table 1 along with solubility data.Table 1 Kidney Stone Composition Parameters
 Some studies indicate that potassium citrate, taken orally, may prevent the formation of calcium oxalate crystals, the most abundant component of kidney stones. Oxalic acid, shown in Figure 2, is significantly more soluble than calcium oxalate.
Some studies indicate that potassium citrate, taken orally, may prevent the formation of calcium oxalate crystals, the most abundant component of kidney stones. Oxalic acid, shown in Figure 2, is significantly more soluble than calcium oxalate.
 Figure 2 Structure of oxalic acid and its associated anions with increasing pHPotassium citrate alkalinizes the urine, potentially causing a decrease in oxalate solubility and the formation of more crystals. However, potassium citrate can also react with calcium oxalate according to the unbalanced equation shown in Reaction 1:CaC2O4 + K3(C6H5O7) → Ca3(C6H5O7)2 + K2C2O4Reaction 1Calcium citrate and potassium oxalate are both hundreds of times more soluble than calcium oxalate, so the presence of citrate and potassium ions can help maintain calcium and oxalate ions in solution. This effect may be sufficient to overcome the decreased solubility that occurs at higher pH levels.
Figure 2 Structure of oxalic acid and its associated anions with increasing pHPotassium citrate alkalinizes the urine, potentially causing a decrease in oxalate solubility and the formation of more crystals. However, potassium citrate can also react with calcium oxalate according to the unbalanced equation shown in Reaction 1:CaC2O4 + K3(C6H5O7) → Ca3(C6H5O7)2 + K2C2O4Reaction 1Calcium citrate and potassium oxalate are both hundreds of times more soluble than calcium oxalate, so the presence of citrate and potassium ions can help maintain calcium and oxalate ions in solution. This effect may be sufficient to overcome the decreased solubility that occurs at higher pH levels.
Adapted from Primiano A, Persichilli S, Gambaro G, et al. FT-IR analysis of urinary stones: a helpful tool for clinician comparison with the chemical spot test. Dis Markers. 2014;2014:176165.
At which pH would oxalate adopt the greatest net dipole moment?
A)1
B)3
C)5
D)7
Kidney stones are a common ailment affecting approximately 10% of adults in the United States. They form when solutes precipitate out of solution as crystals in the urinary tract, and they can cause severe pain in the side, back, abdomen, and groin. Individuals who have been previously diagnosed with kidney stones have an increased probability of developing new stones relative to unaffected individuals. Different measures may help prevent the formation of different kinds of stones, so analysis of the composition of stones that have been passed or removed can aid in preventing recurrence. Stones can be ground into fine powders, dissolved in a small amount of solvent, and analyzed by infrared (IR) spectroscopy, as shown in Figure 1.
 Figure 1 Schematic of kidney stone analysis by IR spectroscopyIR analysis of kidney stones from 50 individuals revealed the percentage of stones that contain each component, shown in Table 1 along with solubility data.Table 1 Kidney Stone Composition Parameters
Figure 1 Schematic of kidney stone analysis by IR spectroscopyIR analysis of kidney stones from 50 individuals revealed the percentage of stones that contain each component, shown in Table 1 along with solubility data.Table 1 Kidney Stone Composition Parameters Some studies indicate that potassium citrate, taken orally, may prevent the formation of calcium oxalate crystals, the most abundant component of kidney stones. Oxalic acid, shown in Figure 2, is significantly more soluble than calcium oxalate.
Some studies indicate that potassium citrate, taken orally, may prevent the formation of calcium oxalate crystals, the most abundant component of kidney stones. Oxalic acid, shown in Figure 2, is significantly more soluble than calcium oxalate. Figure 2 Structure of oxalic acid and its associated anions with increasing pHPotassium citrate alkalinizes the urine, potentially causing a decrease in oxalate solubility and the formation of more crystals. However, potassium citrate can also react with calcium oxalate according to the unbalanced equation shown in Reaction 1:CaC2O4 + K3(C6H5O7) → Ca3(C6H5O7)2 + K2C2O4Reaction 1Calcium citrate and potassium oxalate are both hundreds of times more soluble than calcium oxalate, so the presence of citrate and potassium ions can help maintain calcium and oxalate ions in solution. This effect may be sufficient to overcome the decreased solubility that occurs at higher pH levels.
