Deck 2: Chemical Formulas, Equations, and Reaction Yields
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/33
العب
ملء الشاشة (f)
Deck 2: Chemical Formulas, Equations, and Reaction Yields
1
If the relative atomic mass of 56Fe is 55.935 (on the 12C scale), what is the mass of one 56Fe atom?
A) 3.37×10-25 g
B) 1.66×10-24 g
C) 7.74×10-24 g
D) 9.29×10-23 g
E) 3.37×10-22 g
A) 3.37×10-25 g
B) 1.66×10-24 g
C) 7.74×10-24 g
D) 9.29×10-23 g
E) 3.37×10-22 g
D
2
Which has the greatest number of hydrogen atoms?
A) 1020 hydrogen atoms
B) 100 g of water
C) 5 g of an unknown compound
D) 20 g of hydrogen gas
E) 100 g of a substance that is 2% H by mass
A) 1020 hydrogen atoms
B) 100 g of water
C) 5 g of an unknown compound
D) 20 g of hydrogen gas
E) 100 g of a substance that is 2% H by mass
D
3
Which of these carbohydrates has the largest molecular mass?
A) threose (C4H8O4)
B) ribose (C5H10O5)
C) glucose (C6H12O6)
D) sucrose (C12H22O11)
E) raffinose (C18H32O16)
A) threose (C4H8O4)
B) ribose (C5H10O5)
C) glucose (C6H12O6)
D) sucrose (C12H22O11)
E) raffinose (C18H32O16)
E
4
What is the relative molecular mass of the compound trinitrotoluene, C7H5N3O6 (on the 12C scale)?
A) 43.03
B) 205.13
C) 215.13
D) 227.13
E) 278.03
A) 43.03
B) 205.13
C) 215.13
D) 227.13
E) 278.03

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
5
Assume a kernel of wheat has a volume of 8 mm3. How many moles of wheat kernels can fit in the world's largest grain elevator with a capacity of 20 million bushels (1 bushel = 35.24 L)?
A) 1.17×10-12
B) 1.42×10-12
C) 1.46×10-10
D) 1.46×10-7
E) 1.42×10-6
A) 1.17×10-12
B) 1.42×10-12
C) 1.46×10-10
D) 1.46×10-7
E) 1.42×10-6

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
6
Vitamin B12, cyanocobalamin, has the molecular formula, C68H88CoN14O14P. What is the percent mass of cobalt in this compound?
A) 1.02%
B) 4.35%
C) 10.3%
D) 22.3%
E) 23.2%
A) 1.02%
B) 4.35%
C) 10.3%
D) 22.3%
E) 23.2%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
7
Which of the following carbohydrates has the largest percent mass of carbon?
A) threose (C4H8O4)
B) ribose (C5H10O5)
C) glucose (C6H12O6)
D) sucrose (C12H22O11)
E) raffinose (C18H32O16)
A) threose (C4H8O4)
B) ribose (C5H10O5)
C) glucose (C6H12O6)
D) sucrose (C12H22O11)
E) raffinose (C18H32O16)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
8
Magnetite is a mineral compound that is 72.36% iron and 27.64% oxygen by mass. What is the empirical formula for the compound that makes up magnetite?
A) FeO
B) Fe2O
C) Fe2O3
D) Fe3O2
E) Fe3O4
A) FeO
B) Fe2O
C) Fe2O3
D) Fe3O2
E) Fe3O4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
9
You have 25.0 g of a compound that contains only silicon and nitrogen. Chemical analysis reveals that your sample contains 15.0 g of silicon. What is the empirical formula for your compound?
A) SiN
B) Si3N4
C) Si2N3
D) SiN2
E) Si2N
A) SiN
B) Si3N4
C) Si2N3
D) SiN2
E) Si2N

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
10
You have a compound that contains only carbon and hydrogen. If you burn the compound at high temperature in the presence of oxygen it is completely converted to CO2 and H2O. When you react 20.0 g of your sample you generate 62.7 g of CO2 and 25.7 g of H2O. Which of the following is a possible formula for your compound?
A) C2H2
B) C2H4
C) C2H6
D) C3H5
E) C3H7
A) C2H2
B) C2H4
C) C2H6
D) C3H5
E) C3H7

