Deck 8: Binding in Transition Metal Compounds and Coordination Complexes
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/33
العب
ملء الشاشة (f)
Deck 8: Binding in Transition Metal Compounds and Coordination Complexes
1
Which would you expect to have a lower melting temperature CrF2 or CrF5?
A) CrF2
B) CrF5
C) they will be approximately the same
D) they will be exactly the same
A) CrF2
B) CrF5
C) they will be approximately the same
D) they will be exactly the same
B
2
What is the name of this compound, NbO2?
A) Niobium (II) oxide
B) Niobium (III) oxide
C) Niobium (IV) oxide
D) Oxygen Niobate
E) Dioxygen Niobate
A) Niobium (II) oxide
B) Niobium (III) oxide
C) Niobium (IV) oxide
D) Oxygen Niobate
E) Dioxygen Niobate
C
3
The oxylate ion is a 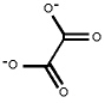
A) monodentate ligand.
B) bidentate ligand.
C) tridentate ligand.
D) tetradentate ligand.
E) will not act as a metal ligand.

A) monodentate ligand.
B) bidentate ligand.
C) tridentate ligand.
D) tetradentate ligand.
E) will not act as a metal ligand.
B
4
What is the oxidation state of Cr in Cr(CO)6?
A) 0
B) +1
C) +3
D) +6
E) -6
A) 0
B) +1
C) +3
D) +6
E) -6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
5
What is the chemical formula of pentaamminechloroplatinum(IV) bromide?
A) [Pt(NH3)5Cl]Br
B) [Pt(NH3)5]ClBr
C) [Pt5(NH3)Cl]Br
D) [Pt(NH3)5Cl]Br3
E) [Pt2(NH3)5Cl]Br
A) [Pt(NH3)5Cl]Br
B) [Pt(NH3)5]ClBr
C) [Pt5(NH3)Cl]Br
D) [Pt(NH3)5Cl]Br3
E) [Pt2(NH3)5Cl]Br

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
6
Name the following compound, Pt(H2NCH2CH2NH2)Cl4]Cl2
A) hexachloro(ethylenediamine)platinum (IV)
B) hexachloride(ethylenediamine)platinum (VI)
C) tetrachloro(ethylenediamine)platinum (IV) chloride
D) tetrachloro(ethylenediamine)platinum (VI) chloride
E) Platinum(ethylenediamine)hexachloride
A) hexachloro(ethylenediamine)platinum (IV)
B) hexachloride(ethylenediamine)platinum (VI)
C) tetrachloro(ethylenediamine)platinum (IV) chloride
D) tetrachloro(ethylenediamine)platinum (VI) chloride
E) Platinum(ethylenediamine)hexachloride

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
7
What is the chemical formula of potassium hexafluorocobaltate(III)?
A) [CoK]F6
B) K[CoF6]
C) K[CoF6]3
D) K3[CoF6]
E) K[Co3F6]
A) [CoK]F6
B) K[CoF6]
C) K[CoF6]3
D) K3[CoF6]
E) K[Co3F6]

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
8
Name the following compound, Na2[NiCl4].
A) disodium tetrachloronickel (II)
B) sodium tetrachloronickelate (II)
C) sodium tetrachloronickelate (IV)
D) sodium tetrachloronickel (II)
E) sodium nickel (II) chloride
A) disodium tetrachloronickel (II)
B) sodium tetrachloronickelate (II)
C) sodium tetrachloronickelate (IV)
D) sodium tetrachloronickel (II)
E) sodium nickel (II) chloride

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
9
How many isomers are there of the triamminetriaquachromium (III) ion (octahedral)?
A) 1
B) 2
C) 3
D) 4
E) 6
A) 1
B) 2
C) 3
D) 4
E) 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
10
How many isomers are there of trisoxaltochromate (III) ion?
A) 1
B) 2
C) 3
D) 4
E) 6
A) 1
B) 2
C) 3
D) 4
E) 6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
11
How many optical isomers are there for the square planar compound diaminedichloroplatinum (II)?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
12
What can you say about the following two compounds? 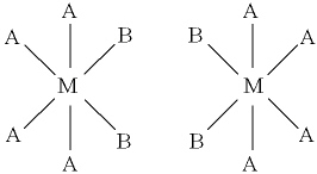
A) they are isomers
B) they are enantiomers
C) they are isomers and enantiomers
D) they are the identical compounds

A) they are isomers
B) they are enantiomers
C) they are isomers and enantiomers
D) they are the identical compounds