Figure 2 Structure of oxalic acid and its associated anions with increasing pHPotassium citrate alkalinizes the urine, potentially causing a decrease in oxalate solubility and the formation of more crystals. However, potassium citrate can also react with calcium oxalate according to the unbalanced equation shown in Reaction 1:CaC2O4 + K3(C6H5O7) → Ca3(C6H5O7)2 + K2C2O4Reaction 1Calcium citrate and potassium oxalate are both hundreds of times more soluble than calcium oxalate, so the presence of citrate and potassium ions can help maintain calcium and oxalate ions in solution. This effect may be sufficient to overcome the decreased solubility that occurs at higher pH levels.Adapted from Primiano A, Persichilli S, Gambaro G, et al. FT-IR analysis of urinary stones: a helpful tool for clinician comparison with the chemical spot test. Dis Markers. 2014;2014:176165.
At which pH would oxalate adopt the greatest net dipole moment?
A)1
B)3
C)5
D)7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
77
Passage
Combustion occurs when an oxidation-reduction reaction takes place between a reduced fuel source and an oxidizer, most frequently oxygen. The reaction between hydrogen and oxygen forms water (Reaction 1) and is highly exothermic and thermodynamically favorable. At the completion of hydrogen combustion, essentially all H2 molecules have been converted to water. Nevertheless, at room temperature and atmospheric pressure, molecular hydrogen and oxygen can coexist quite stably.2 H2 + O2 → 2 H2OReaction 1A group of researchers designed two prototype internal combustion engines, each of which could inject a hydrogen/oxygen mixture into a 5-mL combustion chamber at a total pressure of 1 atm and could repeat this process for several cycles. In one engine design, a spark was generated to initiate combustion as hydrogen and oxygen were injected. In another method, the combustion chamber was coated with a small amount of platinum powder. Platinum dramatically increases the reaction rate at room temperature without the need for a spark. The platinum itself is not altered by the reaction and can be reused. Researchers measured the maximum power output of both engine designs under identical injection and temperature conditions (Figure 1).
 Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2.
Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2.
 Figure 2 Rate of combustion in platinum-coated engines under various conditions
Figure 2 Rate of combustion in platinum-coated engines under various conditions
Adapted from Schultze M, Mantzaras J. Hetero-/homogeneous combustion of hydrogen/air mixtures over platinum: Fuel-lean versus fuel-rich combustion modes doi: 10.1016/j.ijhydene.2013.06.069
In the absence of platinum, a spark initiates combustion by doing which of the following?
A)It shifts the reaction equilibrium toward water formation.
B)It consumes oxygen, driving the reaction toward the products.
C)It increases the average kinetic energy of nearby molecules.
D)It removes electrons from oxygen to form reactive oxygen species.
Combustion occurs when an oxidation-reduction reaction takes place between a reduced fuel source and an oxidizer, most frequently oxygen. The reaction between hydrogen and oxygen forms water (Reaction 1) and is highly exothermic and thermodynamically favorable. At the completion of hydrogen combustion, essentially all H2 molecules have been converted to water. Nevertheless, at room temperature and atmospheric pressure, molecular hydrogen and oxygen can coexist quite stably.2 H2 + O2 → 2 H2OReaction 1A group of researchers designed two prototype internal combustion engines, each of which could inject a hydrogen/oxygen mixture into a 5-mL combustion chamber at a total pressure of 1 atm and could repeat this process for several cycles. In one engine design, a spark was generated to initiate combustion as hydrogen and oxygen were injected. In another method, the combustion chamber was coated with a small amount of platinum powder. Platinum dramatically increases the reaction rate at room temperature without the need for a spark. The platinum itself is not altered by the reaction and can be reused. Researchers measured the maximum power output of both engine designs under identical injection and temperature conditions (Figure 1).
 Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2.
Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2. Figure 2 Rate of combustion in platinum-coated engines under various conditions
Figure 2 Rate of combustion in platinum-coated engines under various conditionsAdapted from Schultze M, Mantzaras J. Hetero-/homogeneous combustion of hydrogen/air mixtures over platinum: Fuel-lean versus fuel-rich combustion modes doi: 10.1016/j.ijhydene.2013.06.069
In the absence of platinum, a spark initiates combustion by doing which of the following?
A)It shifts the reaction equilibrium toward water formation.
B)It consumes oxygen, driving the reaction toward the products.
C)It increases the average kinetic energy of nearby molecules.
D)It removes electrons from oxygen to form reactive oxygen species.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
78
Passage
Combustion occurs when an oxidation-reduction reaction takes place between a reduced fuel source and an oxidizer, most frequently oxygen. The reaction between hydrogen and oxygen forms water (Reaction 1) and is highly exothermic and thermodynamically favorable. At the completion of hydrogen combustion, essentially all H2 molecules have been converted to water. Nevertheless, at room temperature and atmospheric pressure, molecular hydrogen and oxygen can coexist quite stably.2 H2 + O2 → 2 H2OReaction 1A group of researchers designed two prototype internal combustion engines, each of which could inject a hydrogen/oxygen mixture into a 5-mL combustion chamber at a total pressure of 1 atm and could repeat this process for several cycles. In one engine design, a spark was generated to initiate combustion as hydrogen and oxygen were injected. In another method, the combustion chamber was coated with a small amount of platinum powder. Platinum dramatically increases the reaction rate at room temperature without the need for a spark. The platinum itself is not altered by the reaction and can be reused. Researchers measured the maximum power output of both engine designs under identical injection and temperature conditions (Figure 1).
 Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2.
Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2.
 Figure 2 Rate of combustion in platinum-coated engines under various conditions
Figure 2 Rate of combustion in platinum-coated engines under various conditions
Adapted from Schultze M, Mantzaras J. Hetero-/homogeneous combustion of hydrogen/air mixtures over platinum: Fuel-lean versus fuel-rich combustion modes doi: 10.1016/j.ijhydene.2013.06.069
Assuming the combustion of hydrogen behaves like an elementary reaction with respect to its rate, which of the following explains why an increase in the H2/O2 ratio causes an increase in the reaction rate?
A)Pauli exclusion principle
B)Law of mass action
C)Boyle's law
D)Henry's law
Combustion occurs when an oxidation-reduction reaction takes place between a reduced fuel source and an oxidizer, most frequently oxygen. The reaction between hydrogen and oxygen forms water (Reaction 1) and is highly exothermic and thermodynamically favorable. At the completion of hydrogen combustion, essentially all H2 molecules have been converted to water. Nevertheless, at room temperature and atmospheric pressure, molecular hydrogen and oxygen can coexist quite stably.2 H2 + O2 → 2 H2OReaction 1A group of researchers designed two prototype internal combustion engines, each of which could inject a hydrogen/oxygen mixture into a 5-mL combustion chamber at a total pressure of 1 atm and could repeat this process for several cycles. In one engine design, a spark was generated to initiate combustion as hydrogen and oxygen were injected. In another method, the combustion chamber was coated with a small amount of platinum powder. Platinum dramatically increases the reaction rate at room temperature without the need for a spark. The platinum itself is not altered by the reaction and can be reused. Researchers measured the maximum power output of both engine designs under identical injection and temperature conditions (Figure 1).
 Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2.
Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2. Figure 2 Rate of combustion in platinum-coated engines under various conditions
Figure 2 Rate of combustion in platinum-coated engines under various conditionsAdapted from Schultze M, Mantzaras J. Hetero-/homogeneous combustion of hydrogen/air mixtures over platinum: Fuel-lean versus fuel-rich combustion modes doi: 10.1016/j.ijhydene.2013.06.069
Assuming the combustion of hydrogen behaves like an elementary reaction with respect to its rate, which of the following explains why an increase in the H2/O2 ratio causes an increase in the reaction rate?
A)Pauli exclusion principle
B)Law of mass action
C)Boyle's law
D)Henry's law

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
79
Passage
Combustion occurs when an oxidation-reduction reaction takes place between a reduced fuel source and an oxidizer, most frequently oxygen. The reaction between hydrogen and oxygen forms water (Reaction 1) and is highly exothermic and thermodynamically favorable. At the completion of hydrogen combustion, essentially all H2 molecules have been converted to water. Nevertheless, at room temperature and atmospheric pressure, molecular hydrogen and oxygen can coexist quite stably.2 H2 + O2 → 2 H2OReaction 1A group of researchers designed two prototype internal combustion engines, each of which could inject a hydrogen/oxygen mixture into a 5-mL combustion chamber at a total pressure of 1 atm and could repeat this process for several cycles. In one engine design, a spark was generated to initiate combustion as hydrogen and oxygen were injected. In another method, the combustion chamber was coated with a small amount of platinum powder. Platinum dramatically increases the reaction rate at room temperature without the need for a spark. The platinum itself is not altered by the reaction and can be reused. Researchers measured the maximum power output of both engine designs under identical injection and temperature conditions (Figure 1).
 Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2.
Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2.
 Figure 2 Rate of combustion in platinum-coated engines under various conditions
Figure 2 Rate of combustion in platinum-coated engines under various conditions
Adapted from Schultze M, Mantzaras J. Hetero-/homogeneous combustion of hydrogen/air mixtures over platinum: Fuel-lean versus fuel-rich combustion modes doi: 10.1016/j.ijhydene.2013.06.069
Which of the following would most likely improve the power output of a platinum-coated combustion chamber?
A)Coating the chamber with finer platinum powder
B)Lining the chamber with larger platinum particles
C)Polishing the platinum on the chamber wall to make it smooth
D)Decreasing the amount of platinum to make more room for reactants
Combustion occurs when an oxidation-reduction reaction takes place between a reduced fuel source and an oxidizer, most frequently oxygen. The reaction between hydrogen and oxygen forms water (Reaction 1) and is highly exothermic and thermodynamically favorable. At the completion of hydrogen combustion, essentially all H2 molecules have been converted to water. Nevertheless, at room temperature and atmospheric pressure, molecular hydrogen and oxygen can coexist quite stably.2 H2 + O2 → 2 H2OReaction 1A group of researchers designed two prototype internal combustion engines, each of which could inject a hydrogen/oxygen mixture into a 5-mL combustion chamber at a total pressure of 1 atm and could repeat this process for several cycles. In one engine design, a spark was generated to initiate combustion as hydrogen and oxygen were injected. In another method, the combustion chamber was coated with a small amount of platinum powder. Platinum dramatically increases the reaction rate at room temperature without the need for a spark. The platinum itself is not altered by the reaction and can be reused. Researchers measured the maximum power output of both engine designs under identical injection and temperature conditions (Figure 1).
 Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2.
Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2. Figure 2 Rate of combustion in platinum-coated engines under various conditions
Figure 2 Rate of combustion in platinum-coated engines under various conditionsAdapted from Schultze M, Mantzaras J. Hetero-/homogeneous combustion of hydrogen/air mixtures over platinum: Fuel-lean versus fuel-rich combustion modes doi: 10.1016/j.ijhydene.2013.06.069
Which of the following would most likely improve the power output of a platinum-coated combustion chamber?
