Deck 22: Introduction to Oxygen and Carbon Dioxide in Physiology
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال
سؤال

فتح الحزمة
قم بالتسجيل لفتح البطاقات في هذه المجموعة!
Unlock Deck
Unlock Deck
1/65
العب
ملء الشاشة (f)
Deck 22: Introduction to Oxygen and Carbon Dioxide in Physiology
1
When a diving beetle is underwater with its air bubble, oxygen diffuses from the
A) animal into the bubble.
B) water directly into the animal.
C) bubble into the surrounding water.
D) water into the bubble.
A) animal into the bubble.
B) water directly into the animal.
C) bubble into the surrounding water.
D) water into the bubble.
D
2
Respiratory gases move from place to place principally by
A) diffusion.
B) convection.
C) active transport.
D) both diffusion and convective transport.
A) diffusion.
B) convection.
C) active transport.
D) both diffusion and convective transport.
D
3
The diffusion of an uncharged solute in aqueous solution
A) always diffuses from regions of high concentration to regions of low concentration.
B) will diffuse at a rate exponential to the difference in concentration between regions.
C) involves a passive diffusion component as well as an active diffusion component.
D) diffuses faster compared to charged solutes.
A) always diffuses from regions of high concentration to regions of low concentration.
B) will diffuse at a rate exponential to the difference in concentration between regions.
C) involves a passive diffusion component as well as an active diffusion component.
D) diffuses faster compared to charged solutes.
A
4
Which statement regarding the diffusion of materials between gas mixtures and aqueous solutions is false?
A) Diffusion can be predicted by means of partial pressure measurements.
B) Diffusion can be predicted by using concentrations.
C) Respiratory gases can exist in the gas phase as well as dissolved in an aqueous solution.
D) Each gas has a unique solubility in aqueous solution.
A) Diffusion can be predicted by means of partial pressure measurements.
B) Diffusion can be predicted by using concentrations.
C) Respiratory gases can exist in the gas phase as well as dissolved in an aqueous solution.
D) Each gas has a unique solubility in aqueous solution.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
5
Which molecules are respiratory gases or chemical forms of respiratory gases?
I) O2
II) O3
III) CO2
IV) HCO3-
V) N2
A) I, II, and III
B) I, II, III, and IV
C) I, III, and IV
D) I, III, and V
I) O2
II) O3
III) CO2
IV) HCO3-
V) N2
A) I, II, and III
B) I, II, III, and IV
C) I, III, and IV
D) I, III, and V

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
6
From the list below, choose the correct order of "physiological urgency" for each element in a given terrestrial animal, beginning with the most urgent.
I) The need for water
II) The need to void CO2
III) The need to obtain oxygen
IV) The need for food
A) I, III, II, IV
B) III, II, I, IV
C) II, III, I, IV
D) II, III, IV, I
I) The need for water
II) The need to void CO2
III) The need to obtain oxygen
IV) The need for food
A) I, III, II, IV
B) III, II, I, IV
C) II, III, I, IV
D) II, III, IV, I

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
7
The strength of a chemical substance's tendency to undergo a physical or chemical change is called
A) diffusion.
B) its partial pressure.
C) its chemical potential.
D) its potential energy.
A) diffusion.
B) its partial pressure.
C) its chemical potential.
D) its potential energy.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
8
Which statement regarding gas mixtures in aqueous solutions is false?
A) When a gas dissolves in a solution, it becomes incorporated into the liquid phase.
B) When a gas dissolves in a solution, the molecules become distributed among the H2O molecules in much the same way as glucose molecules do.
C) Gas in solution can appear as microscopic bubbles.
D) When a large bubble rises to the surface of a solution, the solution is considered to be in the gas phase.
A) When a gas dissolves in a solution, it becomes incorporated into the liquid phase.
B) When a gas dissolves in a solution, the molecules become distributed among the H2O molecules in much the same way as glucose molecules do.
C) Gas in solution can appear as microscopic bubbles.
D) When a large bubble rises to the surface of a solution, the solution is considered to be in the gas phase.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
9
The partial pressure of any given gas
A) cannot be calculated from the universal gas law.
B) is dependent on any other gas present.
C) is the individual pressure exerted by the gas in a gas mixture.
D) is proportional to the size of the gas molecule.
A) cannot be calculated from the universal gas law.
B) is dependent on any other gas present.
C) is the individual pressure exerted by the gas in a gas mixture.
D) is proportional to the size of the gas molecule.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
10
Which factor is a constant according to the universal gas law?
A) Moles of gas
B) Temperature
C) The universal gas constant
D) Partial pressure
A) Moles of gas
B) Temperature
C) The universal gas constant
D) Partial pressure

