Deck 7: Bonding in Organic Molecules
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/33
العب
ملء الشاشة (f)
Deck 7: Bonding in Organic Molecules
1
The octane number for gasoline is
A) the percentage of the gasoline that is alkanes with eight carbon.
B) the percentage of the gasoline that is 2,2,4 trimethyl pentane.
C) the percentage of the gasoline that is not heptane.
D) the percentage of the gasoline that is not heptane or hexane.
E) none of the above
A) the percentage of the gasoline that is alkanes with eight carbon.
B) the percentage of the gasoline that is 2,2,4 trimethyl pentane.
C) the percentage of the gasoline that is not heptane.
D) the percentage of the gasoline that is not heptane or hexane.
E) none of the above
E
2
Naptha is
A) liquefied naphthalene.
B) a mixture of gases including methane, ethane, and propane.
C) a mixture of hydrocarbons with >C20 used in asphalt.
D) a mixture of C5-C12 hydrocarbons used in gasoline.
E) the name for the boat conformation of cyclohexane.
A) liquefied naphthalene.
B) a mixture of gases including methane, ethane, and propane.
C) a mixture of hydrocarbons with >C20 used in asphalt.
D) a mixture of C5-C12 hydrocarbons used in gasoline.
E) the name for the boat conformation of cyclohexane.
D
3
Increasing the chain length of straight chain alkanes causes the boiling point to increase because
A) the longer molecule assume a "balled up" shape.
B) the longer molecule assume a "stretched" shape.
C) the longer molecules have more dispersion forces.
D) the longer molecules have large dipole moments.
E) none of the above
A) the longer molecule assume a "balled up" shape.
B) the longer molecule assume a "stretched" shape.
C) the longer molecules have more dispersion forces.
D) the longer molecules have large dipole moments.
E) none of the above
C
4
The C-C-C angle in cyclopropane must be
A) 45°
B) 60°
C) 90°
D) 109.5°
E) 120°
A) 45°
B) 60°
C) 90°
D) 109.5°
E) 120°

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
5
Name the following compound. 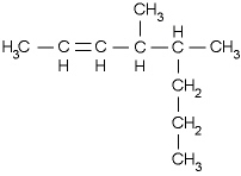
A) 3-methyl-2-propylhexane
B) 4-methyl-6-propyl-2-hexene
C) 2-propyl-3-methyl-4-hexene
D) 4,5-dimethyl-2-octene
E) 4-pentyl-2-pentene

A) 3-methyl-2-propylhexane
B) 4-methyl-6-propyl-2-hexene
C) 2-propyl-3-methyl-4-hexene
D) 4,5-dimethyl-2-octene
E) 4-pentyl-2-pentene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
6
Name the following compound. 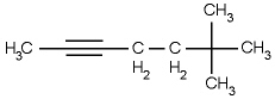
A) 6,6,6-trimethyl-2-hexyne
B) 5,5,5-trimethyl-2-hexyne
C) 6,6-dimethyl-2-heptyne
D) 1,5,5,5-tetrametyl-1-pentyne
E) 5,5-dimethyl-2-hexyne

A) 6,6,6-trimethyl-2-hexyne
B) 5,5,5-trimethyl-2-hexyne
C) 6,6-dimethyl-2-heptyne
D) 1,5,5,5-tetrametyl-1-pentyne
E) 5,5-dimethyl-2-hexyne

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
7
Name the following compound. 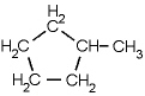
A) 1-methylcyclopentane
B) 1-methylcyclohexane
C) 1-cylcopentylmethane
D) n-hexane
E) toluene

A) 1-methylcyclopentane
B) 1-methylcyclohexane
C) 1-cylcopentylmethane
D) n-hexane
E) toluene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
8
The following molecule contains how many carbons with sp3 hybridization? 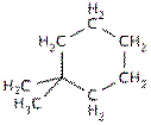
A) 2
B) 5
C) 6
D) 7
E) 8

A) 2
B) 5
C) 6
D) 7
E) 8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
9
In C60 each carbon is bonded to three other carbons. What is the hybridization of the carbons in C60?
A) sp
B) sp2
C) sp3
D) 50% sp2 and 50% sp3
E) the ones on the hexagonal faces are sp2 and the pentagon faces are sp3
A) sp
B) sp2
C) sp3
D) 50% sp2 and 50% sp3
E) the ones on the hexagonal faces are sp2 and the pentagon faces are sp3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
10
The complete combustion of 1 mole of cyclobutanol into carbon dioxide and water will yield how many moles of water?
A) 1
B) 3.5
C) 4
D) 4.5
E) 9
A) 1
B) 3.5
C) 4
D) 4.5
E) 9