A)Coating the chamber with finer platinum powder
B)Lining the chamber with larger platinum particles
C)Polishing the platinum on the chamber wall to make it smooth
D)Decreasing the amount of platinum to make more room for reactants

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck
80
Passage
An automated external defibrillator (AED) is a medical device used to send an electric shock to the heart after cardiac arrest. A key component of the AED is the power source, or battery. Batteries used in AEDs need to have a good charge-to-weight ratio; they must be safe and reliable as well as rechargeable.Two types of rechargeable batteries used in early models of AEDs are the lead storage (also called lead-acid) battery and the nickel-cadmium (NiCd) battery. These batteries consist of multiple electrochemical cells that are connected in series to deliver a potential between 9 V and 18 V. A capacitor allows the AED to accumulate charge so that it can deliver between 300 V and 1,000 V.Some AEDs use a sealed lead storage battery. Lead storage batteries are robust and hold a charge for a long time. However, they have a low energy-to-weight ratio. Each lead storage cell delivers approximately 2.0 V, and a battery of four cells weighing 1000 g can provide 30 W∙h of energy.
 Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2:
Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2:
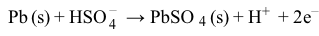 Reaction 1
Reaction 1
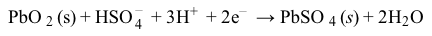 Reaction 2
Reaction 2
NiCd batteries have a higher energy-to-weight ratio than lead storage batteries but cannot hold as much charge. Each NiCd cell delivers approximately 1.3 V, and a single-cell battery weighing 120 g can provide 7.2 W∙h of energy.The half reactions at the anode and cathode for a NiCd battery in KOH are shown in Reactions 3 and 4:
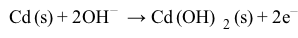 Reaction 3
Reaction 3
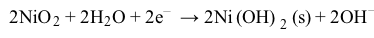 Reaction 4
Reaction 4
To charge the NiCd cell, the applied external potential must be:
A)equal to 1.3 V.
B)greater than 1.3 V.
C)equal to −1.3 V.
D)less than −1.3 V.
An automated external defibrillator (AED) is a medical device used to send an electric shock to the heart after cardiac arrest. A key component of the AED is the power source, or battery. Batteries used in AEDs need to have a good charge-to-weight ratio; they must be safe and reliable as well as rechargeable.Two types of rechargeable batteries used in early models of AEDs are the lead storage (also called lead-acid) battery and the nickel-cadmium (NiCd) battery. These batteries consist of multiple electrochemical cells that are connected in series to deliver a potential between 9 V and 18 V. A capacitor allows the AED to accumulate charge so that it can deliver between 300 V and 1,000 V.Some AEDs use a sealed lead storage battery. Lead storage batteries are robust and hold a charge for a long time. However, they have a low energy-to-weight ratio. Each lead storage cell delivers approximately 2.0 V, and a battery of four cells weighing 1000 g can provide 30 W∙h of energy.
 Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2:
Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2: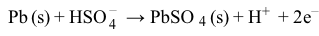 Reaction 1
Reaction 1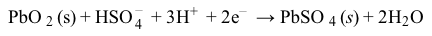 Reaction 2
Reaction 2NiCd batteries have a higher energy-to-weight ratio than lead storage batteries but cannot hold as much charge. Each NiCd cell delivers approximately 1.3 V, and a single-cell battery weighing 120 g can provide 7.2 W∙h of energy.The half reactions at the anode and cathode for a NiCd battery in KOH are shown in Reactions 3 and 4:
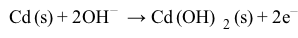 Reaction 3
Reaction 3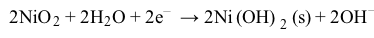 Reaction 4
Reaction 4To charge the NiCd cell, the applied external potential must be:
A)equal to 1.3 V.
B)greater than 1.3 V.
C)equal to −1.3 V.
D)less than −1.3 V.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 317 في هذه المجموعة.
فتح الحزمة
k this deck