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
11
According to the universal gas law, _______ is(are) inversely proportional to the partial pressure.
A) moles of gas
B) temperature
C) volume
D) the universal gas constant
A) moles of gas
B) temperature
C) volume
D) the universal gas constant

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
12
Refer to the figure shown.
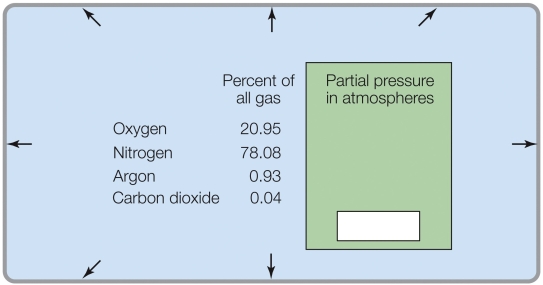 According to the data in the figure, the partial pressures at sea level total
According to the data in the figure, the partial pressures at sea level total
A) 1 atm.
B) 0.5 atm.
C) 100%.
D) 1 kPa.
 According to the data in the figure, the partial pressures at sea level total
According to the data in the figure, the partial pressures at sea level totalA) 1 atm.
B) 0.5 atm.
C) 100%.
D) 1 kPa.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
13
Refer to the figure shown.
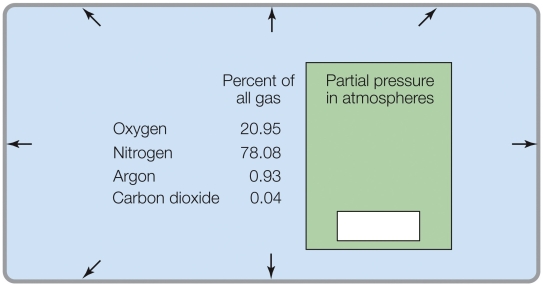 If the total pressure was 2 atm, the partial pressure of CO2 would be _______ atm.
If the total pressure was 2 atm, the partial pressure of CO2 would be _______ atm.
A) 0.04
B) 0.08
C) 0.0004
D) 0.0008
 If the total pressure was 2 atm, the partial pressure of CO2 would be _______ atm.
If the total pressure was 2 atm, the partial pressure of CO2 would be _______ atm.A) 0.04
B) 0.08
C) 0.0004
D) 0.0008

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
14
Refer to the figure shown.
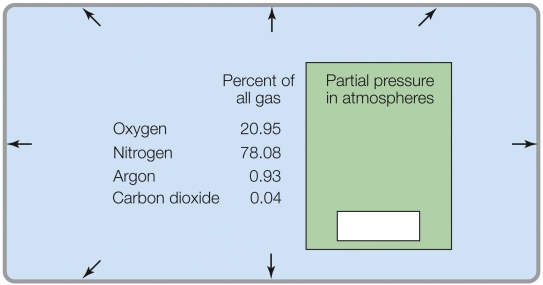 If the total pressure was 0.5 atm, the partial pressure of O2 would be _______ atm.
If the total pressure was 0.5 atm, the partial pressure of O2 would be _______ atm.
A) 20.95
B) 10.48
C) 0.2095
D) 0.1048
 If the total pressure was 0.5 atm, the partial pressure of O2 would be _______ atm.
If the total pressure was 0.5 atm, the partial pressure of O2 would be _______ atm.A) 20.95
B) 10.48
C) 0.2095
D) 0.1048