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
13
A Zn2+ ion in a strong octahedral field will have how many unpaired electrons?
A) 0
B) 2
C) 3
D) 4
E) 8
A) 0
B) 2
C) 3
D) 4
E) 8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
14
A Co2+ ion in a weak octahedral field will have how many unpaired electrons?
A) 0
B) 1
C) 2
D) 3
E) 7
A) 0
B) 1
C) 2
D) 3
E) 7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
15
For a Mn2+ ion the number of unpaired electrons in an octahedral compound will be
A) greater in a weak field.
B) greater in a strong field.
C) the same in weak and strong fields.
D) will never have unpaired electrons.
A) greater in a weak field.
B) greater in a strong field.
C) the same in weak and strong fields.
D) will never have unpaired electrons.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
16
Which of the following statements is true for crystal field theory of an octahedral complex?
A) The dxy, dyz, dxz orbitals are lower in energy than the dz2, and the dx2-y2 because they are unaffected by the ligand field.
B) The dxy, dyz, dxz orbitals are lower in energy than the dz2, and the dx2- because their energy is increased less than the dz2, and the dx2-y2.
C) in low spin complexes the dz2, and the dx2-y2 are lower in energy than the dxy, dyz, dxz orbitals.
D) a & c
E) b & c
A) The dxy, dyz, dxz orbitals are lower in energy than the dz2, and the dx2-y2 because they are unaffected by the ligand field.
B) The dxy, dyz, dxz orbitals are lower in energy than the dz2, and the dx2- because their energy is increased less than the dz2, and the dx2-y2.
C) in low spin complexes the dz2, and the dx2-y2 are lower in energy than the dxy, dyz, dxz orbitals.
D) a & c
E) b & c

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
17
What is the crystal field stabilization energy for a high spin Co2+ octahedral complex?
A) 0
B) -(2/5)Do
C) -(4/5)Do
D) -(7/5)Do
E) -(9/5)Do
A) 0
B) -(2/5)Do
C) -(4/5)Do
D) -(7/5)Do
E) -(9/5)Do

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
18
The octahedral compound [FeCl3(NH3)3] is found to be paramagnetic. This means
A) it must be a high spin complex.
B) it must be a low spin complex.
C) it could be either a high spin or low spin complex.
D) it has a crystal field stabilization that is zero.
E) a & d
A) it must be a high spin complex.
B) it must be a low spin complex.
C) it could be either a high spin or low spin complex.
D) it has a crystal field stabilization that is zero.
E) a & d

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
19
The octahedral complex [Co(H2O)6]3+ has an absorption at 549 nm. Based on this where do you think the absorption will be for [Co(NH3)6]3+ might be?
A) 590 nm
B) 437 nm
C) 549 nm
D) It won't have an absorption because it will be a high spin complex.
E) There is no way to predict.
A) 590 nm
B) 437 nm
C) 549 nm
D) It won't have an absorption because it will be a high spin complex.
E) There is no way to predict.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
20
Compared to the [Co(NH3)4Cl2]+ ion, the [Co(NH3)4(ox)]+ ion has an absorption that is farther to the blue. This means
A) the oxylate ion is a stronger field ligand than Cl-.
B) the oxylate ion is a stronger field ligand than NH3.
C) the oxylate ion is a weaker field ligand than Cl-.
D) the oxylate ion is a weaker field ligand than NH3.
E) both c & d
A) the oxylate ion is a stronger field ligand than Cl-.
B) the oxylate ion is a stronger field ligand than NH3.
C) the oxylate ion is a weaker field ligand than Cl-.
D) the oxylate ion is a weaker field ligand than NH3.
E) both c & d

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
21
[Co(H2O)6]3+ absorbs at 549 nm while [V(H2O)6]2+ absorbs at 806 nm.
A) Do is larger for the Vanadium compound
B) Do is smaller for the Vanadium compound
C) Do is the same for the two compounds because they have the same ligands
D) there is no way to compare Do for the two compounds from the data given
A) Do is larger for the Vanadium compound
B) Do is smaller for the Vanadium compound
C) Do is the same for the two compounds because they have the same ligands
D) there is no way to compare Do for the two compounds from the data given

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
22
Given that [Ni(NH3)6]2+ has an absorption at 926 nm and [Cr(NH3)6]3+ has an absorption at 463 nm, what is the ratio of Do in the nickel compound compared to the chromium compound
A) Do in the nickel compound is 2 times that of the chromium compound
B) Do in the nickel compound is 0.5 times that of the chromium compound
C) Do in the nickel compound is 4 times that of the chromium compound
D) Do in the nickel compound is 0.25 times that of the chromium compound
E) Do is the same as they have the same ligands
A) Do in the nickel compound is 2 times that of the chromium compound
B) Do in the nickel compound is 0.5 times that of the chromium compound
C) Do in the nickel compound is 4 times that of the chromium compound
D) Do in the nickel compound is 0.25 times that of the chromium compound
E) Do is the same as they have the same ligands