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
11
Oxidation of a primary alcohol will result in the formation of
A) a secondary alcohol.
B) an aldehyde.
C) a ketone.
D) a carboxylic acid.
E) an ester.
A) a secondary alcohol.
B) an aldehyde.
C) a ketone.
D) a carboxylic acid.
E) an ester.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
12
Oxidation of an aldehyde will result in the formation of
A) a primary alcohol.
B) a secondary alcohol.
C) a ketone.
D) a carboxylic acid.
E) an ester.
A) a primary alcohol.
B) a secondary alcohol.
C) a ketone.
D) a carboxylic acid.
E) an ester.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
13
Which of the following molecules is chiral
A)
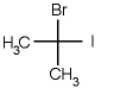
B)
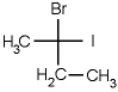
C)
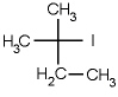
D)
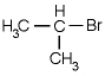
E) none of the above
A)

B)

C)

D)

E) none of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
14
The following compound is an 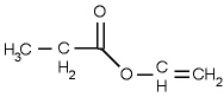
A) ketone
B) ether
C) alcohol
D) alkene
E) ester

A) ketone
B) ether
C) alcohol
D) alkene
E) ester

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
15
In dimethyl ether, shown below, what would you predict for the C-O-C bond angle? 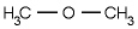
A) 109.5°
B) 180°
C) 120°
D) 105° (the same as water)
E) 110°
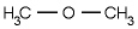
A) 109.5°
B) 180°
C) 120°
D) 105° (the same as water)
E) 110°

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
16
The structure of DDT is given below. What is its molecular formula? 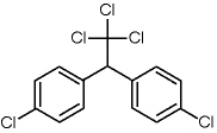
A) C13H9Cl5
B) C14H9Cl5
C) C14H8Cl5
D) C14Cl5
E) C13Cl5

A) C13H9Cl5
B) C14H9Cl5
C) C14H8Cl5
D) C14Cl5
E) C13Cl5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
17
The structure of acetaminophen is given below. How many chiral centers does the molecule have? 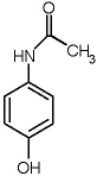
A) 0
B) 1
C) 2
D) 3
E) 8

A) 0
B) 1
C) 2
D) 3
E) 8

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
18
The molecule naphthalene is shown below. How would you describe the bonding in naphthalene using a mix of VB and MO? 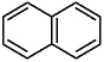
A) 12 C-C s bonds (sp2-sp2), 12 C-H s bonds (sp2-1s), 6p MO's
B) 12 C-C s bonds (sp2-sp2), 10 C-H s bonds (sp2-1s), 6p MO's
C) 11 C-C s bonds (sp2-sp2), 11 C-H s bonds (sp2-1s), 5.5p MO's
D) 11 C-C s bonds (sp2-sp2), 8 C-H s bonds (sp2-1s), 5p MO's
E) 10 C-C s bonds (sp2-sp2), 10 C-H s bonds (sp2-1s), 5p MO's

A) 12 C-C s bonds (sp2-sp2), 12 C-H s bonds (sp2-1s), 6p MO's
B) 12 C-C s bonds (sp2-sp2), 10 C-H s bonds (sp2-1s), 6p MO's
C) 11 C-C s bonds (sp2-sp2), 11 C-H s bonds (sp2-1s), 5.5p MO's
D) 11 C-C s bonds (sp2-sp2), 8 C-H s bonds (sp2-1s), 5p MO's
E) 10 C-C s bonds (sp2-sp2), 10 C-H s bonds (sp2-1s), 5p MO's

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
19
How many different isomers exist for Dichloro-ethene, C2H2Cl2?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
20
In the hydrogenation reaction of ethene a hydrogen is added to each carbon in the double bond. If trans-dichloroethene is reacted with H2 how many isomers of dichloroethane are formed? 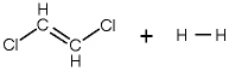
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which would you expect to be most reactive?
A) cyclopropane
B) cyclobutane
C) cyclopentane
D) cyclohexane
E) cyclooctane
A) cyclopropane
B) cyclobutane
C) cyclopentane
D) cyclohexane
E) cyclooctane