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
15
Refer to the figure shown.
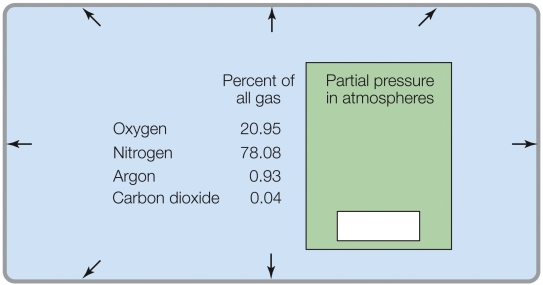 If the total pressure was 0.1 atm, the percent of O2 in the gas mixture would be _______%.
If the total pressure was 0.1 atm, the percent of O2 in the gas mixture would be _______%.
A) 20.95
B) 2.095
C) 0.2095
D) 0.02095
 If the total pressure was 0.1 atm, the percent of O2 in the gas mixture would be _______%.
If the total pressure was 0.1 atm, the percent of O2 in the gas mixture would be _______%.A) 20.95
B) 2.095
C) 0.2095
D) 0.02095

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
16
The fraction of the total moles of gas in a gas mixture is called the
A) mole fractional concentration.
B) volume fractional concentration.
C) partial pressure.
D) absorption coefficient.
A) mole fractional concentration.
B) volume fractional concentration.
C) partial pressure.
D) absorption coefficient.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
17
Suppose that at a temperature of 25°C and pressure of 1 atm, all the O2 is removed from 10 L of dry atmospheric air, and the remaining gas is restored to the original temperature and pressure. The final volume will be
A) 10 L.
B) about 7.9 L.
C) about 5 L.
D) about 2.1 L.
A) 10 L.
B) about 7.9 L.
C) about 5 L.
D) about 2.1 L.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
18
Consider two gas mixtures that are identical in temperature. The concentration of O2 in mixture #1 is 20 mmol/L, and the concentration of O2 in mixture #2 is 60 mmol/L. Which statement about the mixtures is true?
A) The partial pressure of O2 in mixture #2 is three times higher than it is in mixture #1.
B) The partial pressure of O2 in mixture #2 is 3 times lower than it is in mixture #1.
C) The percent of O2 in mixture #2 is three times higher than it is in mixture #1.
D) The partial pressures of O2 in both mixtures are the same.
A) The partial pressure of O2 in mixture #2 is three times higher than it is in mixture #1.
B) The partial pressure of O2 in mixture #2 is 3 times lower than it is in mixture #1.
C) The percent of O2 in mixture #2 is three times higher than it is in mixture #1.
D) The partial pressures of O2 in both mixtures are the same.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
19
Henry's law relates _______ in aqueous solutions.
A) partial pressure and concentration
B) partial pressure and volume
C) pressure and volume
D) temperature and concentration
A) partial pressure and concentration
B) partial pressure and volume
C) pressure and volume
D) temperature and concentration

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
20
The _______ of a particular gas in a mixture is the dissolved concentration of that gas when the partial pressure is 1 atm.
A) gas tension
B) absorption coefficient
C) volume fractional concentration
D) density
A) gas tension
B) absorption coefficient
C) volume fractional concentration
D) density

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
21
Which statement about gases dissolved in aqueous solutions is true?
A) Different gases have different solubilities.
B) Gas solubilities increase strongly with increasing temperature.
C) Gas solubilities increase with increasing salinity.
D) Gas solubility decreases with increasing molecular size.
A) Different gases have different solubilities.
B) Gas solubilities increase strongly with increasing temperature.
C) Gas solubilities increase with increasing salinity.
D) Gas solubility decreases with increasing molecular size.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
22
Which scenarios will result in dissolved gas coming out of solution?
I) Collecting cold creek water, sealing the container, and allowing the solution to warm
II) Collecting warm water, sealing the container, and allowing the solution to cool
III) Collecting cold creek water, adding salt, sealing the container, and allowing the solution to warm
IV) Collecting cold creek water, adding salt, sealing the container, and keeping the solution at the original temperature
A) I, II, and III
B) I, III, and IV
C) II, III, and IV
D) III and IV
I) Collecting cold creek water, sealing the container, and allowing the solution to warm
II) Collecting warm water, sealing the container, and allowing the solution to cool
III) Collecting cold creek water, adding salt, sealing the container, and allowing the solution to warm
IV) Collecting cold creek water, adding salt, sealing the container, and keeping the solution at the original temperature
A) I, II, and III
B) I, III, and IV
C) II, III, and IV
D) III and IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
23
If a water beetle has used up half of the oxygen in its air bubble, the concentration of oxygen in the bubble is _______ the concentration of oxygen in the water. The partial pressure of oxygen in the bubble is _______ the partial pressure of oxygen in the water. Therefore, oxygen will move from the _______.
A) greater than; less than; water into the bubble
B) less than; less than; bubble into the water
C) less than; less than; water into the bubble
D) greater than; less than; bubble into the water
A) greater than; less than; water into the bubble
B) less than; less than; bubble into the water
C) less than; less than; water into the bubble
D) greater than; less than; bubble into the water