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
11
Methane CH4 reacts with molecular oxygen, O2, to form carbon dioxide, CO2, and water, H2O. In a balanced chemical equation for this reaction there will always be
A) the same number of moles of CO2 and H2O.
B) twice as many moles of CO2 as H2O.
C) twice as many moles of H2O as CO2.
D) twice as many moles of H2O as O2.
E) twice as many moles of CO2 as CH4.
A) the same number of moles of CO2 and H2O.
B) twice as many moles of CO2 as H2O.
C) twice as many moles of H2O as CO2.
D) twice as many moles of H2O as O2.
E) twice as many moles of CO2 as CH4.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
12
For the given unbalanced reaction
X CaO(s) + y H2O(l) → z Ca(OH)2
The correct stiochiometric coefficients x, y, & z are
A) 1,1,1
B) 1,2,1
C) 2,1,2
D) 2,1,1
E) 1,1,2
X CaO(s) + y H2O(l) → z Ca(OH)2
The correct stiochiometric coefficients x, y, & z are
A) 1,1,1
B) 1,2,1
C) 2,1,2
D) 2,1,1
E) 1,1,2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
13
Barium hydroxide reacts with hydrochloric acid to produce barium chloride and water. A balanced chemical equation for this reaction is?
A) Ba(OH)2 + HCL → BACL2 + H2O
B) Ba(OH) + HCL → BACL + H2O
C) Ba(OH)2 + 2HCL → BACL2 + 2H2O
D) Ba(OH)2 + 2HCL → BACL2 + H2O
E) Ba(OH) + 2HCL → BACL + H2O
A) Ba(OH)2 + HCL → BACL2 + H2O
B) Ba(OH) + HCL → BACL + H2O
C) Ba(OH)2 + 2HCL → BACL2 + 2H2O
D) Ba(OH)2 + 2HCL → BACL2 + H2O
E) Ba(OH) + 2HCL → BACL + H2O

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
14
Which of the following is not correctly balanced?
A) 2 Al + 6 HCl → 2 AlCl3 + 3 H2
B) 2 C6H6 + 15 O2 → 12 CO2 + 6 H2O
C) 16 Cr + 3 S8 → 8 Cr2S3
D) 2 NaHCO3 → Na2CO3 + 2 CO2 + H2O
E) Fe2O3 + 2 Al → 2 Fe + Al2O3
A) 2 Al + 6 HCl → 2 AlCl3 + 3 H2
B) 2 C6H6 + 15 O2 → 12 CO2 + 6 H2O
C) 16 Cr + 3 S8 → 8 Cr2S3
D) 2 NaHCO3 → Na2CO3 + 2 CO2 + H2O
E) Fe2O3 + 2 Al → 2 Fe + Al2O3

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
15
In fermentation, sucrose, C12H22O11, reacts with water to form ethanol, C2H5OH, and carbon dioxide. A balanced chemical equation for this reaction is?
A) C12H22O11(aq) + 3 H2O(l) → 5 C2H5OH(aq) + 2 Co2(g)
B) C12H22O11(aq) + 7 H2O(l) → 2 C2H5OH(aq) + 8 Co2(g)
C) C12H22O11(aq) + 2 H2O(l) → 6 C2H5OH(aq) + Co2(g)
D) C12H22O11(aq) + 2 H2O(l) → 4 C2H5OH(aq) + 4 Co2(g)
E) C12H22O11(aq) + H2O(l) → 4 C2H5OH(aq) + 4 Co2(g)
A) C12H22O11(aq) + 3 H2O(l) → 5 C2H5OH(aq) + 2 Co2(g)
B) C12H22O11(aq) + 7 H2O(l) → 2 C2H5OH(aq) + 8 Co2(g)
C) C12H22O11(aq) + 2 H2O(l) → 6 C2H5OH(aq) + Co2(g)
D) C12H22O11(aq) + 2 H2O(l) → 4 C2H5OH(aq) + 4 Co2(g)
E) C12H22O11(aq) + H2O(l) → 4 C2H5OH(aq) + 4 Co2(g)

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
16
Tetrasilane (Si4H10) is a liquid with can react with oxygen to form SiO2 and water. How many moles of water are produced from each mole of tetrasilane that reacts?
A) 0.1
B) 2.5
C) 4
D) 5
E) 10
A) 0.1
B) 2.5
C) 4
D) 5
E) 10