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
22
Why is there an energetic barrier to rotation of the C=C bond in ethene?
A) The trans isomer is more stable than the cis isomer.
B) Rotation breaks the s C-C bond.
C) Rotation breaks the p C-C bond.
D) Steric repulsion between the hydrogen atoms.
E) There is no energy barrier to rotation of this bond.
A) The trans isomer is more stable than the cis isomer.
B) Rotation breaks the s C-C bond.
C) Rotation breaks the p C-C bond.
D) Steric repulsion between the hydrogen atoms.
E) There is no energy barrier to rotation of this bond.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
23
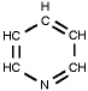
Which of the following is a primary amines?
A)
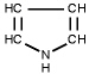
B)
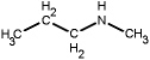
C)
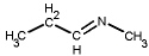
D)
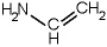
E)
A)

B)

C)

D)

E)


فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
24
An amide bond is formed from the condensation of a carboxylic acid with a primary or secondary amine. What other product is formed in this reaction?
A) H2
B) CO2
C) NH3
D) H2O
E) there are no other products
A) H2
B) CO2
C) NH3
D) H2O
E) there are no other products

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
25
The structure of aspirin is shown below. What functional groups does aspirin possess? 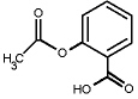
A) ether
B) ester
C) carboxylic acid
D) aldehyde
E) a & b
F) b & c
G) a & c

A) ether
B) ester
C) carboxylic acid
D) aldehyde
E) a & b
F) b & c
G) a & c

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
26
Benzene has a p electron system composed of the 6 pz orbitals on the carbon atoms? How many bonding and antibonding can be formed from these six atomic orbitals?
A) 6 bonding, 0 antibonding
B) 4 bonding, 2 antibonding
C) 3 bonding, 3 antibonding
D) 2 bonding, 4 antibonding
E) 6 bonding, 6 antibonding
A) 6 bonding, 0 antibonding
B) 4 bonding, 2 antibonding
C) 3 bonding, 3 antibonding
D) 2 bonding, 4 antibonding
E) 6 bonding, 6 antibonding

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
27
In a saturated hydrocarbon
A) the sample is all liquid.
B) the C-C bonds are all single bonds.
C) the oxygens are all in alcohol functional groups.
D) the double and triple C-C bonds are in the middle of the molecule.
E) the molecules are straight chains (no branching).
A) the sample is all liquid.
B) the C-C bonds are all single bonds.
C) the oxygens are all in alcohol functional groups.
D) the double and triple C-C bonds are in the middle of the molecule.
E) the molecules are straight chains (no branching).

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
28
Name the following compound. 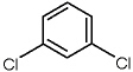
A) o-dichloroxylene
B) o-dichlorobenezne
C) m-dichloroxylene
D) m-dichlorobenzene
E) p-dichloroxylene

A) o-dichloroxylene
B) o-dichlorobenezne
C) m-dichloroxylene
D) m-dichlorobenzene
E) p-dichloroxylene

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
29
Which of the following functional groups does not contain a C-O double bond?
A) aldehyde
B) carboxylic acid
C) ester
D) ether
E) ketone
A) aldehyde
B) carboxylic acid
C) ester
D) ether
E) ketone

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
30
How many p electrons are in C70?
A) 0
B) 7
C) 35
D) 70
E) 140
A) 0
B) 7
C) 35
D) 70
E) 140

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
31
Which of the following is a tertiary alcohol?
A) n-butanol
B) isopropyl alcohol
C) 2-methyl-2-propanol
D) phenol
E) ethylene glycol
A) n-butanol
B) isopropyl alcohol
C) 2-methyl-2-propanol
D) phenol
E) ethylene glycol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
32
Which of the following, when oxidized, will generally result in the formation of a ketone?
A) alkane
B) primary alcohol
C) secondary alcohol
D) tertiary alcohol
E) phenol
A) alkane
B) primary alcohol
C) secondary alcohol
D) tertiary alcohol
E) phenol

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following generally serves as chemical catalysts for biochemical reactions?
A) structural proteins
B) hormones
C) enzymes
D) carboxylic acids
E) all of the above
A) structural proteins
B) hormones
C) enzymes
D) carboxylic acids
E) all of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck