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
24
If a water beetle has been using an air bubble as a gill for 1 hour, the O2 partial pressure will be _______ that of air and the N2 partial pressure will be _______ that of air.
A) less than; less than
B) equal to; equal to
C) less than; equal to
D) greater than; greater than
A) less than; less than
B) equal to; equal to
C) less than; equal to
D) greater than; greater than

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
25
In which situation would a water beetle's gas bubble last the shortest amount of time as a functional gill?
A) Gas bubble = 0.1 atm O2, 0.75 atm N2; water = 0.21 atm of O2
B) Gas bubble = 0.1 atm O2, 0.75 atm N2; water = 0.1 atm of O2
C) Gas bubble = 0.2 atm O2, 0.75 atm N2; water = 0.1 atm of O2
D) Gas bubble = 1 atm O2; water = 0.21 atm of O2
A) Gas bubble = 0.1 atm O2, 0.75 atm N2; water = 0.21 atm of O2
B) Gas bubble = 0.1 atm O2, 0.75 atm N2; water = 0.1 atm of O2
C) Gas bubble = 0.2 atm O2, 0.75 atm N2; water = 0.1 atm of O2
D) Gas bubble = 1 atm O2; water = 0.21 atm of O2

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
26
Which statement regarding the diffusion of gases is false?
A) Within gas mixtures, gases diffuse in net fashion from areas of relatively high partial pressure to areas of relatively low partial pressure.
B) Within aqueous solutions, gases diffuse in net fashion from areas of relatively high partial pressure to areas of relatively low partial pressure.
C) Across gas‒water interfaces, gases diffuse in net fashion from areas of relatively high partial pressure to areas of relatively low partial pressure.
D) Depending on the circumstance, gases can diffuse from low to high partial pressure.
A) Within gas mixtures, gases diffuse in net fashion from areas of relatively high partial pressure to areas of relatively low partial pressure.
B) Within aqueous solutions, gases diffuse in net fashion from areas of relatively high partial pressure to areas of relatively low partial pressure.
C) Across gas‒water interfaces, gases diffuse in net fashion from areas of relatively high partial pressure to areas of relatively low partial pressure.
D) Depending on the circumstance, gases can diffuse from low to high partial pressure.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
27
In the diffusion equation, if the units of K are cm2 ∙ s-1, which of the following best represents the units for J?
A) moles ∙ cm-2 ∙ s-1
B) moles ∙ sec-1
C) M ∙ g-1 ∙ cm-2
D) M ∙ cm2 ∙ s
A) moles ∙ cm-2 ∙ s-1
B) moles ∙ sec-1
C) M ∙ g-1 ∙ cm-2
D) M ∙ cm2 ∙ s

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
28
In the diffusion equation, when X increases,
A) J increases.
B) J decreases.
C) J is not affected.
D) J can increase or decrease.
A) J increases.
B) J decreases.
C) J is not affected.
D) J can increase or decrease.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
29
In the diffusion equation,
A) permeability is integrated into P1-P2.
B) permeability is integrated into K.
C) P1-P2 represents permeability.
D) X represents permeability.
A) permeability is integrated into P1-P2.
B) permeability is integrated into K.
C) P1-P2 represents permeability.
D) X represents permeability.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
30
The Krogh diffusion coefficient (K) for O2 in air is _______ K for O2 in water.
A) slightly greater than
B) slightly lower than
C) about 200,000 times lower than
D) about 200,000 times greater than
A) slightly greater than
B) slightly lower than
C) about 200,000 times lower than
D) about 200,000 times greater than