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
17
Citric acid (C6H8O7) is produced from fermentation of sugars such as sucrose (C12H22O11). In this process oxygen reacts with the sugar to produce citric acid and water as products. How many moles of citric acid can be produced from one mole of sucrose?
A) 2
B) 1
C) 0.5
D) 0.333
E) 0.1
A) 2
B) 1
C) 0.5
D) 0.333
E) 0.1

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
18
Nitrogen gas reacts with hydrogen gas to produce ammonia.
3 H2(g) + N2(g) → 2 NH3(g)
How many grams of nitrogen are required to produce 1.000 g of ammonia?
A) 0.8224
B) 1.644
C) 0.5000
D) 0.6667
E) 0.4112
3 H2(g) + N2(g) → 2 NH3(g)
How many grams of nitrogen are required to produce 1.000 g of ammonia?
A) 0.8224
B) 1.644
C) 0.5000
D) 0.6667
E) 0.4112

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
19
Methane can be reacted with steam to produce carbon dioxide and hydrogen gas in a two step reaction. The resulting overall reaction can be written
CH4(g) + 2 H2O(g) → CO2(g) + 4 H2(g)
How many grams of hydrogen can be produced from each gram of methane?
A) 1.006
B) 0.503
C) 0.377
D) 0.251
E) 4.000
CH4(g) + 2 H2O(g) → CO2(g) + 4 H2(g)
How many grams of hydrogen can be produced from each gram of methane?
A) 1.006
B) 0.503
C) 0.377
D) 0.251
E) 4.000

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
20
Magnesium will react with carbon dioxide to form magnesium oxide and carbon.
2 Mg(s) + CO2(g) → 2MgO(s) + C(s)
20)0 grams of magnesium react completely with excess carbon dioxide. How many grams of solid product should be formed?
A) 20.0 grams
B) 27.5 grams
C) 30.0 grams
D) 38.1 grams
E) 40.0 grams
2 Mg(s) + CO2(g) → 2MgO(s) + C(s)
20)0 grams of magnesium react completely with excess carbon dioxide. How many grams of solid product should be formed?
A) 20.0 grams
B) 27.5 grams
C) 30.0 grams
D) 38.1 grams
E) 40.0 grams

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
21
Calcium carbide, CaC₂, reacts with water to produce calcium hydroxide and acetylene gas, C₂H₂. 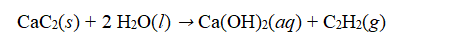 How many grams of acetylene can be produced from 30.0 g of calcium carbide?
How many grams of acetylene can be produced from 30.0 g of calcium carbide?
A) 1.42 g
B) 12.2 g
C) 28.0 g
D) 30.0 g
E) 64.1 g
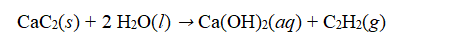 How many grams of acetylene can be produced from 30.0 g of calcium carbide?
How many grams of acetylene can be produced from 30.0 g of calcium carbide? A) 1.42 g
B) 12.2 g
C) 28.0 g
D) 30.0 g
E) 64.1 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
22
Ammonium perchlorate, NH4ClO4, is used with aluminum as rocket fuel. 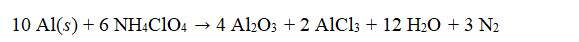 If 100.0 g of aluminum are reacted with 60.0 g of ammonium perchlorate, how many grams of aluminum oxide are produced?
If 100.0 g of aluminum are reacted with 60.0 g of ammonium perchlorate, how many grams of aluminum oxide are produced?
A) 34.71 g
B) 40.00 g
C) 123.8 g
D) 151.1 g
E) 1789 g
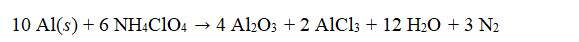 If 100.0 g of aluminum are reacted with 60.0 g of ammonium perchlorate, how many grams of aluminum oxide are produced?
If 100.0 g of aluminum are reacted with 60.0 g of ammonium perchlorate, how many grams of aluminum oxide are produced?A) 34.71 g
B) 40.00 g
C) 123.8 g
D) 151.1 g
E) 1789 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
23
Aluminum sulfide reacts with water to form aluminum hydroxide and hydrogen sulfide by the following reaction 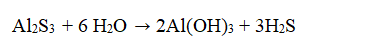 If 25 g of aluminum sulfide is reacted with 25 g of water how many moles of hydrogen sulfide will be formed?
If 25 g of aluminum sulfide is reacted with 25 g of water how many moles of hydrogen sulfide will be formed?
A) 0.12 moles
B) 0.50 moles
C) 0.78 moles
D) 2.0 moles
E) 3.0 moles
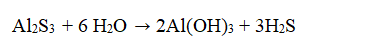 If 25 g of aluminum sulfide is reacted with 25 g of water how many moles of hydrogen sulfide will be formed?
If 25 g of aluminum sulfide is reacted with 25 g of water how many moles of hydrogen sulfide will be formed? A) 0.12 moles
B) 0.50 moles
C) 0.78 moles
D) 2.0 moles
E) 3.0 moles