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
23
[Cr(CN)6]3- absorbs at 376 nm. Given that D0 in [Co(NH3)6]3+ is 1.23 time smaller than D0 in [Co(CN)6]3-, where to you think the compound D0 in [Co(NH3)6]3+ will absorb?
A) 253 nm
B) 278 nm
C) 311 nm
D) 452 nm
E) 463 nm
A) 253 nm
B) 278 nm
C) 311 nm
D) 452 nm
E) 463 nm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
24
Pt(NH3)2Cl2 is a square planar complex. How many unpaired electrons does it have?
A) 0
B) 1
C) 2
D) 4
E) 8
A) 0
B) 1
C) 2
D) 4
E) 8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
25
Generally speaking which is the least stable geometry for coordination complexes?
A) octahedral
B) square planar
C) tetrahedral
D) tetrahedral and octahedral are about the same and both less stable then square planar
E) square planer and octahedral are about the same and both less stable than tetrahedral
A) octahedral
B) square planar
C) tetrahedral
D) tetrahedral and octahedral are about the same and both less stable then square planar
E) square planer and octahedral are about the same and both less stable than tetrahedral

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
26
In the VB picture what is the hybridization of the metal in an octahedral complex?
A) sp
B) sp2
C) sp3
D) dsp2
E) d2sp3
A) sp
B) sp2
C) sp3
D) dsp2
E) d2sp3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
27
Why are Zn2+ ions colorless in water?
A) they don't form coordination complexes with water.
B) the Zn2+/H2O coordination complexes have filled d orbitals.
C) the Zn2+/H2O coordination complexes are all paramagnetic.
D) the Zn2+/H2O coordination complexes are all tetrahedral.
E) none of the above
A) they don't form coordination complexes with water.
B) the Zn2+/H2O coordination complexes have filled d orbitals.
C) the Zn2+/H2O coordination complexes are all paramagnetic.
D) the Zn2+/H2O coordination complexes are all tetrahedral.
E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
28
The vibrational frequency of CO when bound to a metal ion is lower than that of free CO because
A) the crystal field shifts the energy of the vibration.
B) CO is a weak field ligand.
C) the high electronegativity of the metal ion.
D) the metal donates an electron to a bonding orbital of CO.
E) the metal donates an electron to an antibonding orbital of CO.
A) the crystal field shifts the energy of the vibration.
B) CO is a weak field ligand.
C) the high electronegativity of the metal ion.
D) the metal donates an electron to a bonding orbital of CO.
E) the metal donates an electron to an antibonding orbital of CO.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
29
Would it be better to use Cr3+ or Fe3+ octahedral complexes when trying to determine if a ligand is a strong or weak field ligand?
A) Cr3+
B) Fe3+
C) either would be fine
D) neither would work
A) Cr3+
B) Fe3+
C) either would be fine
D) neither would work

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
30
[Cu(CF3)4]- is found to be diamagnetic. What is its structure?
A) square planar
B) tetrahedral
C) either square planar or tetrahedral
D) octahedral (with two other water ligands)
E) it will be an unusual shape with a steric number of 8
A) square planar
B) tetrahedral
C) either square planar or tetrahedral
D) octahedral (with two other water ligands)
E) it will be an unusual shape with a steric number of 8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
31
Using the Lewis classification of acids and bases, a hard base prefers to be attached to
A) a soft base
B) another hard base
C) a soft acid
D) a hard acid
E) a compound that exhibits "borderline" tendencies
A) a soft base
B) another hard base
C) a soft acid
D) a hard acid
E) a compound that exhibits "borderline" tendencies

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
32
Which of the following best describes the lanthanide contraction?
A) f-orbitals are more directional than d-orbitals and therefore less effective at screening nuclear charge
B) f-orbitals are more diffuse than d-orbitals and therefore less effective at screening nuclear charge
C) the increase in atomic and ionic radii as one goes from left to right across the lanthanides
D) A and C
E) B and C
A) f-orbitals are more directional than d-orbitals and therefore less effective at screening nuclear charge
B) f-orbitals are more diffuse than d-orbitals and therefore less effective at screening nuclear charge
C) the increase in atomic and ionic radii as one goes from left to right across the lanthanides
D) A and C
E) B and C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
33
The enthalpy of hydration for a metal ion is
A) equal to the energy associated with the hydration of the gas phase metal ion
B) equal to the ionization energy of the neutral metal atom
C) always positive
D) always negative
E) A and D
A) equal to the energy associated with the hydration of the gas phase metal ion
B) equal to the ionization energy of the neutral metal atom
C) always positive
D) always negative
E) A and D

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck