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
31
Refer to the figure shown.
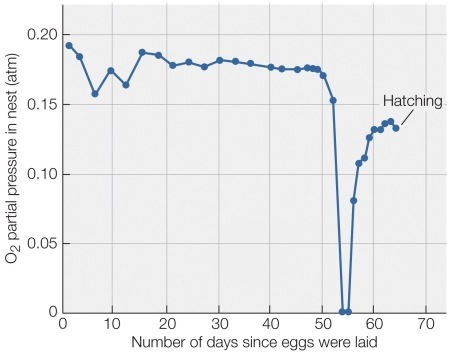 The eggs of which animal would most likely have the data profile in the figure?
The eggs of which animal would most likely have the data profile in the figure?
A) Bald eagle
B) Snapping turtle
C) Bullfrog
D) Largemouth bass
 The eggs of which animal would most likely have the data profile in the figure?
The eggs of which animal would most likely have the data profile in the figure?A) Bald eagle
B) Snapping turtle
C) Bullfrog
D) Largemouth bass

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
32
Refer to the figure shown.
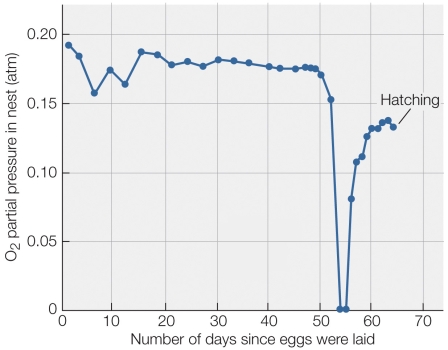 According to the figure, what was the most likely cause of the zeroing of oxygen partial pressure in the nest?
According to the figure, what was the most likely cause of the zeroing of oxygen partial pressure in the nest?
A) The eggs grew rapidly and used up the available oxygen.
B) The nest was flooded with water.
C) An algal bloom rapidly used up the available oxygen.
D) The eggs hatched and rapidly used up the available oxygen.
 According to the figure, what was the most likely cause of the zeroing of oxygen partial pressure in the nest?
According to the figure, what was the most likely cause of the zeroing of oxygen partial pressure in the nest?A) The eggs grew rapidly and used up the available oxygen.
B) The nest was flooded with water.
C) An algal bloom rapidly used up the available oxygen.
D) The eggs hatched and rapidly used up the available oxygen.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
33
Which of the following contributes to a respiratory gas partial pressure in solution?
I) O2
II) CO2
III) O2 bound to hemoglobin
IV) HCO3- dissolved in plasma
A) I and II
B) I, II, and III
C) I, II, and IV
D) I, II, III, and IV
I) O2
II) CO2
III) O2 bound to hemoglobin
IV) HCO3- dissolved in plasma
A) I and II
B) I, II, and III
C) I, II, and IV
D) I, II, III, and IV

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
34
If the diameter of the body of a larval fish exceeded _______, diffusion alone would not be able to meet the demands of its oxygen consumption.
A) 0.1 mm
B) 1.0 mm
C) 5 mm
D) 1 cm
A) 0.1 mm
B) 1.0 mm
C) 5 mm
D) 1 cm

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
35
A scuba diver remaining at a depth of 50 m for a significant amount of time needs to worry about the possible effects of which gas when he or she ascends?
A) Oxygen
B) Carbon dioxide
C) Nitrogen
D) Nitric oxide
A) Oxygen
B) Carbon dioxide
C) Nitrogen
D) Nitric oxide