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
24
Iron oxide can be reduced to iron by a reaction with carbon to form carbon monoxide 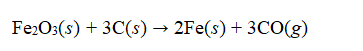 If 95.0 grams of iron oxide is reacted with excess carbon yields 63 g of iron, what is the percent yield of this reaction?
If 95.0 grams of iron oxide is reacted with excess carbon yields 63 g of iron, what is the percent yield of this reaction?
A) 12%
B) 59%
C) 66%
D) 95%
E) 100%
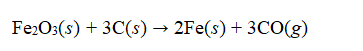 If 95.0 grams of iron oxide is reacted with excess carbon yields 63 g of iron, what is the percent yield of this reaction?
If 95.0 grams of iron oxide is reacted with excess carbon yields 63 g of iron, what is the percent yield of this reaction?A) 12%
B) 59%
C) 66%
D) 95%
E) 100%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
25
Acetonitrile, CH₃CN, can be synthesized from carbon monoxide, hydrogen, and ammonia in the presence of a catalyst at high temperatures by the following reaction  If 20 g of carbon monoxide, 20 g of hydrogen, and 10 g of ammonia are reacted, which will be the limiting reagent?
If 20 g of carbon monoxide, 20 g of hydrogen, and 10 g of ammonia are reacted, which will be the limiting reagent?
A) carbon monoxide
B) hydrogen
C) ammonia
D) none of them
E) there is no way to know
 If 20 g of carbon monoxide, 20 g of hydrogen, and 10 g of ammonia are reacted, which will be the limiting reagent?
If 20 g of carbon monoxide, 20 g of hydrogen, and 10 g of ammonia are reacted, which will be the limiting reagent?A) carbon monoxide
B) hydrogen
C) ammonia
D) none of them
E) there is no way to know

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which has a larger molar volume (the volume occupied by one mole): gold (density = 19.3 g cm⁻³) or tin (density = 7.31 g cm⁻³)?
A) gold
B) tin
C) they are exactly the same
D) there is no way to know without other information
A) gold
B) tin
C) they are exactly the same
D) there is no way to know without other information

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
27
Copper oxide ore, CuO, can be smelted with carbon to make copper metal and carbon dioxide. 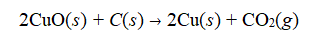 If 100.0 of a mixed ore is smelted and produces 75.9 grams of pure copper. What percentage of the mixed ore is CuO? (You can assume the reaction yield is 100%, there is excess carbon, and that CuO is the only source of copper.)
If 100.0 of a mixed ore is smelted and produces 75.9 grams of pure copper. What percentage of the mixed ore is CuO? (You can assume the reaction yield is 100%, there is excess carbon, and that CuO is the only source of copper.)
A) 28.0%
B) 48.5%
C) 75.9%
D) 79.9%
E) 95.0%
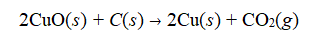 If 100.0 of a mixed ore is smelted and produces 75.9 grams of pure copper. What percentage of the mixed ore is CuO? (You can assume the reaction yield is 100%, there is excess carbon, and that CuO is the only source of copper.)
If 100.0 of a mixed ore is smelted and produces 75.9 grams of pure copper. What percentage of the mixed ore is CuO? (You can assume the reaction yield is 100%, there is excess carbon, and that CuO is the only source of copper.) A) 28.0%
B) 48.5%
C) 75.9%
D) 79.9%
E) 95.0%