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
36
The "bends" refers to a physiological condition sustained by a scuba diver in which
A) oxygen gas is too concentrated in the blood due to a dive that lasts too long at a deep depth.
B) nitrogen gas is too concentrated in the blood due to a dive that lasts too long at a deep depth.
C) oxygen gas comes out of solution in the blood due to a rapid ascent.
D) nitrogen gas comes out of solution in the blood due to a rapid ascent.
A) oxygen gas is too concentrated in the blood due to a dive that lasts too long at a deep depth.
B) nitrogen gas is too concentrated in the blood due to a dive that lasts too long at a deep depth.
C) oxygen gas comes out of solution in the blood due to a rapid ascent.
D) nitrogen gas comes out of solution in the blood due to a rapid ascent.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
37
The process that occurs when a gas mixture or an aqueous solution flows and gas molecules in the gas or liquid phase are carried from place to place by the fluid flow is called
A) diffusion.
B) convection.
C) tidal flow.
D) partial pressure.
A) diffusion.
B) convection.
C) tidal flow.
D) partial pressure.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
38
The principal process(es) by which animals use convection to transport gases is(are)
A) ventilation.
B) the pumping of blood.
C) digestion.
D) ventilation and the pumping of blood.
A) ventilation.
B) the pumping of blood.
C) digestion.
D) ventilation and the pumping of blood.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
39
The rate of convective gas transport depends on the
A) partial pressure of a particular gas in solution.
B) rate of fluid flow.
C) total concentration of gas in the fluid.
D) rate of fluid flow and the total concentration of gas in the fluid.
A) partial pressure of a particular gas in solution.
B) rate of fluid flow.
C) total concentration of gas in the fluid.
D) rate of fluid flow and the total concentration of gas in the fluid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
40
The movement of water across a fish gill is a good example of
A) permeability.
B) tidal flow.
C) unidirectional flow.
D) Henry's law.
A) permeability.
B) tidal flow.
C) unidirectional flow.
D) Henry's law.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
41
Refer to the figure shown.
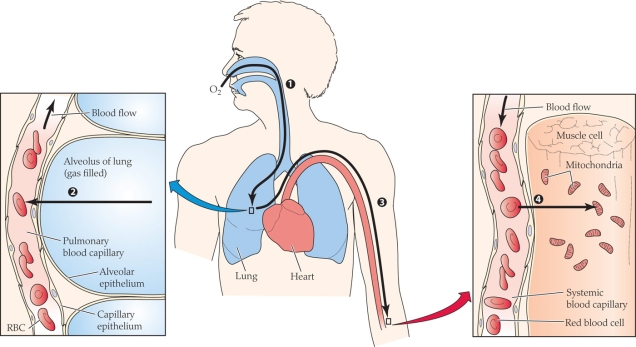 What process(es) is(are) occurring in the figure?
What process(es) is(are) occurring in the figure?
A) Diffusion
B) Convection
C) Active transport
D) Both diffusion and convection
 What process(es) is(are) occurring in the figure?
What process(es) is(are) occurring in the figure?A) Diffusion
B) Convection
C) Active transport
D) Both diffusion and convection

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
42
Refer to the figure shown.
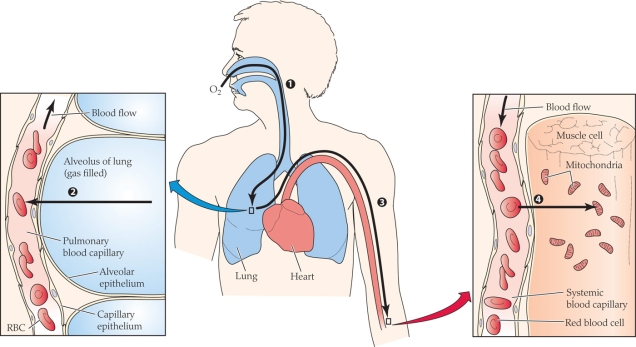 Which of the following correctly matches the process with the numerical label?
Which of the following correctly matches the process with the numerical label?
A) 1 = diffusion; 2 = diffusion; 3 = convection; 4 = diffusion
B) 1 = convection; 2 = diffusion; 3 = diffusion; 4 = diffusion
C) 1 = diffusion; 2 = convection; 3 = diffusion; 4 = convection
D) 1 = convection; 2 = diffusion; 3 = convection; 4 = diffusion
 Which of the following correctly matches the process with the numerical label?
Which of the following correctly matches the process with the numerical label?A) 1 = diffusion; 2 = diffusion; 3 = convection; 4 = diffusion
B) 1 = convection; 2 = diffusion; 3 = diffusion; 4 = diffusion
C) 1 = diffusion; 2 = convection; 3 = diffusion; 4 = convection
D) 1 = convection; 2 = diffusion; 3 = convection; 4 = diffusion

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
43
Refer to the figure shown.
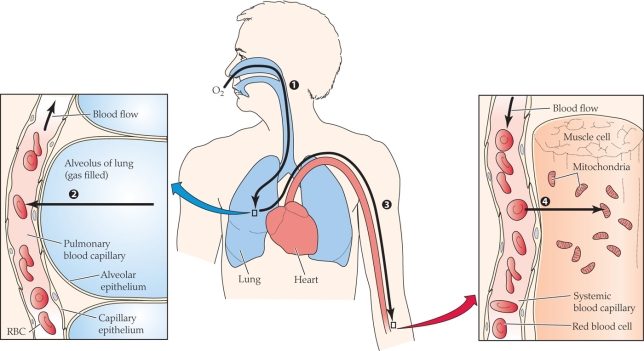 At what point(s) in the figure is the partial pressure of oxygen the lowest?
At what point(s) in the figure is the partial pressure of oxygen the lowest?
A) 1
B) 2
C) 3
D) 4
 At what point(s) in the figure is the partial pressure of oxygen the lowest?
At what point(s) in the figure is the partial pressure of oxygen the lowest?A) 1
B) 2
C) 3
D) 4

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
44
Refer to the figure shown.
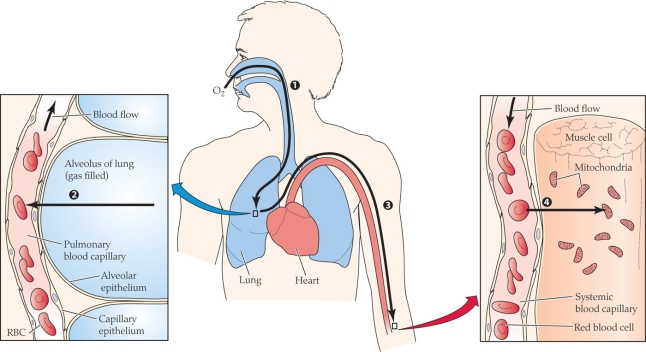 Which of the following have the most similar O2 partial pressures?
Which of the following have the most similar O2 partial pressures?
A) Ambient air and alveolar gas
B) Alveolar gas and arterial blood
C) Arterial blood and average systemic capillary blood
D) Average systemic capillary blood and mitochondria
 Which of the following have the most similar O2 partial pressures?
Which of the following have the most similar O2 partial pressures?A) Ambient air and alveolar gas
B) Alveolar gas and arterial blood
C) Arterial blood and average systemic capillary blood
D) Average systemic capillary blood and mitochondria