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
28
The hard ceramic boron carbide, B₄C, can be made the reaction of boron oxide, B₂O₃, with carbon in an arc furnace. How many grams of boron carbide can be made from 10.0 g of boron oxide?
A) 3.10 g
B) 3.97 g
C) 5.00 g
D) 10.0 g
E) 12.6 g
A) 3.10 g
B) 3.97 g
C) 5.00 g
D) 10.0 g
E) 12.6 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
29
A vanadium oxide contains 56.02% vanadium by mass. What is its empirical formula?
A) V₂O
B) VO
C) V₂O₃
D) VO₂
E) V₂O₅
A) V₂O
B) VO
C) V₂O₃
D) VO₂
E) V₂O₅

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
30
Caffeine has the molecular formula, C₈H₁₀ N₄ O₂. If it is burned in excess oxygen it will form carbon dioxide, water vapor, and nitrogen gas. Which gas will be most abundant by mass?
A) CO₂
B) H₂O
C) N₂
D) they will all the be the same
E) it depends on the mass of caffeine
A) CO₂
B) H₂O
C) N₂
D) they will all the be the same
E) it depends on the mass of caffeine

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
31
Triphenylene is an organic compound containing only carbon and hydrogen with 3 carbon atoms for every two hydrogen atoms. Which of the following is a possible molecular mass for triphenylene?
A) 34.0 g/mol
B) 228.29 g/mol
C) 366.8 g/mol
D) 400.0 g/mol
E) b or c could be correct
A) 34.0 g/mol
B) 228.29 g/mol
C) 366.8 g/mol
D) 400.0 g/mol
E) b or c could be correct

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
32
Sodium perbromate can be synthesized via the following reaction that gives off Xenon gas.  A reaction is started with 200.0g of NaBrO₃, 250.0g of XeF₂ and 100.0g of water. Assuming that the reaction goes to completion, and that all of the formed xenon gas is allowed to escape into the atmosphere, what will be the minimum mass of products and reactants remaining in the reaction vessel?
A reaction is started with 200.0g of NaBrO₃, 250.0g of XeF₂ and 100.0g of water. Assuming that the reaction goes to completion, and that all of the formed xenon gas is allowed to escape into the atmosphere, what will be the minimum mass of products and reactants remaining in the reaction vessel?
A) 173.4 g
B) 193.9 g
C) 376.0 g
D) 356.1 g
E) None of the above
 A reaction is started with 200.0g of NaBrO₃, 250.0g of XeF₂ and 100.0g of water. Assuming that the reaction goes to completion, and that all of the formed xenon gas is allowed to escape into the atmosphere, what will be the minimum mass of products and reactants remaining in the reaction vessel?
A reaction is started with 200.0g of NaBrO₃, 250.0g of XeF₂ and 100.0g of water. Assuming that the reaction goes to completion, and that all of the formed xenon gas is allowed to escape into the atmosphere, what will be the minimum mass of products and reactants remaining in the reaction vessel? A) 173.4 g
B) 193.9 g
C) 376.0 g
D) 356.1 g
E) None of the above

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck
33
When acetaldehyde is treated with aqueous sodium hydroxide (in excess), aldol is formed via the following reaction in a 50% yield. 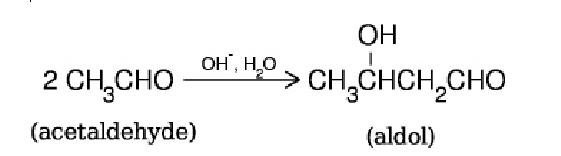 What is the minimum mass of acetaldehyde that must be used if one wants to make 100 g of aldol?
What is the minimum mass of acetaldehyde that must be used if one wants to make 100 g of aldol?
A) 88 g
B) 176 g
C) 200 g
D) 400 g
E) 50.0 g
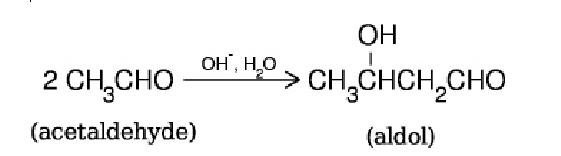 What is the minimum mass of acetaldehyde that must be used if one wants to make 100 g of aldol?
What is the minimum mass of acetaldehyde that must be used if one wants to make 100 g of aldol? A) 88 g
B) 176 g
C) 200 g
D) 400 g
E) 50.0 g

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 33 في هذه المجموعة.
فتح الحزمة
k this deck