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
45
If active oxygen transport existed, one of its advantages would be
A) that it is a completely passive and therefore energy free process.
B) it could move oxygen down partial pressure gradients.
C) it could move oxygen against partial pressure gradients.
D) it would negate the need for convection.
A) that it is a completely passive and therefore energy free process.
B) it could move oxygen down partial pressure gradients.
C) it could move oxygen against partial pressure gradients.
D) it would negate the need for convection.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
46
In the analogy of the cascading waterfall, the height of each waterfall is analogous to
A) the heart rate.
B) the rate of blood flow.
C) the partial pressure difference between the corresponding two cell surfaces.
D) the energy difference between the air and the mitochondria.
A) the heart rate.
B) the rate of blood flow.
C) the partial pressure difference between the corresponding two cell surfaces.
D) the energy difference between the air and the mitochondria.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
47
Oxygen enters the mitochondria
A) by diffusion, at a rate that is dependent on the flow of the oxygenated capillary blood.
B) by diffusion, at a rate that is dependent on the difference in O2 partial pressure between the blood systemic capillaries and the mitochondria.
C) by convection, at a rate that is dependent on the difference in O2 partial pressure between the blood systemic capillaries and the mitochondria.
D) by convection, at a rate that is dependent on the flow of the oxygenated capillary blood.
A) by diffusion, at a rate that is dependent on the flow of the oxygenated capillary blood.
B) by diffusion, at a rate that is dependent on the difference in O2 partial pressure between the blood systemic capillaries and the mitochondria.
C) by convection, at a rate that is dependent on the difference in O2 partial pressure between the blood systemic capillaries and the mitochondria.
D) by convection, at a rate that is dependent on the flow of the oxygenated capillary blood.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
48
Which of the following is not a unit of pressure?
A) Millimeters of mercury
B) Pascals
C) Pounds per square inch
D) mL O2/L
A) Millimeters of mercury
B) Pascals
C) Pounds per square inch
D) mL O2/L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
49
STP refers to a(n)
A) temperature of 37°C.
B) pressure of 1 atm.
C) volume of 22.4 L.
D) oxygen percent of 20.95.
A) temperature of 37°C.
B) pressure of 1 atm.
C) volume of 22.4 L.
D) oxygen percent of 20.95.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
50
Why are volumes of gases expressed at standard conditions of temperature and pressure (STP)?
A) Because animals live at different temperatures and pressures.
B) Because the internal conditions of animals are always at different temperatures and pressures.
C) Because the volume occupied by any given molar amount of gas depends on temperature and pressure.
D) Because gases are always expressed as volume.
A) Because animals live at different temperatures and pressures.
B) Because the internal conditions of animals are always at different temperatures and pressures.
C) Because the volume occupied by any given molar amount of gas depends on temperature and pressure.
D) Because gases are always expressed as volume.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
51
If 2 L of air at 0°C contains 420 mL of O2, how many mL of O2 does it contain if the air is warmed to 24°C?
A) 210 mL
B) 384 mL
C) 420 mL
D) about 600 mL
A) 210 mL
B) 384 mL
C) 420 mL
D) about 600 mL

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
52
If air at 0°C contains 210 mL O2 per L, how much O2 does it contain if it is heated to 24°C?
A) 210 mL O2 per L
B) 420 mL O2 per L
C) 192 mL O2 per L
D) 105 mL O2 per L
A) 210 mL O2 per L
B) 420 mL O2 per L
C) 192 mL O2 per L
D) 105 mL O2 per L

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
53
Which environment has the lowest amount of O2 per L?
A) Air at 120°C
B) Air at -40°C
C) Freshwater at 24°C
D) Seawater at 40°C
A) Air at 120°C
B) Air at -40°C
C) Freshwater at 24°C
D) Seawater at 40°C

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
54
Which environment would have the lowest available oxygen?
A) At night, a small summer pond filled with algae
B) The summit of Mount Everest
C) The burrow of a black-tailed prairie dog
D) The burrow of a lemming beneath the arctic snow
A) At night, a small summer pond filled with algae
B) The summit of Mount Everest
C) The burrow of a black-tailed prairie dog
D) The burrow of a lemming beneath the arctic snow

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
55
One of the most important aspects of whether a particular microenvironment will experience hypoxia is
A) the initial oxygen content.
B) the initial carbon dioxide content.
C) rate of air or water exchange.
D) whether the respiratory medium is gas or liquid.
A) the initial oxygen content.
B) the initial carbon dioxide content.
C) rate of air or water exchange.
D) whether the respiratory medium is gas or liquid.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
56
Explain the two mechanisms by which oxygen and carbon dioxide move within organisms.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
57
Compare and contrast mole fractional concentration versus volume fractional concentration.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
58
What are the three most important characteristics of gases dissolved in aqueous solutions? Give examples in your answer.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
59
How does diffusion work in allowing a water beetle to use an air bubble as a gill? Why does the bubble not collapse?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
60
Why is it important for scuba divers to understand the concept of diffusion, especially as it pertains to N2?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
61
Gas transport in animals often occurs by alternation of convection and diffusion. Explain.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
62
What is meant by the oxygen cascade?

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
63
What is STP and why is it important when expressing volumes of gases? Include information about the relationship between STP and 1 mole of gas.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
64
Compare and contrast air and water as respiratory media. Include some approximate quantitative data in your comparison.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck
65
Compare and contrast the respiratory stresses of terrestrial versus aquatic environments.

فتح الحزمة
افتح القفل للوصول البطاقات البالغ عددها 65 في هذه المجموعة.
فتح الحزمة
k this deck








